Abstract
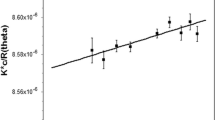
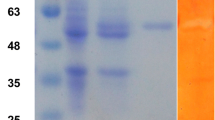
The gene RmGH28 from the organism Rhodothermus marinus, a putative glycosyl hydrolase family 28 polygalacturonase, was expressed in Escherichia coli and biochemically characterized. The gene was found to encode an exopolygalacturonase termed RmGH28, with galacturonic acid monomer and the polymer substrate (n-1) as the products released when acting on de-esterified polygalacturonic acid from citrus pectin. The enzyme at 25 °C had k cat ∼6 s−1 when acting on polygalacturonic acid, with K m ∼0.7 μM and a substrate inhibition constant K si ∼70 μM. The enzyme was hyperthermophilic, with one half initial enzyme activity remaining after 1-h incubation at 93.9 °C. Since the enzyme can function at high temperatures where reaction rates are increased and the risk of bacterial contamination is decreased, this indicates that RmGH28 can be useful in industry for generating galacturonic acid from pectin. The amino acid sequence of RmGH28 is highly homologous to the known hyperthermophilic exopolygalacturonases TtGH28 and Tm0437, which together can serve as starting points for structure-function studies and molecular breeding enzyme engineering approaches.




Similar content being viewed by others
Abbreviations
- RmGH28:
-
Enzyme in this study
- monoGalUA:
-
Galacturonic acid
- diGalUA:
-
Digalacturonic acid
- triGalUA:
-
Trigalacturonic acid
- polyGalUA:
-
Polygalacturonic acid
References
Mohnen, D. (2008). Pectin structure and biosynthesis. Current Opinion in Plant Biology, 11, 266–277.
Wong, D. (2008). Enzymatic deconstruction of backbone structures of the ramified regions in pectin. Protein Journal, 27, 30–42.
Lin, C. S. K., Pfaltzgraff, L. A., Herrero-Davila, L., Mubofu, E. B., Abderrahim, S., Clark, J. H., Koutinas, A. A., Kopsahelis, N., Stamatelatou, K., Dickson, F., Thankappan, S., Mohamed, Z., Brocklesby, R., & Luque, R. (2013). Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy and Environmental Science, 6, 426–464.
Rezzadori, K., Benedetti, S., & Amante, E. R. (2012). Proposals for the residues recovery: orange waste as raw material for new products. Food and Bioproducts Processing, 90, 606–614.
Pfaltzgraff, L. A., De Bruyn, M., Cooper, E. C., Budarin, V., & Clark, J. H. (2013). Food waste biomass: a resource for high-value chemicals. Green Chemistry, 15, 307–314.
Yapo, B. M., Lerouge, P., Thibault, J.-F., & Ralet, M.-C. (2007). Pectins from citrus peel cell walls contain homogalacturonans homogenous with respect to molar mass, rhamnogalacturonan I and rhamnogalacturonan II. Carbohydrate Polymers, 69, 426–435.
Wagschal, K., Jordan, D. B., Lee, C. C., Younger, A., Braker, J. D., & Chan, V. J. (2015). Biochemical characterization of uronate dehydrogenases from three Pseudomonads, Chromohalobacter salixigens, and Polaromonas naphthalenivorans. Enzyme and Microbial Technology, 69, 62–68.
Smith, T. N., Hash, K., Davey, C. L., Mills, H., Williams, H., & Kiely, D. E. (2012). Modifications in the nitric acid oxidation of d-glucose. Carbohydrate Research, 350, 6–13.
Kuivanen, J., Wang, Y. M. J., & Richard, P. (2016). Engineering Aspergillus niger for galactaric acid production: elimination of galactaric acid catabolism by using RNA sequencing and CRISPR/Cas9. Microbial Cell Factories, 15, 210.
