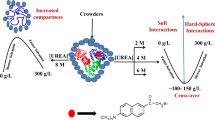
Abstract
The folding and unfolding of proteins inside a cell take place in the presence of macromolecules of various shapes and sizes. Such crowded conditions can significantly affect folding, stability, and biophysical properties of proteins. Thus, to logically mimic the intracellular environment, the thermodynamic stability of two different proteins (lysozyme and α-lactalbumin) was investigated in the presence of mixtures of three crowding agents (ficoll 70, dextran 70, and dextran 40) at different pH values. These crowders possess different shapes and sizes. It was observed that the stabilizing effect of mixtures of crowders is more than the sum effects of the individual crowder, i.e., the stabilizing effect is non-additive in nature. Moreover, dextran 40 (in the mixture) has been found to exhibit the greatest stabilization when compared with other crowders in the mixture. In other words, the small size of the crowder has been observed to be a dominant factor in stabilization of the proteins.

Graphical Abstract



Similar content being viewed by others
Abbreviations
- GdmCl:
-
Guanidinium chloride
- UV:
-
Ultra-violet
- T m :
-
Midpoint of thermal denaturation
- ΔH m :
-
Enthalpy change at Tm
- ΔC p :
-
Constant-pressure heat capacity change
- ∆G D°:
-
Gibbs free energy change at 25 °C
- F70:
-
Ficoll 70
- D70:
-
Dextran 70
- D40:
-
Dextran 40
References
Fulton, A. (1982). How crowded is the cytoplasm ? Cell, 30(2), 345–347.
Minton, A. P. (1983). The effect of volume occupancy upon the thermodynamic activity of proteins: some biochemical consequences. Molecular and Cellular Biochemistry, 55(2), 119–140.
Zimmerman, S. B., & Trach, S. O. (1991). Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli. Journal of Molecular Biology, 222(3), 599–620.
Medalia, O., Weber, I., Frangakis, A. S., Nicastro, D., Gerisch, G., & Baumeister, W. (2002). Macromolecular architecture in eukaryotic cells visualized by cryoelectron tomography. Science, 298(5596), 1209–1213.
Ellis, R. J., & Minton, A. P. (2003). Cell biology: join the crowd. Nature, 425(6953), 27–28.
Rivas, G., Ferrone, F., & Herzfeld, J. (2004). Life in a crowded world. EMBO Reports, 5(1), 23–27.
Minton, A. P. (1981). Excluded volume as a determinant of macromolecular structure and reactivity. Biopolymers, 20(10), 2093–2120.
Ellis, R. J. (2001). Macromolecular crowding: an important but neglected aspect of the intracellular environment. Current Opinion in Structural Biology, 11(1), 114–119.
Zimmerman, S. B., & Minton, A. P. (1993). Macromolecular crowding: biochemical, biophysical, and physiological consequences. Annual Review of Biophysics and Biomolecular Structure, 22(1), 27–65.
Ellis, R. J. (2001). Macromolecular crowding: obvious but underappreciated. Trends in Biochemical Sciences, 26(10), 597–604.
Goodsell, D. S. (1991). Inside a living cell. Trends in Biochemical Sciences, 16(6), 203–206.
Chebotareva, N. A., Kurganov, B. I., & Livanova, N. B. (2004). Biochemical effects of molecular crowding. Biochemistry (Moscow), 69(11), 1239–1251.
Christiansen, A., Wang, Q., Cheung, M. S., & Wittung-Stafshede, P. (2013). Effects of macromolecular crowding agents on protein folding in vitro and in silico. Biophysical Reviews, 5(2), 137–145.
Kuznetsova, I., Turoverov, K., & Uversky, V. (2014). What macromolecular crowding can do to a protein. International Journal of Molecular Sciences, 15(12), 23090–23140.
Kuznetsova, I., Zaslavsky, B., Breydo, L., Turoverov, K., & Uversky, V. (2015). Beyond the excluded volume effects: mechanistic complexity of the crowded milieu. Molecules, 20(1), 1377–1409.
