Abstract
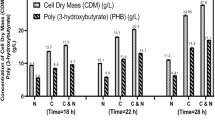
Bioplastic production from microbial sources is an emerging area which provides opportunities even to convert the wastes into bioplastics. Poly (3-hydroxybutyric acid), commonly called as PHB, is a bioplastic, which is stored as intracellular cytoplasmic inclusions in microorganisms. The objectives of this study are to calorimetrically monitor the PHB production and evaluate the thermokinetic data in a bioreaction calorimeter (BioRC1e). Thus, a well-known PHB-producing bacteria Ralstonia eutropha was selected for batch process in a bioreaction calorimeter. The metabolic heat generated was found to be correlated with the biomass, substrate consumption, oxygen uptake rate (OUR), carbon dioxide evolution rate (CER) and PHB production. The OUR pattern explained the oxidative metabolism of the strain R. eutropha. The heat yields due to biomass and glucose consumption during PHB production were found to be 12.56 and 13.56 kJ/g, respectively. The oxycalorific value obtained for the PHB production was 443.80 kJ/mol of O2. The concentration of PHB obtained in BioRC1e was 4.33 g/L with a production rate of 0.09 g/L/h. The chemical structure of the extracted PHB by R. eutropha was confirmed using fourier transform infrared spectroscopy (FT-IR) and 1H and 13C nuclear magnetic resonance (NMR) analysis.









Similar content being viewed by others
Abbreviations
- A :
-
Heat transfer area (m2)
- DO:
-
Dissolved oxygen
- PHB:
-
Poly (3-hydroxybutyric acid)
- Q :
-
Heat generated (kJ)
- Q met (t):
-
Cumulative metabolic heat (kJ)
- OUR:
-
Oxygen uptake rate (W)
- q :
-
Heat evolution rate (W)
- q a :
-
Heat of acid or base addition (W)
- q ac :
-
Heat accumulation in the bulk (W)
- q bl :
-
Baseline heat (W)
- q CO2 :
-
Heat of CO2 vaporization (W)
- q e :
-
Heat loss to the environment (W)
- q f :
-
Heat loss or gain from the feed (W)
- q g :
-
Heat loss due to aeration (W)
- q j :
-
Heat transfer through the reactor wall to the jacket oil (W)
- q s :
-
Heat input due to stirring (W)
- q r :
-
Heat generated by the reaction (W)
- T j :
-
Temperature of jacket oil (°C)
- T r :
-
Temperature of reactor contents (°C)
- U :
-
Overall heat transfer coefficient (W/m2K)
- Y Q/X :
-
Heat yield coefficient with respect to biomass (kJ/g)
- Y X/S :
-
Biomass yield coefficient with respect to substrate
- Y Q/O :
-
Heat generated due to oxygen consumed (kJ/mol of O2)
- Y Q/S :
-
Heat yield due to substrate consumption (kJ/g)
- O:
-
Oxygen
- S:
-
Substrate
- X:
-
Biomass
References
Atlas, R.M., & Bartha, R. (1993). In microbial ecology: Fundamentals and applications, The Benjamin/Publishing company, Redwood city,3, 39–43.
Philip, S., Keshavarz, T., & Roy, I. (2007). Polyhydroxyalkanoates: biodegradable polymers with a range of applications. Journal of Chemical Technology and Biotechnology, 82(3), 233–247. doi:10.1002/jctb.1667.
Reinecke, F., & Steinbuchel, A. (2009). Ralstonia eutropha strain H16 as model organism for PHA metabolism and for biotechnological production of technically interesting biopolymers. Journal of Molecular Microbiology and Biotechnology, 16(1–2), 91–108. doi:10.1159/000142897.
Islam Mozumder, M. S., Garcia-Gonzalez, L., Wever, H. D., & Volcke, E. I. P. (2015). Poly (3-hydroxybutyrate) (PHB) production from CO2: model development and process optimization. Biochemical Engineering Journal, 98, 107–116. doi:10.1016/j.bej.2015.02.031.
Venkateswar Reddy, M., Mawatari, Y., Yajima, Y., Seki, C., Hoshino, T., & Chang, Y.-C. (2015). Poly-3-hydroxybutyrate (PHB) production from alkylphenols, mono and poly-aromatic hydrocarbons using Bacillus sp. CYR1: a new strategy for wealth from waste. Bioresource Technology, 192, 711–717. doi:10.1016/j.biortech.2015.06.043.
Prabisha, T. P., Sindhu, R., Binod, P., Sankar, V., Raghu, K. G., & Pandey, A. (2015). Production and characterization of PHB from a novel isolate Comamonas sp. from a dairy effluent sample and its application in cell culture. Biochemical Engineering Journal, 101(August), 150–159. doi:10.1016/j.bej.2015.05.012.
García, A., Segura, D., Espín, G., Galindo, E., Castillo, T., & Peña, C. (2014). High production of poly-β-hydroxybutyrate (PHB) by an Azotobacter vinelandii mutant altered in PHB regulation using a fed-batch fermentation process. Biochemical Engineering Journal, 82, 117–123. doi:10.1016/j.bej.2013.10.020.
