Abstract
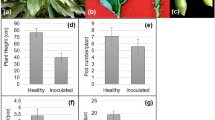
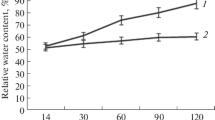
A laboratory study was delineated to ascertain the impact and the extent of Dolichos yellow mosaic virus (DYMV) on biochemical constituents and various enzyme levels in the leaves of hyacinth bean. DYMV-infected leaves of all the genotypes used in the study revealed significant and consistent changes in activities of CAT, APX, PPO, DHAR, and MDHAR paralleled with a compelling hike in proline levels. Unlike that in non-infected leaves of the genotypes VRSEM-301 and VRSEM-749, VRSEM-894 and VRSEM-855, the enzyme level did not alter much which extended equally with increased phenolics, suggesting a well-coordinated generation of free radicals thereby suppressing oxidative stress in the latter. The genotypes were also evaluated at molecular level for elucidating the presence of the virus by using five sets of primer pairs. Two primers viz., DAC1 and DAC2 witnessed the presence of the virus in both non-infected and infected leaves. The difference in the appearance and/or disappearance of bands according to non-infected to infect reverberates the variation between genotypes in defense against infection.



Similar content being viewed by others
Abbreviations
- DYMV:
-
Dolichos yellow mosaic virus
- DHAR:
-
Dehydroascorbate reductase
- MDHAR:
-
Monodehydroascorbate reductase
- APX:
-
Ascorbate peroxidase
- PPO:
-
Polyphenol oxidase
- ROS:
-
Reactive oxygen species
- CAT:
-
Catalase
- Chl a:
-
Chlorophyll a
- Chl b:
-
Chlorophyll b
- CAR:
-
Carotenoid
- Pro:
-
Proline
- DAS:
-
Days after sowing
References
Arnon, D. I. (1949). Copper enzymes in isolated chloroplast. Polyphenoxidase in beta vulgaris. Plant Physiology, 24, 1–15.
Atwal, A. K., Ramandeep Munshi, S. K., & Mann, A. P. S. (2003). Biochemical changes in relation to Alternaria leaf blight in Indian mustard. Plant Diseases Research (Ludhiana), 19, 57–59.
Awasthi, R.P. (1988). Comparative study of some exotic and indigenous oleiferous Brassica crop species in relation to their reaction to three Alternaria species PhD thesis (Plant Pathology, GBPUAT, Pantnagar). 290p.
Barka, D. M., Maaty, E., & Sabah, A. A. (2008). Quantitative and qualitative changes of phytochemical composition in viral-infested Nicotiana tabaccum. Journal of Applied Sciences Research, 4(9), 1083–1091.
Bates, L. S., Waldren, R. P., & Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil, 39, 205–207.
Chang, C., Yang, M., Wen, H., & Chern, J. (2002). Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Journal of Food and Drug Analysis, 10, 178–182.
Doyle, J. J., & Doyle, J. L. (1987). A rapid DNA isolation procedure from small quantity of fresh leaf material. Phytochemical Bulletin, 119, 11–15.
Durango, D., Quiones, W., Torres, F., Rosero, Y., Gil, J., & Echeverri, F. (2002). Phytoalexin accumulation in Colombian bean varieties and amino sugars as elicitors. Molecules, 7, 817–832.
El-Khallal, S. M. (2007). Induction and modulation of resistance in tomato plants against Fusarium wilt disease by bioagent fungi (arbuscular mycorrhiza) and/or hormonal elicitors (Jasmonic acid & Salicylic acid): 2-changes in the antioxidant enzymes, phenolic compounds and pathogen related-proteins. Australian Journal of Basic and Applied Sciences, 1, 717–732.
Gautam, R., Sthapit, B., Subedi, A., Poudel, D., Shrestha, P., & Eyzaguirre, P. (2008). Home gardens management of key species in Nepal: a way to maximize the use of useful diversity for the well-being of poor farmers. Plant Genetic Resources, 7, 142–153.
Gill, S. S., & Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry, 48, 909–930.
Hare, P. D., & Cress, W. A. (1997). Metabolic implications of stress induced proline accumulation in plants. Plant Growth Regulation, 21, 79–102.
Imeh, U., & Khokhar, S. (2002). Distribution of conjugated and free phenols in fruits: antioxidant activity and cultivar variations. Journal of Agricultural and Food Chemistry, 50, 6301–6306.
Jaypal, R., & Mahadevan, A. (1968). Biochemical changes in banana leaves in response to leaf spot pathogenesis. Indian Phytopathology, 21, 43–48.
Karpinski, S., Escobar, C., Karpinska, B., Creissen, G., & Mullineaux, P. (1997). Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell, 9, 627–640.
Lakshminarayan, S., Singh, P. S., & Singh, D. S. (2007). Relative occurrence of whitefly and yellow mosaic virus on some genotypes of Vigna radiate. Annals of Plant Protection Sciences, 15, 438–440.
Li, C. S. (1991). Identification of clover yellow vein virus infecting Dolichos lablab. Virologica Sinica, 6, 223–226.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193(1), 265–275.
Maass, B. L. (2006). Changes in seed morphology, dormancy and germination from wild to cultivated hyacinth bean germplasm (Lablab purpureus: Papilionoideae). Genetic Resources and Crops Evolution, 53, 1127–1135.
