Abstract
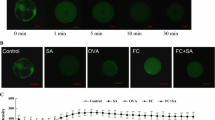
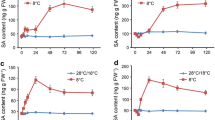
Ca2+ serves as a second messenger in plant responses to different signals, and salicylic acid (SA) has been recognized as a signal mediating plant responses to many stresses. We recently found that SA treatment led to the cytoplasmic acidification of Salvia miltiorrhiza cells and alkalinization of extracellular medium. Here, we demonstrate that SA can rapidly induce Ca2+ mobilization in protoplasts, but the induction can be blocked with a channel blocker of either plasma or organellar membranes. Following SA, A 23187, or 10 mmol/L Ca2+ treatment, rosmarinic acid (RA) accumulation reached the highest level at 16 h, whereas the peak was found at 10 h if plasma membrane channel blockers were used. By contrast, the highest accumulation of RA occurred at 16 h when organellar channels were blocked, exhibiting the same tendency with SA-induced cells. In agreement with these observations, both phenylalanine ammonia-lyase (PAL) activity and its gene expression detected by real-time PCR also showed the same patterns. These results indicate that SA treatment firstly results in calcium release from internal stores, which in turn leads to PAL activity increase, RA accumulation, and a large amount of Ca2+ influx from apoplast after 10 h of SA induction.












Similar content being viewed by others
References
Chandra, S., & Chandra, R. (2011). Engineering secondary metabolite production in hairy roots. Phytochemistry Review, 10, 371–395.
Kai, G., Xu, H., Zhou, C., Liao, P., Xiao, J., Luo, X., You, L., & Zhang, L. (2011). Metabolic engineering tanshinone biosynthetic pathway in Salvia miltiorrhiza hairy root cultures. Metabolic Engineering, 13, 319–327.
Yan, Q., Shi, M., Ng, J., & Wu, J. Y. (2006). Elicitor-induced rosmarinic acid accumulation and secondary metabolism enzyme activities in Salvia miltiorrhiza hairy roots. Plant Science, 170, 853–858.
Zhang, Y., Yan, Y. P., Wu, Y. C., Hua, W. P., Chen, C., Ge, Q., & Wang, Z. Z. (2014). Pathway engineering for phenolic acid accumulation in Salvia miltiorrhiza by combinational genetic manipulation. Metabolic Engineering, 21, 71–80.
Dong, J., Wan, G., & Liang, Z. (2010). Accumulation of salicylic acid-induced phenolic compounds and raised activities of secondary metabolic and antioxidative enzymes in Salvia miltiorrhiza cell culture. Journal of Biotechnology, 148, 99–104.
Vicente, M. R. S., & Plasencia, J. (2011). Salicylic acid beyond defence: its role in plant growth and development. Journal of Experimental Botany, 62, 3321–3338.
Vijaya, S. N., Udayasri, P., Aswani Kumar, V. V. V., Ravi, B. B., Phani, K. Y., & Vijay, V. M. (2010). Advancements in the production of secondary metabolites. Journal of Natural Products, 3, 112–123.
Durrant, W. E., & Dong, X. (2004). Systemic acquired resistance. Annual Review of Phytopathology, 24, 185–209.
Shah, J. (2003). The salicylic acid loop in plant defense. Current Opinion on Plant Biology, 6, 365–371.
Vlot, A. C., Dempsey, D. M. A., & Klessing, D. F. (2009). Salicylic acid, a multifaceted hormone to combat disease. Nature Review of Phytopathology, 47, 177–206.
Batistič, O., & Kudla, J. (2012). Analysis of calcium signaling pathways in plants. Biochimica et Biophysia Acta, 1820, 1283–1293.
Dodd, A. N., Kudla, J., & Sanders, D. (2010). The language of calcium signaling. Annual Review of Plant Biology, 61, 593–620.
Kudla, J., Batistič, O., & Hashimoto, K. (2010). Calcium signals: the lead currency of plant information processing. Plant Cell, 22, 541–563.
Sanders, D., Brownlee, C., & Harper, J. F. (1999). Communicating with calcium. Plant Cell, 11, 691–706.
Petersen, M., & Simmonds, M. S. J. (2003). Rosmarinic acid. Phytochemistry, 62, 121–125.
Knight, M. R., Smith, S. M., & Trewavas, A. J. (1992). Wind-induced plant motion immediately increases cytosolic calcium. Proceedings of National Academy of Science of the United States of America, 89, 4967–4971.
Song, J., & Wang, Z. Z. (2009). Molecular cloning, expression and characterization of a phenylalanine ammonia-lyase gene (SmPAL1) from Salvia miltiorrhiza. Molecular Biology Reports, 36, 939–952.
Sticher, L., Mauch-Mani, B., & Métreaux, J. P. (1997). Systemic acquired resistance. Annual Review of Phytopathology, 35, 235–270.
Van Camp, W., Van Montagu, M., & Inzé, D. (1998). H2O2 and NO: redox signals in disease resistance. Trend in Plant Science, 3, 330–334.
Chen, H., Liu, L., Dong, J., & Xia, G. (2012). Hydrogen peroxide is involved in the signal transduction of salicylic acid-induced salvianolic acid B biosynthesis in Salvia miltiorrhiza cell cultures. Chinese Journal of Biotechnology, 28, 834–846.
Guo, B., Liang, Y. C., Zhu, Y. G., & Zhao, F. J. (2007). Role of salicylic acid in alleviating oxidative damage in rice roots (Oryza sativa) subjected to cadmium stress. Environmental Pollution, 147, 743–749.
