Abstract
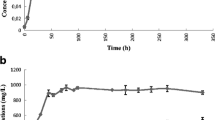
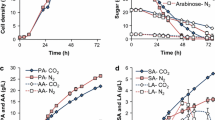
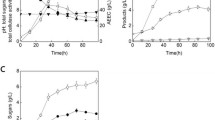
Butanol production from lignocellulosic biomass has a low overall yield due to carbon loss in the form of xylose during the biomass pretreatment and CO2 during the fermentation processes. This research presents a cascading process for producing butanol coupling three bioprocesses. First, an indigenous microbial community performed the direct acidogenesis of raw corn stover producing an H2-CO2 gas stream and volatile fatty acids (VFA) using the most biodegradable fraction. The acidogenesis process had maximum hydrogen productivity of 87 mL/L day and VFA production of 8.5 g/L of acetic acid, 3.7 g/L of butyric acid, and 2.2 g/L of propionic acid. The acidogenesis process experienced a species succession with early colonizing bacteria dominated by Lactobacillus being replaced with more succeeding microbial groups dominated by Enterococcous, Prevotella, and Megasphaera. Second, the spent solids of corn stover were used for producing acetone-butanol-ethanol (ABE) using a mixed culture bioaugmented with Clostridium saccharobutylicum. A simplex centroid mixture design served to elucidate the effects of adding different mixtures of acetic, butyric, and propionic acids on butanol production. Pure butyric acid improved three times the butanol titer compared to the control treatment with no acid addition (610 mg/L versus 230 mg/L of butanol, respectively). Opposite, acetic and propionic acids inhibited the butanol production. Finally, additional butanol was produced using an H2-CO2 gas stream, where the type of inoculum and culture medium affected the process. An inoculum enriched with Spirochaetales, Pseudomonas, Enterobacter, and Proteiniphilum grown on a culture medium with trace metals reached the highest butanol titer of 889 mg/L. This cascading process improved the carbon utilization by producing butanol from VFA and CO2, and not only from cellulose.





Similar content being viewed by others
References
Ren NQ, Zhao L, Chen C, Guo WQ, Cao GL (2016) A review on bioconversion of lignocellulosic biomass to H2: key challenges and new insights. Bioresour Technol 215:92–99. https://doi.org/10.1016/j.biortech.2016.03.124
Bharathiraja B, Jayamuthunagai J, Sudharsanaa T, Bharghavi A, Praveenkumar R, Chakravarthy M, Yuvaraj D (2017) Biobutanol - an impending biofuel for future: a review on upstream and downstream processing techniques. Renew Sustain Energy Rev 68:788–807. https://doi.org/10.1016/j.rser.2016.10.017
Li J, Chi X, Zhang Y, Wang X (2018) Enhanced coproduction of hydrogen and butanol from rice straw by a novel two-stage fermentation process. Int Biodeterior Biodegrad 127:62–68. https://doi.org/10.1016/j.ibiod.2017.11.004
Valdez-Vazquez I, Alatriste-Mondragón F, Arreola-Vargas J, Buitrón G, Carrillo-Reyes J, León-Becerril E, Mendez-Acosta HO, Ortíz I, Weber B (2020) A comparison of biological, enzymatic, chemical and hydrothermal pretreatments for producing biomethane from Agave bagasse. Ind Crops Prod 145:112160. https://doi.org/10.1016/j.indcrop.2020.112160
Atasoy M, Owusu-Agyeman I, Plaza E, Cetecioglu Z (2018) Bio-based volatile fatty acid production and recovery from waste streams: current status and future challenges. Bioresour Technol 268:773–786. https://doi.org/10.1016/j.biortech.2018.07.042
Chu Y, Wei Y, Yuan X, Shi X (2011) Bioconversion of wheat stalk to hydrogen by dark fermentation: effect of different mixed microflora on hydrogen yield and cellulose solubilisation. Bioresour Technol 102(4):3805–3809. https://doi.org/10.1016/j.biortech.2010.11.092