Shishatskaya, E. I., & Volova, T. G. (2004). A comparative investigation of biodegradable polyhydroxyalkanoate films as matrices for in vitro cell cultures. Journal of Materials Science. Materials in Medicine, 15(8), 915–923. doi:10.1023/B:JMSM.0000036280.98763.c1.
Chen, G.-Q., & Wu, Q. (2005). The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials, 26(33), 6565–6578. doi:10.1016/j.biomaterials.2005.04.036.
Michalak, M., Marek, A. A., Zawadiak, J., Kawalec, M., & Kurcok, P. (2013). Synthesis of PHB-based carrier for drug delivery systems with pH-controlled release. European Polymer Journal, 49(12), 4149–4156. doi:10.1016/j.eurpolymj.2013.09.021.
Dawes, E. A., & Senior, P. J. (1973). The role and regulation of energy reserve polymers in micro-organisms. Advances in Microbial Physiology, 10, 135–266.
Byrom, D. (1987). Polymer synthesis by microorganisms: technology and economics. Trends in Biotechnology, 5(9), 246–250. doi:10.1016/0167-7799(87)90100-4.
Rodríguez-Contreras, A., Koller, M., Miranda-de Sousa Dias, M., Calafell-Monfort, M., Braunegg, G., & Marqués-Calvo, M. S. (2013). High production of poly (3-hydroxybutyrate) from a wild Bacillus megaterium Bolivian strain. Journal of Applied Microbiology, 114(5), 1378–1387. doi:10.1111/jam.12151.
Sudesh, K., Abe, H., & Doi, Y. (2000). Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Progress in Polymer Science, 25(10), 1503–1555. doi:10.1016/S0079-6700(00)00035-6.
Wang, J., & Yu, H.-Q. (2007). Biosynthesis of polyhydroxybutyrate (PHB) and extracellular polymeric substances (EPS) by Ralstonia eutropha ATCC 17699 in batch cultures. Applied Microbiology and Biotechnology, 75(4), 871–8. doi:10.1007/s00253-007-0870-7.
Khanna, S., & Srivastava, A. K. (2008). Productivity enhancement of poly-(β-hydroxybutyrate) by fed-batch cultivation of nutrients using variable (decreasing) nutrient rate by Wautersia eutropha. Chemical Engineering Communications, 195(11), 1424–1436. doi:10.1080/00986440801964087.
Xu, Y., Wang, R.-H., Koutinas, A. A., & Webb, C. (2010). Microbial biodegradable plastic production from a wheat-based biorefining strategy. Process Biochemistry, 45(2), 153–163. doi:10.1016/j.procbio.2009.09.001.
Shi, H., Shiraishi, M., & Shimizu, K. (1997). Metabolic flux analysis for biosynthesis of poly (β-hydroxybutyric acid) in Alcaligenes eutrophus from various carbon sources. Journal of Fermentation and Bioengineering, 84(6), 579–587. doi:10.1016/S0922-338X(97)81915-0.
Ahn, W. S., Park, S. J., & Lee, S. Y. (2001). Production of poly (3-hydroxybutyrate) from whey by cell recycle fed-batch culture of recombinant Escherichia coli. Biotechnology Letters, 23, 235–240.
Tamer, I. M., & Chisti, Y. (1998). Optimization of poly (β-hydroxybutyric acid) recovery from Alcaligenes latus : combined mechanical and chemical treatments, 19(January).
Escapa, I. F., García, J. L., Bühler, B., Blank, L. M., & Prieto, M. A. (2012). The polyhydroxyalkanoate metabolism controls carbon and energy spillage in Pseudomonas putida. Environmental Microbiology, 14(4), 1049–1063. doi:10.1111/j.1462-2920.2011.02684.x.
von Stockar, U., & Marison, I. (1989). The use of calorimetry in biotechnology. In Bioprocesses and Engineering SE - 4 (Vol. 40, pp. 93–136). Springer Berlin Heidelberg. doi:10.1007/BFb0009829
Sivaprakasam, S., Mahadevan, S., & Rajakumar, S. (2008). Biocalorimetric studies of the metabolic activity of Pseudomonas aeruginosa aerobically grown in a glucose-limited complex growth medium. Bioscience, Biotechnology, and Biochemistry, 72(4), 936–942. doi:10.1271/bbb.70476.
Dhandapani, B., Mahadevan, S., & Mandal, A. B. (2012). Energetics of growth of Aspergillus tamarii in a biological real-time reaction calorimeter. Applied Microbiology and Biotechnology, 93(5), 1927–1936. doi:10.1007/s00253-011-3722-4.
Rajendran, K., Sekar, S., Mahadevan, S., Kumar Shanmugam, B., Jeyaprakash, R., Paramasamy, G., & Mandal, A. B. (2014). Biological real-time reaction calorimeter studies for the production of penicillin G acylase from Bacillus badius. Applied Biochemistry and Biotechnology, 172(8), 3736–3747. doi:10.1007/s12010-014-0800-y.
