Abstract
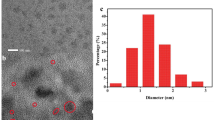
We report a surfactant-free synthesis of monodispersed gold nanoparticles (AuNPs) with average size of 15 nm. An approach for visual and fluorescent sensing of urea in aqueous solution based on shift in surface plasmon band (SPB) maxima as well as quench in fluorescence intensity. To enable the urea detection, we functionalized the thiol-capped gold nanoparticles with urease, the enzyme specific to urea using carbodiimide chemistry. The visible color changed of the gold colloidal solution from red to blue (or purple); this was evident from quenching in absorbance and fluorescence intensity, is the principle applied here for the sensing of urea. The solution turns blue when the urea concentration exceeds 8 mg/dL which reveals visual lower detection limit. The lower detection limits governed by the fluorescence quenching were found 5 mg/dL (R 2 = 0.99) which is highly sensitive and selective compared to shift in SPB maxima. The approach depicted here seems to be important in clinical diagnosis.










Similar content being viewed by others
References
Daniel, M., & Astruc, D. (2004). Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chemical Reviews, 104(1), 293–346.
Genzel, L., Martin, T. P., & Kreibig, U. (1975). Dielectric function and plasma resonances of small metal particles. Zeitschrift fur Physik B: Condensed Matter and Quanta, 21(4), 339–346. doi:10.1007/BF01325393.
Balk, S. P., Ko, Y.-J., & Bubley, G. J. (2003). Biology of prostate-specific antigen. Journal of Clinical Oncology, 21(2), 383–391. doi:10.1200/JCO.2003.02.083.
Lal, S., Westcott, S. L., Taylor, R. N., Jackson, J. B., Nordlander, P., & Halas, N. J. (2002). Light interaction between gold nanoshells plasmon resonance and planar optical waveguides. The Journal of Physical Chemistry B, 106(22), 5609–5612. doi:10.1021/jp014154s.
Link, S., Wang, Z. L., & El-Sayed, M. A. (1999). Alloy formation of gold−silver nanoparticles and the dependence of the plasmon absorption on their composition. The Journal of Physical Chemistry B, 103(18), 3529–3533. doi:10.1021/jp990387w.
Goldys, E. M., & Sobhan, M. A. (2012). Fluorescence of colloidal gold nanoparticles is controlled by the surface adsorbate. Advanced Functional Materials, 22(9), 1906–1913. doi:10.1002/adfm.201102057.
Jiang, Y., Horimoto, N. N., Imura, K., Okamoto, H., Matsui, K., & Shigemoto, R. (2009). Bioimaging with two-photon-induced luminescence from triangular nanoplates and nanoparticle aggregates of gold. Advanced Materials, 21(22), 2309–2313. doi:10.1002/adma.200802312.
Burda, C., Chen, X., Narayanan, R., & El-Sayed, M. A. (2005). Chemistry and properties of nanocrystals of different shapes. Chemical Reviews, 105(4), 1025–1102. doi:10.1021/cr030063a.
Cushing, B. L., Kolesnichenko, V. L., & O’Connor, C. J. (2004). Recent advances in the liquid-phase syntheses of inorganic nanoparticles. Chemical Reviews, 104(9), 3893–3946. doi:10.1021/cr030027b.
Brust, M., Walker, M., Bethell, D., Schiffrin, D. J., & Whyman, R. (1994). Synthesis of thiol-derivatised gold nanoparticles in a two-phase Liquid? Liquid system. Journal of the Chemical Society, Chemical Communications, (7), 801. doi:10.1039/c39940000801.
Enustun, B. V., & Turkevich, J. (1963). Coagulation of colloidal gold. Journal of the American Chemical Society, 85(21), 3317–3328. doi:10.1021/ja00904a001.
Frens, G. (1973). Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature Physical Science, 241(105), 20–22. doi:10.1038/10.1038/physci241020a0.
Chow, M. K., & Zukoski, C. F. (1994). Gold sol formation mechanisms: role of colloidal stability. Journal of Colloid and Interface Science, 165(1), 97–109. doi:10.1006/jcis.1994.1210.
Shao, Y., Jin, Y., & Dong, S. (2004). Synthesis of gold nanoplates by aspartate reduction of gold chloride. Chemical Communications, 9, 1104–1105. doi:10.1039/b315732f.
Goia, D. V., & Matijević, E. (1998). Preparation of monodispersed metal particles. New Journal of Chemistry, 22(11), 1203–1215. doi:10.1039/a709236i.
Jana, N. R., Gearheart, L., & Murphy, C. J. (2001). Evidence for seed-mediated nucleation in the chemical reduction of gold salts to gold nanoparticles. Chemistry of Materials, 13(7), 2313–2322. doi:10.1021/cm000662n.
Schmid, G. (2005). Nanoparticles: From theory to application. Wiley-VCH Verlag GmbH & Co. KGaA. doi:10.1002/3527602399.
