Abstract
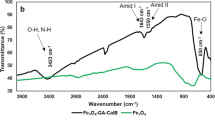
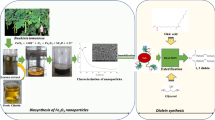
The present study describes grafting of zinc oxide (ZnO) nanoparticles with polyethyleneimine (PEI) followed by modification with glutraldehyde used as the bridge for binding the enzyme to support. The prepared nanocomposites were then characterized using Fourier transform infrared spectroscopy, thermogravimetric analysis, and transmission electron microscopy, utilized for synthesis of geranyl acetate in n-hexane. Among all the three prepared nanocomposites (ZnO + PEI, ZnO + PEI + SAA, ZnO + PEI + GLU), Candida rugosa lipase immobilized on ZnO-PEI-GLU was found to be best for higher ester synthesis. The operating conditions that maximized geranyl acetate resulted in the highest yield of 94 % in 6 h, molar ratio of 0.1:0.4 M (geraniol/vinyl acetate) in the presence of n-hexane as reaction medium. Various kinetic parameters such as V max, K i(G), K m(G), and K m(VA) were determined using nonlinear regression analysis for order bi–bi mechanism. The kinetic study showed that reaction followed order bi–bi mechanism with inhibition by geraniol. Activation energy (E a ) was found to be lower for immobilized lipase (12.31 kJ mol−1) than crude lipase (19.04 kJ mol−1) indicating better catalytic efficiency of immobilized lipase. Immobilized biocatalyst demonstrated 2.23-fold increased catalytic activity than crude lipase and recycled 20 times. The studies revealed in this work showed a promising perspective of using low-cost nanobiocatalysts to overcome the well-known drawbacks of the chemical-catalyzed route.










Similar content being viewed by others
References
Motevalizadeha, S. F., Khoobi, M., Sadighi, A., Khalilvand-Sedagheh, M., Pazhouhandeh, M., Ramazani, A., et al. (2015). Lipase immobilization onto polyethylenimine coated magnetic nanoparticles assisted by divalent metal chelated ions. Journal of Molecular Catalysis B: Enzymatic, 120, 75–83.
Fernandez-Lorente, G., Cabrera, Z., Godoy, C., Fernandez-Lafuente, R., Palomo, J. M., & Guisan, J. M. (2008). Interfacially activated lipases against hydrophobic supports: effect of the support nature on the biocatalytic properties. Process Biochemistry, 43, 1061–1067.
Hasan-Beikdashti, M., Forootanfar, H., Safiarian, M. S., Ameri, A., Ghahremani, M. H., Khoshayand, M. R., et al. (2012). Optimization of culture conditions for production of lipase by a newly isolated bacterium Stenotrophomonas maltophilia. Journal of the Taiwan Institute of Chemical Engineers, 43, 670–677.
Adlercreutz, P. (2013). Immobilization and application of lipases in organic media. Chemical Society Reviews, 42, 6406–6436.
Kapoor, M., & Gupta, M. N. (2012). Lipase promiscuity and its biochemical applications. Process Biochemistry, 47, 555–569.
Manoel, E. A., dos Santos, J. C. S., Freire, D. M. G., Rueda, N., & Fernandez-Lafuente, R. (2015). Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzyme and Microbial Technology, 71, 53–57.
Zhou, G., Wu, C., Jiang, X., Ma, J., Zhang, H., & Song, H. (2012). Active biocatalysts based on Candida rugosa lipase immobilized in vesicular silica. Process Biochemistry, 47, 953–959.
Fernandez-Lafuente, R. (2010). Lipase from Thermomyces lanuginosus: Uses and prospects as an industrial biocatalyst. Journal of Molecular Catalysis B: Enzymatic, 62, 197–212.
Khoobi, M., Motevalizadeh, S. F., Asadgol, Z., Forootanfarc, H., Shafiee, A., & Faramarzi, M. A. (2014). Synthesis of functionalized polyethylenimine-grafted mesoporous silica spheres and the effect of side arms on lipase immobilization and application. Biochemical Engineering Journal, 88, 131–141.
Zucca, P., & Sanjust, E. (2014). Inorganic materials as supports for covalent enzyme immobilization: Methods and mechanisms. Molecules, 19, 14139–14194.