Benz, J. P., Protzko, R. J., Andrich, J. M. S., Bauer, S., Dueber, J. E., & Somerville, C. R. (2014). Identification and characterization of a galacturonic acid transporter from Neurospora crassa and its application for Saccharomyces cerevisiae fermentation processes. Biotechnology for Biofuels, 7.
Mojzita, D., Wiebe, M., Hilditch, S., Boer, H., Penttilä, M., & Richard, P. (2010). Metabolic engineering of fungal strains for conversion of D-galacturonate to meso-Galactarate. Applied and Environmental Microbiology, 76, 169–175.
Moon, T. S., Yoon, S.-H., Lanza, A. M., Roy-Mayhew, J. D., & Prather, K. L. J. (2009). Production of glucaric acid from a synthetic pathway in recombinant Escherichia coli. Applied and Environmental Microbiology, 75, 589–595.
Werpy, T., & Petersen, G. (2004). Top value added chemicals from biomass, volume 1-results of screening for potential candidates from sugars and synthesis gas. US dept of energy, Washington DC. http://www1.eere.energy.gov/bioenergy/pdfs/35523.pdf , 1.
Abbadi, A., Gotlieb, K. F., Meiberg, J. B. M., Peters, J. A., & Van Bekkum, H. (1999). New Ca-sequestering materials: based on the oxidation of the hydrolysis products of lactose. Green Chemistry, 1, 231–235.
Li, X., Wu, D., Lu, T., Yi, G., Su, H., & Zhang, Y. (2014). Highly efficient chemical process to convert mucic acid into adipic acid and DFT studies of the mechanism of the rhenium-catalyzed deoxydehydration. Angewandte Chemie International Edition, 53, 4200–4204.
Kiely, D. E., Chen, L., & Lin, T. H. (2000). Synthetic polyhydroxypolyamides from galactaric, xylaric, D-glucaric, and D-mannaric acids and alkylenediamine monomers—some comparisons. Journal of Polymer Science, Part A: Polymer Chemistry, 38, 594–603.
Lavilla, C., Alla, A., Martínez De Ilarduya, A., Benito, E., García-Martín, M. G., Galbis, J. A., & Muñoz-Guerra, S. (2011). Carbohydrate-based polyesters made from bicyclic acetalized galactaric acid. Biomacromolecules, 12, 2642–2652.
Lewkowski, J. (2001). Convienient synthesis of furan-2,5-dicarboxylic acid and its derivatives. Polish Journal of Chemistry, 75, 1943–1946.
Taguchi, Y., Oishi, A., & Ida, H. (2008). One-step synthesis of dibutyl furandicarboxylates from galactaric acid. Chemistry Letters, 37, 50–51.
de Jong, E., Dam, M. A., Sipos, L., & Gruter, G. J. M. (2012), in Biobased monomers, polymers, and materials. ACS Symposium Series, American Chemical Society, vol. 1105, pp. 1–13.
Isherwood, F. A., Chen, Y. T., & Mapson, L. W. (1954). Synthesis of L-ascorbic acid in plants and animals. The Biochemical Journal, 56, 1–15.
Kuivanen, J., Penttilä, M., & Richard, P. (2015). Metabolic engineering of the fungal D-galacturonate pathway for L-ascorbic acid production. Microbial Cell Factories, 14, 2.
Tayi, L., Maku, R. V., Patel, H. K., & Sonti, R. V. (2016). Identification of pectin degrading enzymes secreted by Xanthomonas oryzae pv. Oryzae and determination of their role in virulence on rice. PLoS ONE, 11.
Kirsch, R., Heckel, D. G., & Pauchet, Y. (2016). How the rice weevil breaks down the pectin network: enzymatic synergism and sub-functionalization. Insect Biochemistry and Molecular Biology, 71, 72–82.
Yadav, S., Yadav, P. K., Yadav, D., & Yadav, K. D. S. (2009). Pectin lyase: a review. Process Biochemistry, 44, 1–10.
