Abstract
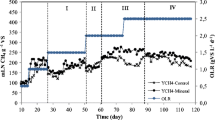
Silage and dry are the two typical cornstalk forms. Either form could be used as substrate in biogas plants and might be replaced by another when shortage occurred. This study focused on the feeding sequence of these two kinds of feedstocks, aiming to discuss their specific methane potential (SMP). A 15-day hydraulic retention time was chosen for semi-continuous experiments based on the batch test results. In semi-continuous experiments, before and after feedstocks were exchanged, the significantly decreased and comparable SMPs of silage and dry cornstalks indicated that a basis of unstable digestion would result in incomplete methane release from the subsequent digestion. A higher similarity of bacterial community structure and greater quantity of bacteria were shown in acidified silage cornstalk digestion through band similarity analysis. Methanosaetaceae and methanomicrobiales were the predominant methanogens, and aceticlastic methanogenesis was the main route for methane production. The different feeding sequences affected the hydrolysis course and further influenced the methanogenic proliferation. Our work suggests that silage cornstalk digestion should be conducted before dry cornstalk digestion.




Similar content being viewed by others
References
Amon, T., Amon, B., Kryvoruchko, V., Machmuller, A., Hopfner-Sixt, K., Bodiroza, V., Hrbek, R., Friedel, J., Potsch, E., Wagentristl, H., Schreiner, M., & Zollitsch, W. (2007). Methane production through anaerobic digestion of various energy crops grown in sustainable crop rotations. Bioprocess Technology, 98, 3204–3212.
Gupta, A., Kumar, A., Sharma, S., & Vijay, V. K. (2013). Comparative evaluation of raw and detoxified mahua seed cake for biogas production. Applied Energy, 102, 1514–1521.
Mei, Z. L., Liu, X. F., Huang, X. B., Li, D., Yan, Z. Y., Yuan, Y. X., & Huang, Y. J. (2016). Anaerobic mesophilic codigestion of rice straw and chicken manure: effects of organic loading rate on process stability and performance. Applied Biochemistry and Biotechnology. doi:10.1007/s12010-016-2035-6.
Gao, R. F., Yuan, X. F., Zhu, W. B., Wang, X. F., Chen, S. J., Cheng, X., & Cui, Z. J. (2012). Methane yield through anaerobic digestion for various maize varieties in China. Bioprocess Technology, 118, 611–614.
Weiland, P. (2010). Biogas production: current state and perspectives. Applied Microbiology and Biotechnology, 85, 849–860.
Herrmann, A. (2013). Biogas production from maize: current state, challenges and prospects. 2. Agronomic and environmental aspects. Bioenergy Research, 6, 372–387.
Holm-Nielsen, J. B., Al Seadi, T., & Oleskowicz-Popiel, P. (2009). The future of anaerobic digestion and biogas utilization. Bioprocess Technology, 100, 5478–5484.
Klimiuk, E., Pokój, T., Budzyński, W., & Dubis, B. (2010). Theoretical and observed biogas production from plant biomass of different fibre contents. Bioprocess Technology, 101, 9527–9535.
Sambusiti, C., Monlau, F., Ficara, E., Carrère, H., & Malpei, F. (2013). A comparison of different pre-treatments to increase methane production from two agricultural substrates. Applied Energy, 104, 62–70.
Jiang, D., Zhuang, D. F., Fu, J. Y., Huang, Y. H., & Wen, K. G. (2012). Bioenergy potential from crop residues in China: availability and distribution. Renewable & Sustainable Energy Reviews, 16(3), 1377–1382.
Chen, X., Zhang, Y., Gu, Y., Liu, Z., Shen, Z., Chu, H., & Zhou, X. (2014). Enhancing methane production from rice straw by extrusion pretreatment. Applied Energy, 122, 34–41.
Liu, W., Lund, H., Mathiesen, B. V., & Zhang, X. (2011). Potential of renewable energy systems in China. Applied Energy, 88, 518–525.
Wang, R., Sun, Y., Zhang, S., & Lu, X. (2012). Two-step pretreatment of corn stalk silage for increasing sugars production and decreasing the amount of catalyst. Bioprocess Technology, 120, 290–294.