Dhandapani, B., Mahadevan, S., Dhilipkumar, S. S., Rajkumar, S., & Mandal, A. B. (2012). Impact of aeration and agitation on metabolic heat and protease secretion of Aspergillus tamarii in a real-time biological reaction calorimeter. Applied Microbiology and Biotechnology, 94(6), 1533–1542. doi:10.1007/s00253-012-3974-7.
Sivaprakasam, S., Mahadevan, S., & Bhattacharya, M. (2007). Biocalorimetric and respirometric studies on metabolic activity of aerobically grown batch culture of Pseudomonas aeruginosa. Biotechnology and Bioprocess Engineering, 12(4), 340–347. doi:10.1007/BF02931054.
Shanmugam, B. K., & Mahadevan, S. (2015). Metabolism and biotransformation of azo dye by bacterial consortium studied in a bioreaction calorimeter. Bioresource Technology, 196, 500–508. doi:10.1016/j.biortech.2015.07.108.
Voisard, D., Pugeaud, P., Kumar, A. R., Jenny, K., Jayaraman, K., Marison, I. W., & von Stockar, U. (2002). Development of a large-scale biocalorimeter to monitor and control bioprocesses. Biotechnology and Bioengineering, 80(2), 125–138. doi:10.1002/bit.10351.
Spanjers, H., Vanrolleghem, P., Olsson, G., & Dold, P. (1996). Respirometry in control of the activated sludge process. Water Science and Technology, 34(3–4), 117–126. doi:10.1016/0273-1223(96)84211-9.
Gustafsson, L. (1991). Microbiological calorimetry. Thermochimica Acta, 193, 145–171. doi:10.1016/0040-6031(91)80181-H.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31(3), 426–428. doi:10.1021/ac60147a030.
Solórzano, L. (1969). Determination of ammonia in natural waters by the phenol hypochlorite method. Limnology and Oceanography, 14(5), 799–801.
Law, J. H., & Slepecky, R. A. (1961). Assay of poly-β-hydroxybutyric acid. Journal of Bacteriology, 82(1), 33–36. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC279110/
Aslim, B., Yüksekdağ, Z. N., & Beyatli, Y. (2002). Determinationof PHB growth quantities of certain Bacillus species isolated from soil. Turkish Electronic Journal of Biotechnology, 6, 24–30.
Doran, P. M. (1995). Bioprocess engineering principles (p. 439). London: Academic Press Ltd.
Birou, B., Marison, I. W., & Stockar, U. V. (1987). Calorimetric investigation of aerobic fermentations. Biotechnology and Bioengineering, 30(5), 650–660. doi:10.1002/bit.260300509.
Kim, B. S., Lee, S. C., Lee, S. Y., Chang, H. N., Chang, Y. K., & Woo, S. I. (1994). Production of poly (3-hydroxybutyric acid) by fed-batch culture of Alcaligenes eutrophus with glucose concentration control. Biotechnology and Bioengineering, 43(9), 892–898. doi:10.1002/bit.260430908.
Pinches, A., & Pallent, L. J. (1986). Rate and yield relationships in the production of xanthan gum by batch fermentations using complex and chemically defined growth media. Biotechnology and Bioengineering, 28(10), 1484–1496. doi:10.1002/bit.260281006.
Schneiders, M. M., Grosshans, U., Busch, C., & Eigenberger, G. (1995). Biocalorimetry-supported analysis of fermentation processes. Applied Microbiology and Biotechnology, 43(3), 431–439.
Maskow, T., & Babel, W. (2000). Calorimetrically recognized maximum yield of poly-3-hydroxybutyrate (PHB) continuously synthesized from toxic substrates. Journal of biotechnology, 77(2–3), 247–53. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10682283
Karthikeyan, R., Surianarayanan, M., Sudharshan, S., Gunasekaran, P., & Asit Baran, M. (2011). Biocalorimetric and respirometric studies on production of Penicillin G acylase from Bacillus badius pac in E. coli DH5. Biochemical Engineering Journal, 55(3), 223–229. doi:10.1016/j.bej.2011.05.003.
Cooney, C. L., Wang, D. I., & Mateles, R. I. (2000). Measurement of heat evolution and correlation with oxygen consumption during microbial growth. Reprinted from Biotechnology and Bioengineering, Vol. XI, Issue 3, Pages 269–281 (1968). Biotechnology and Bioengineering, 67(6), 691–703.
Acknowledgements
One of the authors (Anusha SM) wishes to acknowledge the CSIR, New Delhi, for the CSIR-GATE fellowship and generous research grants. The authors wish to express their gratitude to Prof. NR Rajagopal for encouragement. This work was carried out with the funding from CSIR-Network project SETCA CSC 0113.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1248 kb)
Rights and permissions
About this article
Cite this article
Anusha, S.M., Leelaram, S. & Surianarayanan, M. Production of Poly (3-Hydroxybutyric Acid) by Ralstonia eutropha in a Biocalorimeter and its Thermokinetic Studies. Appl Biochem Biotechnol 179, 1041–1059 (2016). https://doi.org/10.1007/s12010-016-2049-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2049-0