Lombard, V., Golaconda Ramulu, H., Drula, E., Coutinho, P. M., & Henrissat, B. (2014). The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Research, 42, D490–D495.
Yapo, B. M., Lerouge, P., Thibault, J.-F., & Ralet, M.-C. (2007). Pectins from citrus peel cell walls contain homogalacturonans homogenous with respect to molar mass, rhamnogalacturonan I and rhamnogalacturonan II. Carbohydrate Polymers, 69, 426–435.
Jansen, E. F., & MacDonnell, L. R. (1945). Influence of methoxyl content of pectic substances on the action of polygalacturonase. Archives of Biochemistry, 8, 97–112.
Pijning, T., van Pouderoyen, G., Kluskens, L., van der Oost, J., & Dijkstra, B. W. (2009). The crystal structure of a hyperthermoactive exopolygalacturonase from Thermotoga maritima reveals a unique tetramer. FEBS Letters, 583, 3665–3670.
Iacono, R., Cobucci-Ponzano, B., Strazzulli, A., Giglio, R., Maurelli, L., & Moracci, M. (2016). (Hyper)thermophilic biocatalysts for second generation biorefineries. Chimica Oggi/Chemistry Today, 34, 34–37.
Raddadi, N., Cherif, A., Daffonchio, D., Neifar, M., & Fava, F. (2015). Biotechnological applications of extremophiles, extremozymes and extremolytes. Applied Microbiology and Biotechnology, 99, 7907–7913.
Nelson, K. E., Clayton, R. A., Gill, S. R., Gwinn, M. L., Dodson, R. J., Haft, D. H., Hickey, E. K., Peterson, J. D., Nelson, W. C., Ketchum, K. A., McDonald, L., Utterback, T. R., Malek, J. A., Linher, K. D., Garrett, M. M., Stewart, A. M., Cotton, M. D., Pratt, M. S., Phillips, C. A., Richardson, D., Heidelberg, J., Sutton, G. G., Fleischmann, R. D., Eisen, J. A., White, O., Salzberg, S. L., Smith, H. O., Venter, J. C., & Fraser, C. M. (1999). Evidence for lateral gene transfer between Archaea and Bacteria from genome sequence of Thermotoga maritima. Nature, 399, 323–329.
Kluskens, L. D., Van Alebeek, G. J. W. M., Walther, J., Voragen, A. G. J., De Vos, W. M., & Van Der Oost, J. (2005). Characterization and mode of action of an exopolygalacturonase from the hyperthermophilic bacterium Thermotoga maritima. FEBS Journal, 272, 5464–5473.
Gill, S. C., & von Hippel, P. H. (1989). Calculation of protein extinction coefficients from amino acid sequence data. Analytical Biochemistry, 182, 319–326.
Nolan, M., Tindall, B. J., Pomrenke, H., Lapidus, A., Copeland, A., Glavina Del Rio, T., Lucas, S., Chen, F., Tice, H., Cheng, J.-F., Saunders, E., Han, C., Bruce, D., Goodwin, L., Chain, P., Pitluck, S., Ovchinikova, G., Pati, A., Ivanova, N., Mavromatis, K., Chen, A., Palaniappan, K., Land, M., Hauser, L., Chang, Y.-J., Jeffries, C. D., Brettin, T., Göker, M., Bristow, J., Eisen, J. A., Markowitz, V., Hugenholtz, P., Kyrpides, N. C., Klenk, H.-P., & Detter, J. C. (2009). Complete genome sequence of Rhodothermus marinus type strain (R-10T). Standards in Genomic Sciences, 1, 283–291.
Wagschal, K., Rose Stoller, J., Chan, V. J., Lee, C. C., Grigorescu, A. A., & Jordan, D. B. (2016). Expression and characterization of hyperthermostable exo-polygalacturonase TtGH28 from Thermotoga thermophilus. Molecular Biotechnology, 58, 509–519.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31, 426–428.