Herrmann, C., Heiermann, M., & Idler, C. (2011). Effects of ensiling, silage additives and storage period on methane formation of biogas crops. Bioprocess Technology, 102, 5153–5161.
Himmel, M. E., Ding, S.-Y., Johnson, D. K., Adney, W. S., Nimlos, M. R., Brady, J. W., & Foust, T. D. (2007). Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science, 315(5813), 804–807.
Molinuevo-Salces, B., González-Fernández, C., Gómez, X., García-González, M. C., & Morán, A. (2012). Vegetable processing wastes addition to improve swine manure anaerobic digestion: evaluation in terms of methane yield and SEM characterization. Applied Energy, 91, 36–42.
Sanaei-Moghadam, A., Abbaspour-Fard, M. H., Aghel, H., Aghkhani, M. H., & Abedini-Torghabeh, J. (2014). Enhancement of biogas production by co-digestion of potato pulp with cow manure in a CSTR system. Applied Biochemistry and Biotechnology, 173, 1858–1869.
Wang, X. J., Yang, G. H., Feng, Y. Z., Ren, G. X., & Han, X. H. (2012). Optimizing feeding composition and carbon-nitrogen ratios for improved methane yield during anaerobic co-digestion of dairy, chicken manure and wheat straw. Bioprocess Technology, 120, 78–83.
Wang, X. J., Yang, G. H., Li, F., Feng, Y. Z., Ren, G. X., & Han, X. H. (2013). Evaluation of two statistical methods for optimizing the feeding composition in anaerobic co-digestion: mixture design and central composite design. Bioprocess Technology, 131, 172–178.
Zhang, D. D., Li, J., Guo, P., Li, P., Suo, Y. L., Wang, X. J., & Cui, Z. J. (2011). Dynamic transition of microbial communities in response to acidification in fixed-bed anaerobic baffled reactors (FABR) of two different flow directions. Bioprocess Technology, 102, 4703–4711.
APHA (2005) Standard methods for the examination of water and wastewater. 21th ed. Washington DC: American Public Health Association. American Water Works Association and Water Environment Federation.
Muyzer, G., de Waal, E. C., & Uitterlinden, A. G. (1993). Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Applied and Environmental Microbiology, 59, 695–700.
Lueders, T., & Friedrich, M. W. (2002). Effects of amendment with ferrihydrite and gypsum on the structure and activity of methanogenic populations in rice field soil. Applied and Environmental Microbiology, 68, 2484–2494.
Yu, Y., Lee, C., Kim, J., & Hwang, S. (2005). Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Bioinspiration & Biomimetics, 89, 670–679.
Xie, S., Lawlor, P. G., Frost, J. P., Hu, Z., & Zhan, X. (2011). Effect of pig manure to grass silage ratio on methane production in batch anaerobic co-digestion of concentrated pig manure and grass silage. Bioprocess Technology, 102, 5728–5733.
Panichnumsin, P., Nopharatana, A., Ahring, B., & Chaiprasert, P. (2010). Production of methane by co-digestion of cassava pulp with various concentrations of pig manure. Bioinspiration & Biomimetics, 34, 1117–1124.
Ariunbaatar, J., Panico, A., Esposito, G., Pirozzi, F., & Lens, P. N. L. (2014). Pretreatment methods to enhance anaerobic digestion of organic solid waste. Applied Energy, 123, 143–156.
Siegert, I., & Banks, C. (2005). The effect of volatile fatty acid additions on the anaerobic digestion of cellulose and glucose in batch reactors. Progress in Biocybernetics, 40(11), 3412–3418.
Nielsen, H. B., Uellendahl, H., & Ahring, B. K. (2007). Regulation and optimization of the biogas process: propionate as a key parameter. Bioinspiration & Biomimetics, 31, 820–830.
Speece, R. G. (1996). Anaerobic biotechnology for industrial wastewaters. Tennessee: Archae Press: Vanderbilt University.
Lee, S. H., Kang, H. J., Lee, Y. H., Lee, T. J., Han, K., Choi, Y., & Park, H. D. (2012). Monitoring bacterial community structure and variability in time scale in full-scale anaerobic digesters. Journal of Environmental Monitoring, 14, 1893–1905.
