Abstract
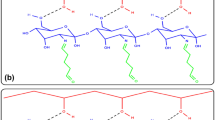
The aim of this paper was to evaluate different strategies of chitosan activation using cross-linking reagent like glycidol, epichlorohydrin, and glutaraldehyde for Thermomyces lanuginosus lipase (TLL) immobilization. Operational activity and stability by esterification of oleic acid with ethanol and thermal inactivation using these derivatives were investigated. Derivative obtained by sequentially activation with glycidol, ethylenediamine, and glutaraldehyde and subsequent TLL immobilization showed the best performance, with high hydrolytic activity value. Its stability was 15-fold higher than solubilized TLL in the evaluated inactivation conditions (60 °C, 25 mM sodium phosphate buffer pH 7). After 5 cycles of oleic acid esterification, only a few percentage of its conversion has reduced. On the other hand, glycidol-activated chitosan derivative showed very low hydrolytic activity value. Epichlorohydrin-activated chitosan derivative showed regular hydrolytic activity value. Both derivatives showed low immobilization yields. Operational stability of this last derivative was very low, where after the first cycle of oleic acid esterification, only 56% of its initial conversion was obtained.

Graphical Abstract





Similar content being viewed by others
References
Hasan, F., Shah, A. A., & Hameed, A. (2009). Methods for detection and characterization of lipases: a comprehensive review. Biotechnology Advances, 27, 782–798.
Castilho L. R, D. M. G. Freire. s.l. : Enzimas em Biotecnologia. Produção, Aplicações e Mercado, Interciência, Rio de Janeiro, Brazil, Vols. 2008; 1; p. 369–385.
Secundo, F., Carrea, G., Tarabiono, C., Gatti-Lafranconi, P., Brocca, S., Lotti, M., Jaeger, K. E., Puls, M., & Eggert, T. (2006). The lid is a structural and functional determinant of lipase activity and selectivity. Journal of Molecular Catalysis B: Enzymatic, 39, 166–170.
Wu, X., Ge, J., Zhu, J., Zhang, Y., Yong, Y., & Liu, Z. (2015). A general method for synthesizing enzyme-polymer conjugates in reverse emulsions using Pluronic as reactive surfactant. Chem. comm., 51(47), 9674–9677.
Zhu, J., Zhang, Y., Lu, D., Zare, R. N., Ge, J., & Liu, Z. (2013). Temperature-responsive enzyme–polymer nanoconjugates with enhanced catalytic activities in organic media. Chemical Communications, 49, 6090–6092.
Hou, M., Wang, R., Wu, X., Zhang, Y., Ge, J., & Liu, Z. (2015). Synthesis of lutein esters by using a reusable lipase-pluronic conjugate as the catalyst. Catalysis Letters, 145(10), 1825–1829.
Wu, X., & Ge, J. (2015). Enzymatic catalysis in melted polymer as green and reusable solvent. Catalysis Letter, 145, 1510–1513.
Royon, D., Daz, M., Ellenrieder, G., & Locatelli, S. (2007). Enzymatic production of biodiesel from cotton seed oil using t-butanol as a solvent. Bioresourse Technol., 98, 648–653.
Wu, X., Wang, R., Zhang, Y., Ge, J., & Liu, Z. (2014). Enantioselective ammonolysis of phenylglycine methyl ester with lipase–pluronic nanoconjugate in tertiary butanol. Catalysis Letters, 144(8), 1407–1410.
Zhang, Y., Dai, Y., Hou, M., Li, T., Ge, J., & Liu, Z. (2013). Chemo-enzymatic synthesis of valrubicin using pluronic conjugated lipase with temperature responsiveness in organic media. RSC Advances, 3(45), 22963–22966.
Ge, J., Lu, D., Wang, J., & Liu, Z. (2009). Lipase nanogel catalyzed transesterification in anhydrous dimethyl sulfoxide. Biomacromolecules, 10(6), 1612–1618.
Garcia-Galan, C., Berenguer-Murcia, A., Fernandez-Lafuente, R., & Rodrigues, R. C. (2011). Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synthesis and Catalysis, 353(16), 2885–2904.
Hung, T. C., Giridhar, R., Chiou, S. H., & Wu, W. T. (2003). Binary immobilization of Candida rugosa lipase on chitosan. Journal of Molecular Catalysis B: Enzymatic, 26, 69–78.
