Abstract
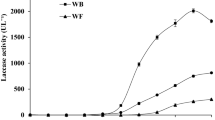
The present study is concerned with the microbiological transformation of L-tyrosine to L-dopa by a newly isolated turkey tail mushroom Coriolus versicolor DOB-4. As tyrosinase (catechol oxidase, EC 1.10.3.1) is an extracellular enzyme, therefore biomass was used as an enzyme source in the reaction mixture. Biomass particles were pretreated with methanol and oven dried at 105 °C for 2 h. The optimal L-dopa production was achieved when 1.5 mg/ml L-tyrosine was used as the basal substrate. Thin layer chromatography and high-performance liquid chromatography analysis depicted that citric acid supports higher substrate conversion and product formation rates. A noticeable enhancement was observed when process parameters viz. L-tyrosine concentration (1.5 mg/ml), citric acid (1.5 mg/ml), time of incubation (50 min), and reaction temperature (60 °C) were optimized using Plackett–Burman design. The maximum production of L-dopa was found to be 0.872 mg/ml with L-tyrosine consumption of 1.002 mg/ml. The model terms were found highly significant (HS, p ≤ 0.05), suggesting the potential commercial utility of the culture (df = 3, LSD = 0.342).









Similar content being viewed by others
References
Buttner, T., Kuhanw, M., & Patzold, T. (2005). Journal of Neurological Transactions, 7, 13–19.
Napolitano, M., Picconi, B., & Centonze, D. (2006). Neuroscience, 398, 211–214.
Karlson, R. H., & Levitan, D. R. (1990). Oecologia, 82, 40–44.
Hyland, K., & Clayton, P. T. (1992). Clinical Chemistry, 38, 2405–2410.
Inamdar, S. A., Joshi, S. M., Jadhav, J. P., & Bapat, V. A. (2012). Natural Products and Bioprospects, 2, 16–20.
Patil, S. A., Surwase, S. N., Jadhav, S. B., & Jadhav, J. P. (2013). Biochemical Engineering Journal, 74, 36–45.
Gurme, S. T., Surwase, S. N., Patil, S. A., Jadhav, S. B., & Jadhav, J. P. (2013). Indian Journal of Microbiology, 53, 194–198.
Ali, S., Jeffry, S., & Haq, I. (2007). BMC Biotechnology, 7, 50–57.
Surwase, S. N., Patil, S. A., Jadhav, S. B., & Jadhav, J. B. (2012). Microbial Biotechnology, 5, 731–737.
Ali, S., & Haq, I. (2012). BMC Biotechnology, 10, 86. doi:10.1186/1472-6750-10-86.
Krishnaveni, R., Vandana-Rathod, M., Thakur, S., & Neelgund, Y. F. (2009). Current Microbiology, 58, 122–128.
Oba, K., Teramukai, S., Kobayashi, M., & Matsui, T. (2007). Canadian Immunology Journal, 56, 905–911.
Raju, B. G., Rao, G. H., & Ayyanna, C. (1993). Visakhapatnam, 82, 106–110.
Pialis, P., Jimenez, M. C., & Bradley, A. S. (2002). Journal of Biotechnology, 51, 141–147.
Rayner, A. D. M., & Todd, N. K. (1978). Transactions of the British Mycological Society, 71, 99–106.
Bergmeyer, H. U. (1974). Methods of enzymatic analysis. New York: Wiley.
Bradley, M., Pialis, P., & Jimenz, C. (1996). Biotechnology & Bioengineering, 51, 141–147.
Dastager, S. G., Li, W. J., Dayanand, A., Tang, S. K., Tain, X. P., Zhi, Y. Z., Xu, L. H., & Jiang, C. L. (2006). African Journal of Biotechnology, 5, 1131–1134.
Marusek, C. M., Trobaugh, N. M., & Flurkey, W. H. (2006). Journal of Biochemistry, 100, 108–123.
Snedecor, G. W., & Cochran, W. G. (1980). Statistical methods. Ames: Iowa State University Press.
Ahuja, S. K., Ferreira, G. M., & Morreira, A. R. (2004). Biotechnology & Bioengineering, 85, 666–675.
Marumo, K., & Waite, J. H. (2003). Journal of Biochemistry, 872, 98–103.
Sih, J., Foss, C., & Rosazza, J. (1969). Journal of American Chemical Society, 91, 6204–6206.
Ros, J. R., Rodriguez, J. N., & Garcia, F. (1993). Journal of Biochemistry, 295, 309–372.
Mason, H. S. (1964). Journal of Biological Chemistry, 172, 83–85.
Hee, S. P., Lee, J. Y., & Kim, H. S. (1998). Biotechnology & Bioengineering, 58, 339–343.
Danial, R. M., Peterson, M. E., & Donson, M. J. (2010). Journal of Biochemistry, 425, 353–360.
Minton, A. P. (2001). Journal of Biological Chemistry, 276, 10577–10580.
Deker, H. (2000). Trends in Biochemical Sciences, 25, 392–397.
Almeida, P. R., Frases, S., Araujo, G. S., de Oliveira, M. M., Gerfen, G. J., Nosanchuk, J. D., & Zancope-Oliveira, R. M. (2012). Applied Environmental Microbiology, 78, 8623–8630.
Madani, W., Kermasha, S., & Biasakawski, B. (1999). Food Science, 52, 1001–1008.
Scribhers, E. T., Tang, E., & Bradley, S. G. (1973). Applied Microbiology, 25, 873–879.
Conn, E., Stumpf, P. K., & Bruening, G. (1987). Outlines of Biochemistry (5th ed.). Singapore: Wiley.
Hornykiewicz, O. (2002). Brazilian Journal of Microbiology, 23, 67–70.
Burkert, J. F. M., Kalil, S. J., Filho, F. M., & Rodrigues, M. I. (2006). Brazilian Journal of Chemical Engineering, 23, 163–170.
Acknowledgments
Director, IIB is acknowledged for moral support. Thanks to Mr. Naeem Akhtar, Department of Botany for his help in the identification of turkey’s tail mushroom (C. versicolor DOB-4).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ali, S., Rizvi, N. Microbiological Transformation of L-Tyrosine to L-Dopa from Methanol Pretreated Biomass of a Novel Coriolus versicolor under Submerged Culture. Appl Biochem Biotechnol 172, 2041–2054 (2014). https://doi.org/10.1007/s12010-013-0658-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0658-4