Minton, A. P. (2001). The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. The Journal of Biological Chemistry, 276(14), 10577–10580.
Samiotakis, A., Wittung-Stafshede, P., & Cheung, M. S. (2009). Folding, stability and shape of proteins in crowded environments: experimental and computational approaches. International Journal of Molecular Sciences, 10(2), 572–588.
Zhou, H.-X., Rivas, G., & Minton, A. P. (2008). Macromolecular crowding and confinement: biochemical, biophysical, and potential physiological consequences. Annual Review of Biophysics, 37(1), 375–397.
Du, F., Zhou, Z., Mo, Z. Y., Shi, J. Z., Chen, J., & Liang, Y. (2006). Mixed macromolecular crowding accelerates the refolding of rabbit muscle creatine kinase: implications for protein folding in physiological environments. Journal of Molecular Biology, 364(3), 469–482.
Fan, J., ZHOU, Z., Chen, J., YANG, Z.-Z. and Liang, Y. (2012) The contrasting effect of macromolecular crowding on protein misfolding. FEBS JOURNAL, pp. 408–408. WILEY-BLACKWELL 111 RIVER ST, HOBOKEN 07030–5774, NJ USA.
Zhou, B.-R., Liang, Y., Du, F., Zhou, Z., & Chen, J. (2004). Mixed macromolecular crowding accelerates the oxidative refolding of reduced, denatured lysozyme: implications for protein folding in intracellular environments. Journal of Biological Chemistry, 279(53), 55109–55116.
Zhou, B. R., Zhou, Z., Hu, Q. L., Chen, J., & Liang, Y. (2008). Mixed macromolecular crowding inhibits amyloid formation of hen egg white lysozyme. Biochimica et Biophysica Acta, 1784(3), 472–480.
Batra, J., Xu, K., & Zhou, H. X. (2009). Nonadditive effects of mixed crowding on protein stability. Proteins, 77(1), 133–138.
Zhou, H. X. (2008). Effect of mixed macromolecular crowding agents on protein folding. Proteins, 72(4), 1109–1113.
Privalov, P. L., & Khechinashvili, N. N. (1974). A thermodynamic approach to the problem of stabilization of globular protein structure: a calorimetric study. Journal of Molecular Biology, 86(3), 665–684.
Beg, I., Minton, A. P., Hassan, M. I., Islam, A., & Ahmad, F. (2015). Thermal stabilization of proteins by mono- and oligosaccharides: measurement and analysis in the context of an excluded volume model. Biochemistry, 54(23), 3594–3603.
Beg, I., Minton, A. P., Islam, A., Hassan, M. I., & Ahmad, F. (2017). The pH dependence of saccharides’ influence on thermal denaturation of two model proteins supports an excluded volume model for stabilization generalized to allow for intramolecular electrostatic interactions. The Journal of Biological Chemistry, 292(2), 505–511.
Beg, I., Minton, A. P., Islam, A., Hassan, M. I., & Ahmad, F. (2018). Comparison of the thermal stabilization of proteins by oligosaccharides and monosaccharide mixtures: measurement and analysis in the context of excluded volume theory. Biophysical Chemistry, 237, 31–37.
Khan, S., Bano, Z., Singh, L. R., Hassan, M. I., Islam, A., & Ahmad, F. (2013). Testing the ability of non-methylamine osmolytes present in kidney cells to counteract the deleterious effects of urea on structure, stability and function of proteins. PLoS One, 8(9), e72533.
Shahid, S., Ahmad, F., Hassan, M. I., & Islam, A. (2015). Relationship between protein stability and functional activity in the presence of macromolecular crowding agents alone and in mixture: an insight into stability-activity trade-off. Archives of Biochemistry and Biophysics, 584, 42–50.
Singh, R., Haque, I., & Ahmad, F. (2005). Counteracting osmolyte trimethylamine N-oxide destabilizes proteins at pH below its pKa: measurements of thermodynamic parameters of proteins in the presence and absence of trimethylamine N-oxide. Journal of Biological Chemistry, 280(12), 11035–11042.