Kayser, H., Pienkoß, F., & Domínguez de María, P. (2014). Chitosan-catalyzed biodiesel synthesis: proof-of-concept and limitations. Fuel, 116, 267–272.
Dutta, P. K., Dutta, J., & Tripathi, V. S. (2004). Chitin and chitosan: chemistry, properties and applications. Journal of Scientific and Industrial Research, 63, 20–31.
Younes, I., Hajji, S., Frachet, V., Rinaudo, M., Jellouli, K., & Nasri, M. (2014). Chitin extraction from shrimp shell using enzymatic treatment. Antitumor, antioxidant and antimicrobial activities of chitosan. International Journal of Biological Macromolecules, 69, 489–498.
Silva, J. A., Macedo, G. P., Rodrigues, D. S., Giordano, R. L. C., & Gonçalves, L. R. B. (2012). Immobilization of Candida antarctica lipase B by covalent attachment on chitosan-based hydrogels using different support activation strategies. Biochemical Engineering J., 60, 16–24.
de Bezerra, T. M. S., Bassan, J. C., de Santos, V. T., O., Ferraz, A., & Monti, R. (2015). Covalent immobilization of laccase in green coconut fiber and use in clarification of apple juice. Process Biochemistry, 50, 417–423.
Nevell, T. P. (1963). In R. L. Whistler (Ed.), S.L. : Methods in carbohydrate chemistry (Vol. 3). NY: Academic Press.
Beppu, M. M., Arruda, E. J., Vieira, R. S., & Santos, N. N. (2004). Adsorption of Cu(II) on porous chitosan membranes functionalized with histidine. J Membrane Scice, 240, 227–235.
dos Santos, J. C. S., Bonazza, H. L., de Matos, L. J. B. L., Carneiro, E. A., Barbosa, O., Fernandez-Lafuente, R., Gonçalves, L. R. B., & de Sant’ Ana, H. B. (2017). Immobilization of CALB on activated chitosan: application to enzymatic synthesis in supercritical and near-critical carbon dioxide. Biotechnology Reports, 14, 16–26.
Adriano, W. S., Costa-Filho, E. H., Silva, J. A., Giordano, R. L. C., & Gonçalves, L. R. B. (2005). Stabilization of penicillin G acylase by immobilization on glutaraldehyde-activated chitosan. Brazilian J. Chem.l Eng., 22, 529–538.
Manoel, E. A., dos Santos, J. C. S., Freire, D. M. G., Rueda, N., & Fernandez-Lafuente, R. (2015). Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzyme and Microbial Technology, 71, 53–57.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Souza, T. C., Fonseca, T. S., Da Costa, J. A., Rocha, M. V. P., Mattos, M. C., Fernandez-Lafuente, R., Gonçalves, L. R. B., & Dos Santos, J. C. S. (2016). Cashew apple bagasse as a support for the immobilization of lipase B from Candida antarctica: application to the chemoenzymatic production of (R)-Indanol. J. of Molecular Catalysis B: Enzymatic, 130, 58–69.
Sadana, A., & Henley, J. P. (1987). Analysis of enzyme deactivations by a series-type mechanism: influence of modification on the activity and stability of enzymes. Annals of the New York Academy of Sciences, 501, 73–79.
Manzo, R. M., Sousa, M., Fenoglio, C. L., Gonçalves, L. R. B., & Mammarella, E. J. (2015). Chemical improvement of chitosan-modified beads for the immobilization of Enterococcus faecium DBFIQ E36 l-arabinose isomerase through multipoint covalent attachment approach. Journal of Industrial Microbiology & Biotechnology, 42, 1325–1340.
Silva, J. A., Macedo, G. P., Rodrigues, D. S., Giordano, R. L. C., & Gonçalves, L. R. B. (2012). Immobilization of Candida antarctica lipase B by covalent attachment on. Biochemical Engineering Journal, 60, 16–24.
Rodrigues, D. S., Mendes, A. A., Adriano, W. S., Gonçalves, L. R. B., & Giordano, R. L. C. (2008). Multipoint covalent immobilization of microbial lipase on chitosan and agarose activated by different methods. Journal of Molecular Catalysis B: Enzymatic, 51, 100–109.