Malhotra, S. S., & Sarkar, S. K. (1979). Effects of sulphur dioxide on sugar and free amino acid content of pine seedlings. Physiologia Plantarum, 47, 223–228.
Maruthi, M. N., Rekha, A. R., Govindappa, M. R., Colvin, J., & Muniyappa, V. (2006). A distinct begomovirus causes Indian dolichos yellow mosaic disease. Plant Pathology, 55, 290.
McKersie, B. D., Hoekstra, F., & Krieg, L. (1990). Differences in the susceptibility of plant membrane lipids to peroxidation. Biochimica et Biophysica Acta, 1030, 119–126.
Melo, G. A., Shimizu, M. M., & Mazzafera, P. (2006). Polyphenoloxidase activity in coffee leaves and its role in resistance against the coffee leaf miner and coffee leaf rust. Phytochemistry, 67, 277–285.
Miller, G. L. (1972). Use of DNS reagent for determination of reducing sugar. Analytical Chemistry, 31, 1012–1013.
Misra, R., Sharma, S., Mishra, K., Kumar, A., & Sriram, S. (2008). Biochemical alterations induced in Taro in response to Phytophthora colocasiae infection. Advances in Natural and Applied Science, 2(3), 112–121.
Morris, J. B. (2009). Morphological and reproductive characterization in hyacinth bean, Lablab purpureus (L.) sweet germplasm with clinically proven nutraceutical and pharmaceutical traits for use as a medicinal food. Journal of Dietary Supplements, 6, 263–279.
Nakano, Y., & Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate specific peroxides in spinach chloroplast. Plant and Cell Physiology, 22, 867–880.
Onifade, A. K., & Agboola, Y. A. J. (2003). Effect of fungal infection on proximate nutrient composition of coconut (Cocosnucifera Linn) fruit. Journal of Food, Agriculture and Enviornment, 1(2), 141–142.
Porra, R. J., Klein, O., & Wright, P. E. (1989). European Journal of Biochemistry, 130, 509–516.
Rani, C. I., Veeraragavathatham, D., & Sanjutha, S. (2008). Analysis on biochemical basis of root-knot nematode (Meloidogyne incognita) resistance in tomato. Research Journal of Agriculture and Biological Sciences, 4, 866–870.
Rivero, R. M., Ruiz, J. M., Garcia, P. C., Lopez-Lefebre, L. R., Sanchy, E., & Romero, L. (2001). Resistance to cold and heat stress: accumulation of phenolic compounds in tomato and water melon plants. Plant Science, 160, 315–321.
Rodriguez Sanchez, E., Rubio-Wilhelmi, M. M., Cervilla, L. M., Blasco, B., Rios, J. J., Rosales, M. A., Romero, L., & Ruiz, J. M. (2010). Genotypic differences in some physiological parameters symptomatic for oxidative stress under moderate drought in tomato plants. Plant Science, 178, 30–40.
Sengooba, T. N., Spence, N. J., Walkey, D. G. A., Allen, D. G., & Femi Lana, A. (1997). The occurrence of bean common mosaic necrosis virus in wild and forage legumes in Uganda. Plant Pathology, 46, 95–103.
Shalitin, D., & Wolf, S. (2000). Cucumber mosaic virus infection affects sugar transport in melon plants. Physiologia Plantarum, 123, 597–604.
Shimoni, M., Bar-Zur, A., & Reuveni, R. (1991). The association of peroxidase activity and resistance of maize to Exserobilum triticum. Journal of Phytopathology, 131, 315–321.
Sobia, A., & Rais, A. (2010). Polymerase chain reaction based detection of dolichos yellow mosaic virus infecting Dolichos. Trends in Biosciences, 3(2), 133–134.
Soliva, R. C., Elez, P., Sebastian, M., & Martın, O. (2001). Evaluation of browning effect on avocado puree preserved by combined methods. Innovative Food Science and Emerging Technologies, 1, 261–268.
Torres, M. A., Jonathan, D. G., & Dangl, J. L. (2006). Reactive oxygen species signaling in response to pathogen. Plant Physiology, 141, 373–378.
Walker, J. R. L. (1975). The biology of plant phenolics. U.K.: Edvard Arnold Publication.
Yang, X., Liangyi, K., & Tien, P. (1996). Resistance of tomato infected with cucumber mosaic virus satellite RNA to potato spindle tuber viroid. Annals of Applied Biology, 129, 543–551.
Acknowledgment
The authors are thankful to the Director, Indian Institute of Vegetable Research, Varanasi for providing all of the necessary funds and facilities for conducting the research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Contribution
Dr Nagendra Rai—Experiment design and manuscript correction, Mr. Krishna Kumar Rai—performed all biochemical, molecular experiment, and preparation of manuscript, Dr V. Venkataravanappa—recorded the meteorological data and DYMV incidence data, Dr Sujoy Saha—manuscript correction.
Rights and permissions
About this article
Cite this article
Rai, N., Rai, K.K., Venkataravanappa, V. et al. Molecular Approach Coupled with Biochemical Attributes to Elucidate the Presence of DYMV in Leaf Samples of Lablab purpureus. L Genotypes. Appl Biochem Biotechnol 178, 876–890 (2016). https://doi.org/10.1007/s12010-015-1915-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1915-5