Nath, N., & Chilkoti, A. (2002). A colorimetric gold nanoparticle sensor to interrogate biomolecular interactions in real time on a surface. Analytical Chemistry, 74(3), 504–509. doi:10.1021/ac015657x.
Xie, J., Zheng, Y., & Ying, J. Y. (2009). Protein-directed synthesis of highly fluorescent gold nanoclusters. Journal of the American Chemical Society, 131(3), 888–889. doi:10.1021/ja806804u.
Radhakumary, C., & Sreenivasan, K. (2011). Naked eye detection of glucose in urine using glucose oxidase immobilized gold nanoparticles. Analytical Chemistry, 83(7), 2829–2833. doi:10.1021/ac1032879.
Wilson, R. (2008). The use of gold nanoparticles in diagnostics and detection. Chemical Society Reviews, 37(9), 2028–2045. doi:10.1039/b712179m.
Dueck, C. L., Neufeld, R. J., & Chang, T. M. S. (1986). Hydrodynamics and urea hydrolysis in a microencapsulated urease, fluidized bed reactor. The Canadian Journal of Chemical Engineering, 64(4), 540–546. doi:10.1002/cjce.5450640403.
Li, X., Tian, J., Nguyen, T., & Shen, W. (2008). Paper-based microfluidic devices by plasma treatment. Analytical Chemistry, 80(23), 9131–9134. doi:10.1021/ac801729t.
Martinez, A. W., Phillips, S. T., Wiley, B. J., Gupta, M., & Whitesides, G. M. (2008). FLASH: a rapid method for prototyping paper-based microfluidic devices. Lab on a Chip, 8(12), 2146–2150. doi:10.1039/b811135a.
Aslan, K., & Pérez-Luna, V. H. (2002). Surface modification of colloidal gold by chemisorption of alkanethiols in the presence of a nonionic surfactant. Langmuir, 18(16), 6059–6065. doi:10.1021/la025795x.
Zhu, T., Vasilev, K., Kreiter, M., Mittler, S., & Knoll, W. (2003). Surface modification of citrate-reduced colloidal gold nanoparticles with 2-mercaptosuccinic acid. Langmuir, 19(22), 9518–9525. doi:10.1021/la035157u.
Gruber, B. A., & Leonard, N. J. (1975). Dynamic and static quenching of 1, N6-ethenoadenine fluorescence in nicotinamide 1, N6-ethenoadenine dinucleotide and in 1, N6-etheno-9-[3-(indol-3-yl)propyl]adenine. Proceedings of the National Academy of Sciences of the United States of America, 72, 3966–3969.
Cheng, P. P. H., Silvester, D., Wang, G., Kalyuzhny, G., Douglas, A., & Murray, R. W. (2006). Dynamic and static quenching of fluorescence by 1–4 nm diameter gold monolayer-protected clusters. Journal of Physical Chemistry B, 110, 4637–4644.
Crispin, X., Geskin, V., Crispin, A., Cornil, J., Lazzaroni, R., Salaneck, W. R., & Brédas, J.-L. (2002). Characterization of the interface dipole at organic/ metal interfaces. Journal of the American Chemical Society, 124(27), 8131–8141. doi:10.1021/ja025673r.
Acknowledgments
Author UKP thankfully acknowledges the financial support from the University Grant Commission (UGC), Govt. of India. Author NRN acknowledges the financial support from UGC (RJNF-JRF-SC-252). Author AS acknowledges the DST and CAS of Department of Physics, B.H.U. Varanasi, India, Author PSS acknowledges CAS of Department of Zoology, B.H.U. Varanasi, India. Author UKP is specially thankful to Dr. Gauthama (IIT Kanpur) for TEM analysis and Dr. A. C. Pandey (NAC, Allahabad) for SEM facilities.
Compliance with Ethical Standards
On behalf of all the authors in the present manuscript, I wish to declare that:
-
This manuscript has not been submitted to more than one journal for simultaneous consideration.
-
No data have been fabricated or manipulated (including images) to support our conclusions.
-
No data, text, or theories by others have been presented in manuscript. The statements in the text have been given with the study of the cited literature.
-
Consent to submit the manuscript has been received explicitly from all coauthors before the work is submitted.
-
Authors whose names appear on the submission have contributed sufficiently to the scientific work and therefore share collective responsibility and accountability for the results.
-
Research grants received from various funding agencies for all the authors have been acknowledged, respectively, in the manuscript.
-
The authors declare that they have no conflict of interest.
-
The authors declare that there is no experiment that has been done on any human or animal during the study of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Upendra Kumar Parashar and Narsingh R. Nirala contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1544 kb)
Rights and permissions
About this article
Cite this article
Parashar, U.K., Nirala, N.R., Upadhyay, C. et al. Urease Immobilized Fluorescent Gold Nanoparticles for Urea Sensing. Appl Biochem Biotechnol 176, 480–492 (2015). https://doi.org/10.1007/s12010-015-1589-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1589-z