Sanchez A, Magaña G, Gomez D, Solís M, Banares-Alcantara R (2014) Bidimensional sustainability analysis of lignocellulosic ethanol production processes. Method and case study. Biofuel Bioprod Bioref 8(5):670–685. https://doi.org/10.1002/bbb.1512
Müller V (2019) New horizons in acetogenic conversion of one-carbon substrates and biological hydrogen storage. Trends Biotechnol 37(12):1344–1354. https://doi.org/10.1016/j.tibtech.2019.05.008
Phillips JR, Atiyeh HK, Tanner RS, Torres JR, Saxena J, Wilkins MR, Huhnke RL (2015) Butanol and hexanol production in Clostridium carboxidivorans syngas fermentation: medium development and culture techniques. Bioresour Technol 190:114–121. https://doi.org/10.1016/j.biortech.2015.04.043
Shen S, Gu Y, Chai C, Jiang W, Zhuang Y, Wang Y (2017) Enhanced alcohol titre and ratio in carbon monoxide-rich off-gas fermentation of Clostridium carboxidivorans through combination of trace metals optimization with variable-temperature cultivation. Bioresour Technol 239:236–243. https://doi.org/10.1016/j.biortech.2017.04.099
Ramió-Pujol S, Ganigué R, Bañeras L, Colprim J (2015) Incubation at 25 °C prevents acid crash and enhances alcohol production in Clostridium carboxidivorans P7. Bioresour Technol 192:296–303. https://doi.org/10.1016/j.biortech.2015.05.077
Chakraborty S, Rene ER, Lens PLN, Veiga MC, Kennes C (2019) Enrichment of a solventogenic anaerobic sludge converting carbon monoxide and syngas into acids and alcohols. Bioresour Technol 272:130–136. https://doi.org/10.1016/j.biortech.2018.10.002
van Soest PJ (1990) Use of detergents in the analysis of fibrous feeds I. Preparation of fiber residues of low nitrogen content. Journal of Association of Official Analytical Chemists 73(4):487–491. https://doi.org/10.1093/jaoac/73.4.487
APHA Standard methods for the examination of water and wastewater, 20th edition. American Public Health Association, 1999. Washington DC.
Pérez-Rangel M, Quiroz-Figueroa FR, González-Castañeda J, Valdez-Vazquez I (2015) Microscopic analysis of wheat straw cell wall degradation by microbial consortia for hydrogen production. Int J Hydrog Energy 40(1):151–160. https://doi.org/10.1016/j.ijhydene.2014.10.050
Valdez-Vazquez I, Morales AL, Escalante AE (2017) History of adaptation determines short-term shifts in performance and community structure of hydrogen-producing microbial communities degrading wheat straw. Microb Biotechnol 10(6):1569–1580. https://doi.org/10.1111/1751-7915.12678
Valdez-Vazquez I, Poggi-Varaldo MH (2009) Hydrogen production by fermentative consortia. Renew Sustain Energy Rev 13(5):1000–1013. https://doi.org/10.1016/j.rser.2008.03.003
Muñoz-Páez KM, Alvarado-Michi EL, Moreno-Andrade I, Buitrón G, Valdez-Vazquez I (2020) Comparison of suspended and granular cell anaerobic bioreactors for hydrogen production from acid agave bagasse hydrolyzates. Int J Hydrog Energy 45(1):275–285. https://doi.org/10.1016/j.ijhydene.2019.10.232
Rachbauer L, Beyer R, Bochmann G, Fuchs W (2017) Characteristics of adapted hydrogenotrophic community during biomethanation. Sci Total Environ 595:912–919. https://doi.org/10.1016/j.scitotenv.2017.03.074
Wolfe BE, Dutton RJ (2015) Fermented foods as experimentally tractable microbial ecosystems. Cell 161(1):49–55. https://doi.org/10.1016/j.cell.2015.02.034
Vorholt JA (2012) Microbial life in the phyllosphere. Nat Rev Microbiol 10:828. https://doi.org/10.1038/nrmicro2910
De Paepe K, Verspreet J, Courtin CM, Van de Wiele T (2020) Microbial succession during wheat bran fermentation and colonisation by human faecal microbiota as a result of niche diversification. ISME J 14:584–596. https://doi.org/10.1038/s41396-019-0550-5