Arakawa, T., & Timasheff, S. N. (1982). Stabilization of protein structure by sugars. Biochemistry, 21(25), 6536–6544.
Mittal, S., & Singh, L. R. (2013). Denatured state structural property determines protein stabilization by macromolecular crowding: a thermodynamic and structural approach. PLoS One, 8(11), e78936.
Sasahara, K., McPhie, P., & Minton, A. P. (2003). Effect of dextran on protein stability and conformation attributed to macromolecular crowding. Journal of Molecular Biology, 326(4), 1227–1237.
Sharma, G. S., Mittal, S., & Singh, L. R. (2015). Effect of dextran 70 on the thermodynamic and structural properties of proteins. International Journal of Biological Macromolecules, 79, 86–94.
Dumitriu, S. (2004). Polysaccharides: Structural diversity and functional versatility, second edition. CRC Press.
Luby-Phelps, K., Castle, P. E., Taylor, D. L., & Lanni, F. (1987). Hindered diffusion of inert tracer particles in the cytoplasm of mouse 3T3 cells. Proceedings of the National Academy of Sciences of the United States of America, 84(14), 4910–4913.
Venturoli, D. and Rippe, B. (2005) Ficoll and dextran vs. globular proteins as probes for testing glomerular permselectivity: effects of molecular size, shape, charge, and deformability. ed.
Hamaguchi, K., & Kurono, A. (1963). Structure of muramidase (lysozyme) I. The effect of guanidine hydrochloride on muramidase. The Journal of Biochemistry, 54, 111–122.
Sugai, S., Yashiro, H., & Nitta, K. (1973). Equilibrium and kinetics of the unfolding of α-lactalbumin by guanidine hydrochloride. Biochimica et Biophysica Acta (BBA) - Protein Structure, 328(1), 35–41.
Nozaki, Y. (1972). The preparation of guanidine hydrochloride. Methods in Enzymology, 26, 43–50.
A guide to multi-detector gel permeation chromatography 2012. Available from: www.agilent.com/chem.
Fissell, W. H., Hofmann, C. L., Smith, R., & Chen, M. H. (2010). Size and conformation of Ficoll as determined by size-exclusion chromatography followed by multiangle light scattering. American Journal of Physiology - Renal Physiology, 298(1), F205–F208.
Sinha, A., Yadav, S., Ahmad, R., & Ahmad, F. (2000). A possible origin of differences between calorimetric and equilibrium estimates of stability parameters of proteins. Biochemical Journal, 345(3), 711–717.
Yadav, S., & Ahmad, F. (2000). A new method for the determination of stability parameters of proteins from their heat-induced denaturation curves. Analytical Biochemistry, 283(2), 207–213.
Becktel, W. J., & Schellman, J. A. (1987). Protein stability curves. Biopolymers, 26(11), 1859–1877.
Hiraoka, Y., & Sugai, S. (1984). Thermodynamics of thermal unfolding of bovine apo-α-lactalbumin. International Journal of Peptide and Protein Research, 23(5), 535–542.
Privalov, P. L. (1979). Stability of proteins: small globular proteins. Advances in Protein Chemistry, 33, 167–241.
Homchaudhuri, L., Sarma, N., & Swaminathan, R. (2006). Effect of crowding by dextrans and Ficolls on the rate of alkaline phosphatase-catalyzed hydrolysis: a size-dependent investigation. Biopolymers, 83(5), 477–486.
Shahid, S., Hassan, M. I., Islam, A., & Ahmad, F. (2017). Size-dependent studies of macromolecular crowding on the thermodynamic stability, structure and functional activity of proteins: in vitro and in silico approaches. Biochimica et Biophysica Acta (BBA) - General Subjects, 1861(2), 178–197.
Alfano, C., Sanfelice, D., Martin, S. R., Pastore, A., & Temussi, P. A. (2017). An optimized strategy to measure protein stability highlights differences between cold and hot unfolded states. Nature Communications, 8, 15428.