Ribeiro, B. D., Castro, A. M., Coelho, M. A. Z., & Freire, D. M. G. (2011). Production and use of lipases in bioenergy: a review from the feedstocks to biodiesel production. Enzyme Research. doi:10.4061/2011/615803 (article ID 615803, 16 pp.)
Babaki, M., Yousefi, M., Habibi, Z., Mohammadi, M., Yousefi, P., Mohammadi, J., & Brask, J. (2016). Enzymatic production of biodiesel using lipases immobilized on silica nanoparticles as highly reusable biocatalysts: effect of water, t-butanol and blue silica gel contents. Renewable Energy, 91, 196–206.
Halling, P. J. (2004). Thermodynamic predictions for biocatalysis in nonconventional media: theory, tests, and recommendations for experimental design and analysis. Enzyme and Microbial Technology, 16, 178–206.
Li, N.-W., Zong, M.-H., & Wu, H. (2009). Highly efficient transformation of waste oil to biodiesel by immobilized lipase from Penicillium expansum. Process Biochemistry, 44, 685–688.
Oliveira, J. F. G., Lucena, I. L., Saboya, R. M. A., Rodrigues, M. L., Torres, A. E. B., Fernandes, F. A. N., Cavalcante, C. L., & Parente, E. J. (2010). Biodiesel production from waste coconut oil by esterification with ethanol: the effect of water removal by adsorption. Renewable Energy, 35, 2581–2584.
Li, Z., Zhang, Y., Su, Y., Ouyang, P., Ge, J., & Liu, Z. (2014). Spatial co-localization of multi-enzymes by inorganic nanocrystal-protein complexes. Chem. Comm, 50, 12465–12468.
Li, Z., Ding, Y., Li, S., Jiang, Y., Liu, Z., & Ge, J. (2016). Highly active, stable and self-antimicrobial enzyme catalysts prepared by biomimetic mineralization of copper hydroxysulfate. Nanoscale, 8, 17440–17445.
Wu, X., Ge, J., Yang, C., Hou, M., & Liu, Z. (2015). Facile synthesis of multiple enzyme-contained metal-organic frameworks in biomolecule-friendly environment. Chem. Comm., 51, 13408–13411.
Wu, X., Yang, C., Ge, J., & Liu, Z. (2015). Polydopamine tethered enzyme/metal-organic framework composites with high stability and reusability. Nanoscale, 7, 18883–18886.
Wu, X., Hou, M., & Ge, J. (2015). Metal-organic frameworks and inorganic nanoflowers: a type of emerging inorganic crystal nanocarriers for enzyme immobilization. Catalysis Science & Technology, 5, 5077–5085.
Acknowledgments
This work was partially sponsored by funds of the projects CAI + D 2011 501 201101 00357 LI (Universidad Nacional del Litoral, Santa Fe, Argentina), Argentina–Brazil Bilateral Cooperation Program BR/12/06 MINCyT-CAPES 2012 (Buenos Aires, Argentina) and CONICET. The authors declare no competing financial interest. The authors would like to thank the financial support of the Brazilian Research Agencies CNPq, CAPES, FINEP, FUNCAP, and FAPESP.
Author information
Authors and Affiliations
Corresponding author
Additional information
Chemical compounds used in this article
Chitosan (PubChem CID: 71853)Epichlorohydrin (PubChem CID: 7835)Glycidol (PubChem CID: 11164)Ethylenediamine (PubChem CID: 3301)p-Nitrophenyl butyrate (PubChem CID: 75834)
Highlights
✓ Thermomyces lanuginosus lipase was immobilized on activated chitosan.
✓ The best activation strategy involved glycidol, ethylenediamine, and glutaraldehyde.
✓ The stability of the best derivative was 15-fold higher than solubilized TLL.
✓ More than 90% of oleic acid conversion was reached after 12 h.
✓ The best derivative lost only 18% activity after 5 cycles of oleic acid esterification.
Rights and permissions
About this article
Cite this article
Bonazza, H.L., Manzo, R.M., dos Santos, J.C.S. et al. Operational and Thermal Stability Analysis of Thermomyces lanuginosus Lipase Covalently Immobilized onto Modified Chitosan Supports. Appl Biochem Biotechnol 184, 182–196 (2018). https://doi.org/10.1007/s12010-017-2546-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2546-9