Klocke, M., Mahnert, P., Mundt, K., Souidi, K., & Linke, B. (2007). Microbial community analysis of a biogas-producing completely stirred tank reactor fed continuously with fodder beet silage as mono-substrate. Systematic and Applied Microbiology, 30, 139–151.
Krause, L., Diaz, N. N., Edwards, R. A., Gartemann, K. H., Kromeke, H., Neuweger, H., Puhler, A., Runte, K. J., Schluter, A., Stoye, J., Szczepanowski, R., Tauch, A., & Goesmann, A. (2008). Taxonomic composition and gene content of a methane-producing microbial community isolated from a biogas reactor. Journal of Biochemistry, 136, 91–101.
McHugh, S., Carton, M., Mahony, T. O., & Flaherty, V. (2003). Methanogenic population structure in a variety of anaerobic bioreactors. FEMS Microbiology Letters, 219, 297–304.
Wang, W., Yan, L., Cui, Z., Gao, Y., Wang, Y., & Jing, R. (2011). Characterization of a microbial consortium capable of degrading lignocellulose. Bioprocess Technology, 102, 9321–9324.
Grabowski, A., Blanchet, D., & Jeanthon, C. (2005). Characterization of long-chain fatty-acid-degrading syntrophic associations from a biodegraded oil reservoir. Research in Microbiology, 156, 814–821.
Bertin, L., Lampis, S., Todaro, D., Scoma, A., Vallini, G., Marchetti, L., Majone, M., & Fava, F. (2010). Anaerobic acidogenic digestion of olive mill wastewaters in biofilm reactors packed with ceramic filters or granular activated carbon. Water Research, 44, 4537–4549.
Biswas, R., Bagchi, S., Bihariya, P., Das, A., & Nandy, T. (2011). Stability and microbial community structure of a partial nitrifying fixed-film bioreactor in long run. Bioprocess Technology, 102, 2487–2494.
Conrad, R., & Klose, M. (2006). Dynamics of the methanogenic archaeal community in anoxic rice soil upon addition of straw. European Journal of Soil Science, 57, 476–484.
Williams, J., Williams, H., Dinsdale, R., Guwy, A., & Esteves, S. (2013). Monitoring methanogenic population dynamics in a full-scale anaerobic digester to facilitate operational management. Bioprocess Technology, 140, 234–242.
Klocke, M., Nettmann, E., Bergmann, I., Mundt, K., Souidi, K., Mumme, J., & Linke, B. (2008). Characterization of the methanogenic archaea within two-phase biogas reactor systems operated with plant biomass. Systematic and Applied Microbiology, 31, 190–205.
Leclerc, M., Delgènes, J., & Godon, J. (2004). Diversity of the archaeal community in 44 anaerobic digesters as determined by single strand conformation polymorphism analysis and 16S rDNA sequencing. Environmental Microbiology, 6, 809–819.
Chin, K. J., Lueders, T., Friedrich, M. W., Klose, M., & Conrad, R. (2004). Archaeal community structure and pathway of methane formation on rice roots. Microbial Ecology, 47, 59–67.
Wu, J. H., Liu, W. T., Tseng, I. C., & Cheng, S. S. (2001). Characterization of microbial consortia in a terephthalate-degrading anaerobic granular sludge system. Environmental Microbiology, 147, 373–382.
Kim, W., Lee, S., Shin, S. G., Lee, C., Hwang, K., & Hwang, S. (2010). Methanogenic community shift in anaerobic batch digesters treating swine wastewater. Water Research, 44, 4900–4907.
Acknowledgments
This work was supported by the special fund for Agro-scientific Research in the Public Interest (No. 2012AA101803), the National Key Technology R&D Program of China (No. 2012BAD14B06), and National Natural Science Foundation of China (Grant No. 51408600).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, Y., Yuan, X., Wen, B. et al. Methane Potential and Microbial Community Dynamics in Anaerobic Digestion of Silage and Dry Cornstalks: a Substrate Exchange Study. Appl Biochem Biotechnol 181, 91–111 (2017). https://doi.org/10.1007/s12010-016-2201-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2201-x