Engelmann U, Weiss N (1985) Megasphaera cerevisiae sp. nov.: a new gram-negative obligately anaerobic coccus isolated from spoiled beer. System Appl Microbiol 6:287–290
Douillard FP, de Vos WM (2014) Functional genomics of lactic acid bacteria: from food to health. Microb Cell Fact 13:S8. https://doi.org/10.1186/1475-2859-13-S1-S8
Castelló E, Ferraz-Junior ADN, Andreani C, Anzola-Rojas MP, Borzacconi L, Buitrón G, Carrillo-Reyes J, Damasceno Gomes S, Maintinguer SI, Moreno-Andrade I, Palomo-Briones R, Razo-Flores E, Schiappacasse Dasati M, Tapia-Venegas E, Valdez-Vazquez I, Vesga-Baron A, Zaiat M, Etchebehere C (2019) Stability problems in the hydrogen production by dark fermentation: possible causes and solutions. Renew Sustain Energy Rev 119:109602. https://doi.org/10.1016/j.rser.2019.109602
García-Depraect O, Díaz-Cruces VF, Rene ER, León-Becerril E (2019) Changes in performance and bacterial communities in a continuous biohydrogen-producing reactor subjected to substrate- and pH-induced perturbations. Bioresour Technol 295:122182. https://doi.org/10.1016/j.biortech.2019.122182
Molina-Guerrero CE, Valdez-Vazquez I, Sánchez A, Vázquez-Castillo JA, Vazquez-Nuñez E (2020) A biorefinery based on the biomechanical configuration of the digestive system of a ruminant for ABE production: a consolidated bioprocessing approach. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-020-00620-5
Zhou Q, Liu Y, Yuan W (2020) Kinetic modeling of butyric acid effects on butanol fermentation by Clostridium saccharoperbutylacetonicum. New Biotechnol 55:118–126. https://doi.org/10.1016/j.nbt.2019.10.004
Al-Shorgani NKN, Kalil MS, Yusoff WMW, Hamid AA (2018) Impact of pH and butyric acid on butanol production during batch fermentation using a new local isolate of Clostridium acetobutylicum YM1. Saudi J Biol Sci 25(2):339–348. https://doi.org/10.1016/j.sjbs.2017.03.020
De Souza MB, Mary dos Santos G, Palladino Delforno T, Tadeu Fuess L, José da Silva A (2019) Enriched microbial consortia for dark fermentation of sugarcane vinasse towards value-added short-chain organic acids and alcohol production. J Biosci Bioeng 127(5):594–601. https://doi.org/10.1016/j.jbiosc.2018.10.008
Zhou Q, Liu Y, Yuan W (2018) Kinetic modeling of lactic acid and acetic acid effects on butanol fermentation by Clostridium saccharoperbutylacetonicum. Fuel 226:181–189. https://doi.org/10.1016/j.fuel.2018.04.019
Lin C-W, Wu C-H, Tran D-T, Shih M-C, Li W-H, Wu C-F (2011) Mixed culture fermentation from lignocellulosic materials using thermophilic lignocellulose-degrading anaerobes. Process Biochem 46(2):489–493. https://doi.org/10.1016/j.procbio.2010.09.024
Wang Z, Cao G, Zheng J, Fu D, Song J, Zhang J, Zhao L, Yang Q (2015) Developing a mesophilic co-culture for direct conversion of cellulose to butanol in consolidated bioprocess. Biotechnol Biofuels 8:84. https://doi.org/10.1186/s13068-015-0266-3
Arslan D, Steinbusch KJJ, Diels L, De Wever H, Buisman CJN, Hamelers HVM (2012) Effect of hydrogen and carbon dioxide on carboxylic acids patterns in mixed culture fermentation. Bioresour Technol 118:227–234. https://doi.org/10.1016/j.biortech.2012.05.003
Arslan D, Steinbusch KJJ, Diels L, De Wever H, Hamelers HVM, Buisman CJN (2013) Selective carboxylate production by controlling hydrogen, carbon dioxide and substrate concentrations in mixed culture fermentation. Bioresour Technol 136:452–460. https://doi.org/10.1016/j.biortech.2013.03.063
Darvekar P, Liang C, Nazmul Karim M, Holtzapple MT (2019) Effect of headspace gas composition on carboxylates production in open culture fermentation of corn stover. Biomass Bioenergy 126:57–61. https://doi.org/10.1016/j.biombioe.2019.04.019