Ghahghaei, A., & Mohammadian, S. (2014). The effect of Arg on the structure perturbation and chaperone activity of α-crystallin in the presence of the crowding agent, dextran. Applied Biochemistry and Biotechnology, 174(2), 739–750.
Kumar, K., Bhargava, P., & Roy, U. (2011). In vitro refolding of Triosephosphate isomerase from L. donovani. Applied Biochemistry and Biotechnology, 164(7), 1207–1214.
Fan, Y.-Q., Liu, H.-J., Li, C., Luan, Y.-S., Yang, J.-M., & Wang, Y.-L. (2013). Inactivation of recombinant human brain-type creatine kinase during denaturation by guanidine hydrochloride in a macromolecular crowding system. Applied Biochemistry and Biotechnology, 169(1), 268–280.
Kumar, R., Sharma, D., Garg, M., Kumar, V., & Agarwal, M. C. (2018). Macromolecular crowding-induced molten globule states of the alkali pH-denatured proteins. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics, 1866(11), 1102–1114.
Benton, L. A., Smith, A. E., Young, G. B., & Pielak, G. J. (2012). Unexpected effects of macromolecular crowding on protein stability. Biochemistry, 51(49), 9773–9775.
Charlton, L. M., Barnes, C. O., Li, C., Orans, J., Young, G. B., & Pielak, G. J. (2008). Residue-level interrogation of macromolecular crowding effects on protein stability. Journal of the American Chemical Society, 130(21), 6826–6830.
Miklos, A. C., Li, C., Sharaf, N. G., & Pielak, G. J. (2010). Volume exclusion and soft interaction effects on protein stability under crowded conditions. Biochemistry, 49(33), 6984–6991.
Miklos, A. C., Sarkar, M., Wang, Y., & Pielak, G. J. (2011). Protein crowding tunes protein stability. Journal of the American Chemical Society, 133(18), 7116–7120.
Sarkar, M., Li, C., & Pielak, G. J. (2013). Soft interactions and crowding. Biophysical Reviews, 5(2), 187–194.
Sarkar, M., Lu, J., & Pielak, G. J. (2014). Protein crowder charge and protein stability. Biochemistry, 53(10), 1601–1606.
Sarkar, M., Smith, A. E., & Pielak, G. J. (2013). Impact of reconstituted cytosol on protein stability. Proceedings of the National Academy of Sciences, 110(48), 19342–19347.
Schlesinger, A. P., Wang, Y., Tadeo, X., Millet, O., & Pielak, G. J. (2011). Macromolecular crowding fails to fold a globular protein in cells. Journal of the American Chemical Society, 133(21), 8082–8085.
Wang, Y., Sarkar, M., Smith, A. E., Krois, A. S., & Pielak, G. J. (2012). Macromolecular crowding and protein stability. Journal of the American Chemical Society, 134(40), 16614–16618.
Ignatova, Z., & Gierasch, L. M. (2004). Monitoring protein stability and aggregation in vivo by real-time fluorescent labeling. Proceedings of the National Academy of Sciences of the United States of America, 101(2), 523–528.
Funding
This work was supported by grant from the Science & Engineering Research Board (SERB), India (SR/FT/LS-48/2010), FIST Program (SR/FST/LSI-541/2012), and Council of Scientific and Industrial Research (CSIR), India (37(1604)/13/EMR-II). SS is thankful to Maulana Azad National Fellowship, University Grants Commission (Government of India), for providing fellowship. FA is grateful to Indian National Science Academy for the award of Senior Scientist Position.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 28 kb)
Rights and permissions
About this article
Cite this article
Shahid, S., Ahmad, F., Hassan, M.I. et al. Mixture of Macromolecular Crowding Agents Has a Non-additive Effect on the Stability of Proteins. Appl Biochem Biotechnol 188, 927–941 (2019). https://doi.org/10.1007/s12010-019-02972-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-02972-9