Min, K., & Yoo, Y. J. (2014). Recent progress in nanobiocatalysis for enzyme immobilization and its application. Biotechnology and Bioprocess Engineering, 19, 553–567.
Stepankova, V., Bidmanova, S., Koudelakova, T., Prokop, Z., Chaloupkova, R., & Damborsky, J. (2013). Strategies for stabilization of enzymes in organic solvents. ACS Catalysis, 3, 2823–2836.
Hwang, E. T., & Gu, M. B. (2013). Enzyme stabilization by nano/microsized hybrid materials. Engineering in Life Science, 13, 49–61.
Gupta, A., Dhakate, S. R., Pahwa, M., Sinha, S., Chand, S., & Mathur, R. B. (2013). Geranyl acetate synthesis catalyzed by Thermomyces lanuginosus lipase immobilized on electrospun polyacrylonitrile nanofiber membrane. Process Biochemistry, 48, 124–132.
Cesar, M., Jose, M. P., Gloria, F. L., Jose, M. G., & Roberto, F. L. (2007). Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme and Microbial Technology, 40, 1451–1463.
Mohamad, N. R., Buanga, N. A., Mahat, N. A., Lok, Y. Y., Huyop, F., Aboul-Eneinc, H. Y., et al. (2015). A facile enzymatic synthesis of geranyl propionate by physically adsorbed Candida rugosa lipase onto multi-walled carbon nanotubes. Enzyme and Microbial Technology, 72, 49–55.
Brady, D., & Jordan, J. (2009). Advances in enzyme immobilization. Biotechnology Letters, 31, 1639–1650.
Garcia-Galann, C., Berenguer-Murcia, A., Fernandez-Lafuente, R., & Rodrigues, R. C. (2011). Potential of different enzyme immobilization strategies to improve enzyme performance. Advanced Synthesis and Catalysis, 353, 2885–2904.
Mogharabi, M., Nassiri-Koopaei, N., Bozorgi-Koushalshahi, M., Nafissi-Varcheh, N., Bagherzadeh, G., & Faramarzi, MA. (2012). Immobilization of laccase in alginate-gelatin mixed gel and decolorization of synthetic dyes. Bioinorganic Chemistry and Applications. 1–6. doi:10.1155/2012/823830
Dandvate, V., Keharia, H., & Madamwar, D. (2011). Ester synthesis using Candida rugosa lipase immobilized on magnetic nanoparticles. Biocatalysis and Biotransformation, 29, 37–45.
Raghavendra, T., Basak, A., Manocha, L., Shah, A., & Madamwar, D. (2013). Robust nanobioconjugates of Candida antarctica lipase B-multiwalled carbon nanotubes: characterization and application for multiple usages in non-aqueous biocatalysis. Bioresource Technology, 140, 103–110.
Tiwari, A., Terada, D., Yoshikawa, C., & Kobayashi, H. (2010). An enzyme-free highly glucose-specific assay using self-assembled amino benzene boronic acid upon polyelectrolytes electrospun nanofibers-mat. Talanta, 82, 1725–1732.
Patel, V., Gajera, H., Gupta, A., Manocha, L., & Madamwar, D. (2015). Synthesis of ethyl caprylate in organic media using Candida rugosa lipase immobilized on exfoliated graphene oxide: Process parameters and reusability studies. Biochemical Engineering Journal, 95, 62–70.
Faramarzi, M. A., & Sadighi, A. (2013). Insights into biogenic and chemical production of inorganic nanomaterials and nanostructures. Advances in Colloid and Interface Science, 189–190, 1–20.
Wang, R. H., Xin, J. H., & Tao, X. M. (2005). UV-blocking property of dumbbell-shaped ZnO crystallites on cotton fabrics. Inorganic Chemistry, 44, 3926–3930.
Selvarajan, E., Mohanasrinivasan, V., Subathra, C., & George, P. (2015). Immobilization of β-galactosidase from Lactobacillus plantarum HF571129 on ZnO nanoparticles: characterization and lactose hydrolysis. Bioprocess and Biosystems Engineering. doi:10.1007/s00449-015-1407-6.
de Lathouder, K. M., van Benthem, D. T. J., Wallin, S. A., Mateo, C., Fernandez- Lafuente, R., Guisan, J. M., et al. (2008). Polyethyleneimine (PEI) functionalized ceramic monoliths as enzyme carriers: preparation and performance. Journal of Molecular Catalysis B: Enzymatic, 50, 20–27.