Liu K, Atiyeh HK, Stevenson BS, Tanner RS, Wilkins MR, Huhnke RL (2014) Mixed culture syngas fermentation and conversion of carboxylic acids into alcohols. Bioresour Technol 152:337–346. https://doi.org/10.1016/j.biortech.2013.11.015
Hu J, Xue Y, Li J, Wang L, Zhang S, Wang Y, Gao M (2016) Characterization of a designed synthetic autotrophic–heterotrophic consortia for fixing CO2 without light. RSC Adv 6:78161–78169. https://doi.org/10.1039/C6RA13118B
Zhang H, Bruns MA, Logan BE (2002) Perchlorate reduction by a novel chemolithoautotrophic, hydrogen-oxidizing bacterium. Environ Microbiol 4(10):570–576. https://doi.org/10.1046/j.1462-2920.2002.00338.x
Valdes N, Soto P, Cottet L, Alarcon P, Gonzalez A, Castillo A, Corsini G, Tello M (2015) Draft genome sequence of Janthinobacterium lividum strain MTR reveals its mechanism of capnophilic behavior. Stand Genomic Sci 10:110. https://doi.org/10.1186/s40793-015-0104-z
Nakayama S, Bando Y, Ohnishi A, Kadokura T, Nakazato A (2013) Decreased hydrogen production leads to selective butanol production in co-cultures of Clostridium thermocellum and Clostridium saccharoperbutylacetonicum strain N1-4. J. Biosci Bioeng 115(2):173–175. https://doi.org/10.1016/j.jbiosc.2012.08.020
Wen Z, Wu M, Lin Y, Yang L, Lin J, Cen P (2014) Artificial symbiosis for acetone-butanol-ethanol (ABE) fermentation from alkali extracted deshelled corn cobs by co-culture of Clostridium beijerinckii and Clostridium cellulovorans. Microb Cell Fact 13(1):92. https://doi.org/10.1186/s12934-014-0092-5
Yang X, Xu M, Yang ST (2015) Metabolic and process engineering of Clostridium cellulovorans for biofuel production from cellulose. Metab Eng 32:39–48. https://doi.org/10.1016/j.ymben.2015.09.001
Valdez-Vazquez I, Pérez-Rangel M, Tapia A, Buitrón G, Molina CE, Hernández G, Amaya-Delgado L (2015) Hydrogen and butanol production from native wheat straw by synthetic microbial consortia integrated by species of Enterococcus and Clostridium. 159:214-222. https://doi.org/10.1016/j.fuel.2015.06.052
Salimi F, Mahadevan R (2013) Characterizing metabolic interactions in a clostridial co-culture for consolidated bioprocessing. BMC Biotechnology 13(1):95. https://doi.org/10.1186/1472-6750-13-95
Tian L, Conway PM, Cervenka ND, Cui J, Maloney M, Olson DG, Lynd LR (2019) Metabolic engineering of Clostridium thermocellum for n-butanol production from cellulose. Biotechnol Biofuels 12:186. https://doi.org/10.1186/s13068-019-1524-6
Chen BY, Chuang FY, Lin CL, Chang JS (2012) Deciphering butanol inhibition to Clostridial species in acclimatized sludge for improving biobutanol production. Biochem Eng J 69:100–105. https://doi.org/10.1016/j.bej.2012.09.005
Acknowledgments
D. G.-T. is grateful to the CONACYT for the scholarship that it provided. Gloria Moreno-Rodríguez, Jaime Perez Trevilla, and Ángel Avizua Hernández Huerta are acknowledged for their technical assistance.
Funding
Financial support of this work was partially received from the DGAPA-UNAM project PAPIIT (Grant No. IA102018) and the Energy Sustainability Fund 2014-05 (CONACYT- SENER), Mexican Bioenergy Innovation Center, Bioalcohols Cluster (Grant No. 249564). K.M. Muñoz-Páez acknowledges the support from CONACYT through the CÁTEDRAS program (Researcher ID 6407, Project 265).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
González-Tenorio, D., Muñoz-Páez, K.M. & Valdez-Vazquez, I. Butanol production coupled with acidogenesis and CO2 conversion for improved carbon utilization. Biomass Conv. Bioref. 12, 2121–2131 (2022). https://doi.org/10.1007/s13399-020-00805-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00805-y