Abstract
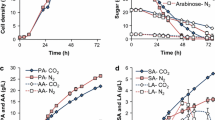
Previously, it was shown that the gas produced in an ethanol fermentor using either corn or barley as feedstock could be sparged directly into an adjacent fermentor as a feedstock for succinic acid fermentation using Escherichia coli AFP184. In the present investigation, it was demonstrated that the CO2 produced in a corn ethanol fermentor could be absorbed in a base solution and the resultant carbonate solution used both for pH control and supply of the CO2 requirement in succinic acid fermentation. Thus, the CO2 produced in a 5-L corn mash containing 30 wt% total solids was absorbed in a packed column containing 2 L of either 5 M NaOH, 5 M KOH, or 15 wt% NH4OH, and the resultant carbonate solutions were used for pH control in a succinic acid fermentor. The results obtained indicated no significant differences between succinic acid production in these experiments and when 2.5 M solutions of Na2CO3, K2CO3, and (NH4)2CO3 from commercial sources were used. In a commercial setting, the demonstrated capture of CO2 in liquid form will allow transportation of the carbonate solutions to locations not in the immediate vicinity of the ethanol plant, and excess carbonate salts can also be recovered as value-added products.




Similar content being viewed by others
References
OECD-FAO (2012).Agricultural Outlook 2012–2021, chapter 3.
Rosentrater, K. A. (2006). BioCycle, 47, 44–52.
Ethanol industry outlook (2014) Washington: Renewable Fuels Association.
Rushing, S. A. (2010). Biofuels Digest, February 17.
Xu, Y., Isom, L., & Hanna, M. A. (2010). Bioresource Technology, 101, 3311–3319.
Chisti, Y. (2007). Biotechnology Advances, 25, 294–306.
Nghiem, N. P., Donnelly, M., & Millard, C. S, (1999). US Patent 5,869,301.
Donnelly, M., & Nghiem, N. P., (2004). US Patent 6,743,610.
McKinlay, J. B., Vieille, C., & Zeikus, J. G. (2007). Applied and Environmental Microbiology, 76, 727–740.
Okino, S. H., Noburyu, R., Suda, M., Jojima, T., Inui, M., & Yukawa, H. (2008). Applied Microbiology and Biotechnology, 81, 459–464.
Werpy, T., & Petersen, G. (2004). Top value added chemicals from biomass. US Department of Energy
Fumagalli, C. (1997). In J. I. Kroschwitz & M. Howe-Grant (Eds.), Kirk–Othmer encyclopedia of chemical technology (Vol. 22, pp. 1072–1102). New York: Wiley.
Nghiem, N. P., Davison, B. H., Donnelly, M. I., Tsai, S. -P., & Frye, J. G. (2000). Chemicals and materials from renewable resources. In J. J. Bozell (Ed.), American Chemical Society Symposium Series 784, 160–173.
Berglund, K. A., Dunuwila, D. D., & Alizadeh, H. (2003). US Patent 6,623,657.
Berglund, K. A., Dunuwila, D. D., & Alizadeh, H. (2003). US Patent 6,635,188.
Millard, C. S., Chao, Y.-P., Liao, J. C., & Donnelly, M. I. (1996). Applied and Environmental Microbiology, 62, 1808–1810.
Nghiem, N. P., Hicks, K. B., & Johnston, D. B. (2010). Applied Biochemistry and Biotechnology, 162, 1915–1928.
De Wulf, P., & Vandamme, E. J. (1997). Applied Microbiology and Biotechnology, 48, 141–148.
Sutton, F. (1911). Systematic handbook of volumetric analysis (10th ed.). Philadelphia: P. Blakiston’s Son and Co.
Acknowledgments
The authors would like to express their sincere thanks to Justin Montanti and Jennifer Thomas of the ERRC, who assisted in the experimental efforts and sample analysis, and DuPont Industrial Biosciences for providing the enzyme products used in this research. The authors also would like to thank Dr. Susanne Kleff of MBI for her critical review and comments on the original manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture (USDA). USDA is an equal employment provider and employer.
Rights and permissions
About this article
Cite this article
Nghiem, N.P., Senske, G.E. Capture of Carbon Dioxide from Ethanol Fermentation by Liquid Absorption for Use in Biological Production of Succinic Acid. Appl Biochem Biotechnol 175, 2104–2113 (2015). https://doi.org/10.1007/s12010-014-1369-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1369-1