Taguchi, G., Yazawa, T., Hayashida, N., & Okazaki, M. (2001). Molecular cloning and heterologous expression of novel glucosyltransferases from tobacco cultured cells that have broad substrates specificity and are induced by salicylic acid and auxin. European Journal of Biochemistry, 268, 4086–4094.
Zhao, J., Davis, L. C., & Verpoorte, R. (2005). Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnology Advance, 23, 283–333.
Mikkelsen, M. D., Petersen, B. L., Glawischnig, E., Jensen, A. B., Andreasson, E., & Halkier, B. A. (2003). Modulation of CYP79 genes and glucosinolate profiles in Arabidopsis by defense signaling pathways. Plant Physiology, 131, 298–308.
Bulgakov, V. P., Tchernoded, G. K., Mischenko, N. P., Khodakovskaya, M. V., Glazunov, V. P., Radchenko, S. V., Zvereva, E. V., Fedoreyev, S. A., & Zhuravlev, Y. N. (2002). Effect of salicylic acid, methyl jasmonate, ethephon and cantharidin on anthraquinone production by Rubia cordifolia callus cultures transformed with rol B and rol C genes. Journal of Biotechnology, 97, 213–221.
Pitta-Alvarez, S. I., Spollansky, T. C., & Giulietti, A. M. (2000). The influence of different biotic and abiotic elicitors on the production and profile of tropane alkoids in hairy root cultures of Brugmansia candida. Enzyme and Microbiology Technology, 26, 252–258.
Peiter, E. (2011). The plant vacuole: emitter and receiver of calcium signals. Cell Calcium, 50, 120–128.
Trewavas, A. J., & Malho, R. (1998). Ca2+ signalling in plant cells: the big network! Current Opinion in Plant Biology, 1, 428–433.
Halliwell, B., & Gutteridge, J. (1999). Free radicals in biology and medicine (3rd ed., p. 936). Oxford: Oxford University Press.
Pei, Z., Murata, Y., Benning, G., Thomine, S., Kluesener, B., Allen, G. J., Grill, E., & Schroeder, J. I. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signaling in guard cells. Nature, 406, 731–734.
Smith, C. J. (1994). Signal transduction in elicitation of phytoalexin synthesis. Biochemical Society Transactions, 22, 414–419.
Lecourieux, D., Mazars, C., Pauly, N., Ranjeva, R., & Pugin, A. (2002). Analysis and effects of cytosolic free calcium increases in response to elicitors in Nicotiana plumbaginifolia cells. Plant Cell, 14, 2627–2641.
Armero, J., & Tena, M. (2001). Possible role of plasma membrane H+-ATPase in the elicitation of phytolexion and related isoflavone root secretion in chickpea (Cicer arietinum L.) seedlings. Plant Science, 161, 791–798.
Boller, T. (1995). Chemoperception of microbial signals in plant cells. Annual Review of Plant Physiology and Plant Molecular Biology, 46, 189–214.
Liu, L., Wang, C., Dong, J., Su, H., Zhuo, Z. Q., & Xue, Y. X. (2013). Effect of calcium on medium alkalinization induced by salicylic acid in Salvia miltiorrhiza suspension cultures. Chinesis Journal of Biotechnology, 29, 986–997.
Sakano, K. (2001). Metabolic regulation of pH in plant cells: role of cytoplasmic pH in defense reaction and secondary metabolism. International Review of Cytology, 206, 1–44.
Hattori, T., & Ohta, Y. (1985). Induction of phenylalanine ammonia-lyase activation and isoflavone glucoside accumulation in suspension-cultured cells of red bean, Vigna angularis, by phytoalexins elicitors, vanadate and elevation of medium pH. Plant Cell and Physiology, 26, 1101–1110.
Hagendoorn, M. J. M., Traas, T. P., Boon, J. J., & van der Plas, L. H. W. (1990). Orthovanadate induced lignin production, in batch and continuous cultures of Petunia hybrida. Journal of Plant Physiology, 137, 72–80.
Van der Luit, A. H., Olivari, C., Haley, A., Knight, M. R., & Trewavas, A. J. (1999). Distinct calcium signaling pathways regulate calmodulin gene expression in tobacco. Plant Physiology, 121, 705–714.
Blume, B., Nurnberger, T., Nass, N., & Scheel, D. (2000). Receptor-mediated increase in cytoplasmic free calcium required for activation of pathogen defense in parsley. Plant Cell, 12, 1425–1440.
Vasconsuelo, A., Morelli, S., Picotto, G., Giulietti, A. M., & Boland, R. (2005). Intracellular calcium mobilization: a key step for chitosan-induced anthraquinone production in Rubia tinctorum L. Plant Science, 169, 712–720.
McAinsh, M. R., Brownlee, C., & Hetherington, A. M. (1992). Visualising changes in cytosolic Ca2+ during the response to stomal guard cells to abscisic acid. Plant Cell, 4, 1113–1122.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 31170274) and Academic Backbone Youth of Northwest A&F University (No. Z111020906).
Author information
Authors and Affiliations
Corresponding author
Additional information
Hongbo Guo and Nan Zhu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Guo, H., Zhu, N., Deyholos, M.K. et al. Calcium Mobilization in Salicylic Acid-Induced Salvia miltiorrhiza Cell Cultures and Its Effect on the Accumulation of Rosmarinic Acid. Appl Biochem Biotechnol 175, 2689–2702 (2015). https://doi.org/10.1007/s12010-014-1459-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1459-0