Abstract
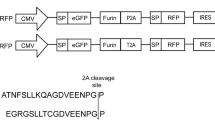
Efficient expression of target protein is one of strategies for gene therapy or vaccine design. Many studies showed that codon optimization could enhance the expression of target proteins. In this paper, a target sequence of about 1.26 kb encoding the major capsid protein VP6 of grass carp reovirus (GCRV) and an optimized counterpart were synthesized and inserted into vectors for expressing VP6. The final constructs (named as pcDV6G and pcDV6YG) were transfected in Ctenopharyngodon idellus kidney (CIK) cells. The fluorescence analysis and the Western blot results showed that the gene fragment was transfected and expressed in CIK cells successfully. Although the qRT-PCR results showed no difference at the messenger RNA (mRNA) levels between the different versions of vp6 in the indicated stages, the enzyme-linked immunosorbent assay (ELISA) results showed that the protein level of VP6 expressed by pcDV6YG was higher than that by pcDV6G in the indicated hours. Taken together, these results suggest that the enhanced expression of GCRV VP6 in CIK cells by relative sequence optimization may be a good choice for making DNA vaccine against GCRV.






Similar content being viewed by others
References
Jiang, Y., & Ahne, W. (1989). Some properties of the etiological agent of the hemorrhagic disease of grass carp and black carp. In W. Ahne & E. Kurstak (Eds.), Viruses of lower vertebrates (pp. 227–239). Berlin: Springer.
Fang, Q., Ke, L. H., & Cai, Y. Q. (1989). Growth characterization and high titre culture of GCHV. Virologica Sinica, 3, 314–319. In Chinese.
Fang, Q., Attoui, H., Francois, J., Biagini, P., Zhu, Z. Y., de Micco, P., et al. (2000). Sequence of genome segments 1, 2 and 3 of the grass carp reovirus (Genus Aquareovirus, Family Reoviridae). Biochemical and Biophysical Research Communications, 274, 762–766.
Fang, Q., Shah, S., Liang, Y., & Zhou, Z. H. (2005). 3D reconstruction and capsid protein characterization of grass carp reovirus. Science in China Series C, 48, 593–600. In Chinese.
Fang, Q., Seng, E. K., Ding, Q. Q., & Zhang, L. L. (2008). Characterization of infectious particles of grass carp reovirus by treatment with proteases. Archives of Virology, 153, 675–682.
Cheng, L., Fang, Q., Shah, S., Atanasov, I. C., & Zhou, Z. H. (2008). Subnanometer resolution structures of the grass carp reovirus core and virion. Journal of Molecular Biology, 382, 213–222.
Cheng, L., Zhu, J., Hui, W. H., Zhang, X., Honig, B., Fang, Q., et al. (2010). Backbone model of an aquareovirus virion by cryoelectron microscopy and bioinformatics. Journal of Molecular Biology, 397, 852–863.
He, Y., Xu, H., Yang, Q., Xu, D., & Lu, L. (2011). The use of an in vitro microneutralization assay to evaluate the potential of recombinant VP5 protein as an antigen for vaccinating against grass carp reovirus. Virology Journal, 8, 132.
Shao, L., Sun, X. Y., & Fang, Q. (2011). Antibodies against outer-capsid proteins of grass carp reovirus expressed in E. coli are capable of neutralizing viral infectivity. Virology Journal, 8, 347.
Xue, R., Liu, L., Cao, G., Xu, S., Li, J., Zou, Y., et al. (2013). Oral vaccination of BacFish-vp6 against grass carp reovirus evoking antibody response in grass carp. Fish and Shellfish Immunology, 34, 348–355.
Fang, Q., Seng, E. K., Wen, D., & Zhang, L. L. (2007). Construction and co-expression of grass carp reovirus VP6 protein and enhanced green fluorescence protein in the insect cells. Virologica Sinica, 22, 397–404.
Zhou, Y., Fan, Y. D., & Zeng, L. B. (2014). Construction of a recombinant eukaryotic vector for a grass carp reovirus VP6 gene and its expression in vitro and in vivo. Virus Disease, 25, 69–77.
Xiao, B., Li, Q., Feng, B., Han, Z., Gao, D., Zhao, R., et al. (2009). Expression of recombinant human nerve growth factor beta in milk of goats by recombinant replication-defective adenovirus. Applied Biochemistry and Biotechnology, 3, 357–366.
Villalobos, A., Ness, J. E., Gustafsson, C., Minshull, J., & Govindarajan, S. (2006). Gene designer: a synthetic biology tool for constructing artificial DNA segments. BMC Bioinformatics, 7, 285.
Puigbò, P., Guzmán, E., Romeu, A., & Garcia-Vallvé, S. (2007). OPTIMIZER: a web server for optimizing the codon usage of DNA sequences. Nucleic Acids Research, 35(Web Server issue), W126–W131.
Fuglsang, A. (2003). Codon optimizer: a freeware tool for codon optimization. Protein Expression and Purification, 31, 247–249.
Wu, G., Bashir-Bello, N., & Freeland, S. J. (2006). The synthetic gene designer: a flexible web platform to explore sequence manipulation for heterologous expression. Protein Expression and Purification, 47, 441–445.
Sandhu, K. S., Pandey, S., MaitiS, S., & Pillai, B. (2008). GASCO: genetic algorithm simulation for codon optimization. In Silicon Biology, 8, 187–192.
Sharp, P. M., & Li, W. H. (1987). The codon adaptation index–a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Research, 15, 1281–1295.
Gustafsson, C., Govindarajan, S., & Minshull, J. (2004). Codon bias and heterologous protein expression. Trends in Biotechnology, 22, 346–353.
Kudla, G., Murray, A. W., Tollervey, D., & Plotkin, J. B. (2009). Coding-sequence determinants of gene expression in Escherichia coli. Science, 324, 255–258.
Allert, M., Cox, J. C., & Hellinga, H. W. (2010). Multifactorial determinants of protein expression in prokaryotic open reading frames. Journal of Molecular Biology, 402, 905–918.
Kozak, M. (1986). Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell, 2, 283–292.
Gao, W., Rzewski, A., Sun, H., Robbins, P. D., & Gambotto, A. (2004). UpGene: Application of a web-based DNA codon optimization algorithm. Biotechnology Progress, 2, 443–448.
Acknowledgments
This study was supported by the Science and Technology Development Project of Yantai, Shandong, China (2011066), and the talent funds of Ludong University (LY2008337).
Conflict of Interest
There is no conflict of interest to disclose. All authors contributed to the preparation of the manuscript and agreed the version.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiao, B., Chi, X., Zhang, L. et al. Enhanced Expression of GCRV VP6 in CIK Cells by Relative Sequence Optimization. Appl Biochem Biotechnol 173, 2129–2139 (2014). https://doi.org/10.1007/s12010-014-1012-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1012-1