Arica, M. Y., & Bayramoglu, G. (2004). Reversible immobilization of tyrosinase onto polyethyleneimine-grafted and Cu(II) chelated poly(HEMA-co-GMA) reactive membranes. Journal of Molecular Catalysis B: Enzymatic, 27, 255–265.
Badgujar, K. C., & Bhanage, B. M. (2014). Synthesis of geranyl acetate in non-aqueous media using immobilized Pseudomonas cepacia lipase on biodegradable polymer film: Kinetic modelling and chain length effect study. Process Biochemistry, 49, 1304–1313.
Winkler, U. K., & Stuckmann, M. (1979). Glycogen, hyaluronate and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. Journal of Bacteriology, 138, 663–670.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the folin phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Nicoletti, G., Cipolatti, E. P., Valerio, A., Carbonera, N. T. G., Soares, N. S., Theilacker, E., et al. (2015). Evaluation of different methods for immobilization of Candida antarctica lipase B (CalB lipase) in polyurethane foam and its application in the production of geranyl propionate. Bioprocess and Biosystems Engineering, 38, 1739–1748.
Barbosa, O., Ortiz, C., Berenguer-Murcia, A., Torres, R., Rodrigues, R. C., & Fernandez-Lafuente, R. (2014). Glutaraldehyde in bio-catalysts design: a useful crosslinker and a versatile tool in enzyme immobilization. RSC Advances, 14, 1583–1600.
Hussain, Q., Shakeel, A. A., Fahad, A., & Ameer, A. (2011). Immobilization of Aspergillus oryzae β-galactosidase on zinc oxide nanoparticles via simple adsorption mechanism. International Journal of Biological Macromolecules, 49, 37–43.
Xin, J. Y., Chen, L. L., Zhang, Y. X., Zhang, S., & Xia, C. G. (2011). Lipase-catalyzed transesterification of ethyl ferulate with triolein in solvent-free medium. Food and Bioproducts Processing, 89, 457–462.
Xiong, J., Huang, Y., Zhang, H., & Hou, L. (2014). Lipase-catalyzed transesterification synthesis of geranyl acetate in organic solvents and its kinetics. Food Science and Technology Research, 20, 207–216.
Dhake, K. P., Karoyo, A. H., Mohamed, M. H., Wilson, L. D., & Bhanage, B. M. (2013). Enzymatic activity studies of Pseudomonas cepacia lipase adsorbed onto copolymer supports containing β-cyclodextrin. Journal of Molecular Catalysis B: Enzymatic, 87, 105–112.
Ozyilmaz, E., Sayin, S., & Yilmaz, M. (2014). Improving catalytic hydrolysis reaction efficiency of sol–gel-encapsulated Candida rugosa lipase with magnetic β-cyclodextrin nanoparticles. Colloids and Surfaces B: Biointerfaces, 113, 182–189.
Yadav, G. D., & Devendran, S. (2012). Lipase catalyzed synthesis of cinnamyl acetate via transesterification in non-aqueous medium. Process Biochemistry, 47, 496–502.
Zhang, S., Shang, W., Yang, X., Zhang, X., Huang, Y., Zhang, S., et al. (2014). Immobilization of lipase with alginate hydrogel beads and the lipase-catalyzed kinetic resolution of a-phenylethanol. Journal of Applied Polymer Science, 131, 4017–4018.
Chua, L. S., & Sarmidi, M. R. (2006). Effect of solvent and initial water content on (R, S)-1-phenylethanol resolution. Enzyme and Microbial Technology, 38, 551–556.
Yadav, G. D., & Borkar, I. V. (2008). Kinetic modelling of immobilized lipase catalysis in synthesis of n-butyl levulinate. Industrial and Engineering Chemistry Research, 47, 3358–3363.
Segel, I. H. (1993). Enzyme kinetics: behavior and analysis of rapid equilibrium and steady-state enzyme systems. New York: Wiley.
Ferraz, L. I. R., Possebom, G., Alvez, E. V., Cansian, R. L., Paroul, N., de Oliveira, D., et al. (2014). Application of home-made lipase in the production of geranyl propionate by esterification of geraniol and propionic acid in solvent-free system. Biocatal. Agri. Biotechnol.. doi:10.1016/j.bcab.2014.07.003i.