Jordan, D. B. (2008). B-D-xylosidase from Selenomonas ruminantium: catalyzed reactions with natural and artificial substrates. Applied Biochemistry and Biotechnology, 146, 137–149.
Stoller, J. R., Wagschal, K., Lee, C. C., & Jordan, D. B. (2017). A general correction to catalytic rates determined for nonprocessive exo-depolymerases acting on both substrate and product in the initial-rate measurement. Analytical Biochemistry, 523, 46–49.
Leatherbarrow, R. J. (2004). GraFit 5. Horley: Erithacus Software Ltd..
Alfredsson, G. A., Kristjansson, J. K., Hjörleifsdottir, S., & Stetter, K. O. (1988). Rhodothermus marinus, gen. nov., sp. nov., a thermophilic, halophilic bacterium from submarine hot springs in Iceland. Microbiology, 134, 299–306.
Karlsson, E. N., Hachem, M. A., Ramchuran, S., Costa, H., Holst, O., Svenningsen, Å. F., & Hreggvidsson, G. O. (2004). The modular xylanase Xyn10A from Rhodothermus marinus is cell-attached, and its C-terminal domain has several putative homologues among cell-attached proteins within the phylum Bacteroidetes. FEMS Microbiology Letters, 241, 233–242.
Liao, C. H., Revear, L., Hotchkiss, A., & Savary, B. (1999). Genetic and biochemical characterization of an exopolygalacturonase and a pectate lyase from Yersinia enterocolitica. Canadian Journal of Microbiology, 45, 396–403.
Abbott, D. W., & Boraston, A. B. (2007). The structural basis for exopolygalacturonase activity in a family 28 glycoside hydrolase. Journal of Molecular Biology, 368, 1215–1222.
Petersen, T. N., Brunak, S., Von Heijne, G., & Nielsen, H. (2011). SignalP 4.0: discriminating signal peptides from transmembrane regions. Nature Methods, 8, 785–786.
Kester, H. C. M., Kusters-Van Someren, M. A., Müller, Y., & Visser, J. (1996). Primary structure and characterization of an exopolygalacturonase from Aspergillus tubingensis. European Journal of Biochemistry, 240, 738–746.
Urbieta, M. S., Donati, E. R., Chan, K.-G., Shahar, S., Sin, L. L., & Goh, K. M. (2015). Thermophiles in the genomic era: biodiversity, science, and applications. Biotechnology Advances, 33, 633–647.
Hiromi, K. (1970). Interpretation of dependency of rate parameters on the degree of polymerization of substrate in enzyme-catalyzed reactions. Evaluation of subsite affinities of exo-enzyme. Biochemical and Biophysical Research Communications, 40, 1–6.
Cook, P. F., & Cleland, W. W. (2007). Enzyme kinetics and mechanism. Ed. New York: Garland Science.
Acknowledgments
This work was supported by US Department of Agriculture (USDA) CRIS 3620-41000-118-00D (D.B.J. and J.R.S.) and USDA CRIS 5325-41000-049-00 (K.C.W. and V.J.C.). This research was also supported in part by an appointment to the Agricultural Research Service (ARS) Research Participation Program administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the US Department of Energy (DOE) and the USDA (J.R.S.). ORISE is managed by Oak Ridge Associated Universities (ORAU) under DOE contract number DE-AC05-06OR23100. All opinions expressed in this paper are the authors’ and do not necessarily reflect the policies and views of USDA, ARS, DOE, or ORAU/ORISE. The mention of firm names or trade products does not imply that they are endorsed or recommended by the USDA over other firms or similar products not mentioned. USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wagschal, K.C., Rose Stoller, J., Chan, V.J. et al. Expression and Characterization of Hyperthermostable Exopolygalacturonase RmGH28 from Rhodothermus marinus . Appl Biochem Biotechnol 183, 1503–1515 (2017). https://doi.org/10.1007/s12010-017-2518-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2518-0