Paroul, N., Grzegozeski, P. L., Chiaradia, V., Treichel, H., Cansian, R. L., Oliveira, J. V., et al. (2010). Production of geranyl propionate by enzymatic esterification of geraniol and propionic acid in solvent-free system. Journal of Chemical Technology and Biotechnology, 85, 1636–1641.
Rizzi, M., Stylos, P., Riek, A., & Reuss, M. (1992). A kinetic study of immobilized lipase catalyzing the synthesis of iso-amyl acetate by transesterification in n-hexane. Enzyme and Microbial Technology, 14, 709–714.
Romero, M. D., Calvo, L., Alba, C., & Daneshfar, A. (2007). A kinetic study of the iso-amyl acetate synthesis by immobilized lipase-catalyzed acetylation in n-hexane. Journal of Biotechnology, 127, 269–277.
Mohamad, N. R., Mahat, N. A., Huyop, F., Aboul-Enein, H. Y., & Wahab, R. A. (2015). Response surface methodological approach for optimizing production of geranyl propionate catalyzed by carbon nanotubes nanobioconjugates. Biotechnology and Biotechnological Equipment, 29, 732–739.
Jiang, Y., Guo, C., Xia, H., Mahmood, I., Liu, C., & Liu, H. (2009). Magnetic nanoparticles supported ionic liquids for lipase immobilization: enzyme activity in catalyzing esterification. Journal of Molecular Catalysis B: Enzymatic, 58, 103–109.
Misiunas, A., Talaikyte, Z., Niaura, G., Razumas, V., & Nylander, T. (2008). Thermomyces lanuginosus lipase in the liquid-crystalline phases of aqueous phytantriol: X-ray diffraction and vibrational spectroscopic studies. Biophysical Chemistry, 134, 144–156.
Mogharabi, M., & Faramarzi, M. A. (2014). Laccase and laccase-mediated systems in the synthesis of organic compounds. Advanced Synthesis and Catalysis, 356, 897–927.
Kumar, D., Nagar, S., Bhushan, I., Kumar, L., Parshad, R., & Gupta, V. K. (2013). Covalent immobilization of organic solvent tolerant lipase on aluminum oxide pellets and its potential application in esterification reaction. Journal of Molecular Catalysis B: Enzymatic, 87, 51–61.
Ozturk, T. K., & Kilinc, A. (2010). Immobilization of lipase in organic solvent in the presence of fatty acid additives. Journal of Molecular Catalysis B: Enzymatic, 67, 214–218.
Salema, J. H., Humeau, C., Chevalot, I., Harscoat-Schiavoa, C., Vanderessec, R., Blancharda, F., et al. (2010). Effect of acyl donor chain length on isoquercitrin acylation and biological activities of corresponding esters. Process Biochemistry, 45, 382–389.
Mateo, C., Palomo, J. M., Fernandez-Lorente, G., Guisan, J. M., & Fernandez-Lafuente, R. (2007). Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme and Microbial Technology, 40, 1451–1463.
Cristina, G. G., Berenguer-Murcia, A., Fernandez-Lafuente, R., & Rodrigues, R. C. (2011). Potential of Different Enzyme Immobilization Strategies to Improve Enzyme Performance. Advanced Synthesis and Catalysis, 353, 2885–2904.
Kasche, V. (1986). Mechanism and yields in enzyme catalysed equilibrium and kinetically controlled synthesis of β-lactam antibiotics, peptides and other condensation products. Enzyme and Microbial Technology, 8, 4–16.
Acknowledgments
The authors would like to acknowledge University Grants Commission (UGC) grant no. F. 42-167/2013 (SR), New Delhi, for financial support. Authors would also like to acknowledge (a) SICART, Vallabh Vidyanagar for FTIR and TEM facility and (b) Department of Physics for extending their TGA facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, V., Shah, C., Deshpande, M. et al. Zinc Oxide Nanoparticles Supported Lipase Immobilization for Biotransformation in Organic Solvents: A Facile Synthesis of Geranyl Acetate, Effect of Operative Variables and Kinetic Study. Appl Biochem Biotechnol 178, 1630–1651 (2016). https://doi.org/10.1007/s12010-015-1972-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1972-9