Abstract
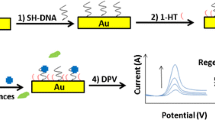
A simple, sensitive aptamer-based biosensor for the detection of phenylalanine is developed using the electrochemical transduction method. For this proposed aptasensor, a 5-thiol-terminated aptamer is covalently attached onto a gold electrode. At the first time, the electrode was evaluated as an electrochemical aptasensor for determination of phenylalanine in aqueous solutions. This sensor was tested in a Tris–HCl buffer with physiological pH = 7.4 by cyclic voltammetry and differential pulse voltammetry. The detection limit and sensitivity of the modified electrode toward phenylalanine were estimated to be 1 nM (S/N = 3) and 0.367 μA nM−1, respectively. The linear range of the signal was observed between 1 and 10 nM of phenylalanine with 0.9914 correlation factor. The herein-described approach is expected to promote the exploitation of aptamer-based biosensors for protein assays in biochemical and biomedical studies.







Similar content being viewed by others
References
Bard, A. J., & Faulkner, L. R. (2001). Electrochemical methods. New York: Wiley. Chapter 12.
Deng, C., Shang, C., Hu, Y., & Zhang, X. (2002). Rapid diagnosis of phenylketonuria and other aminoacidemias by quantitative analysis of amino acids in neonatal blood spots by gas chromatography–mass spectrometry. Journal of Chromatography B: Analytical Technology in the Biomedical and, Life Sciences, 775, 115–120. doi:10.1016/S1570-0232(02)00283-0.
Ellington, A. D., & Szostak, J. W. (1990). In vitro selection of RNA molecules that bind specific ligands. Nature, 346, 818–822. doi:10.1038/346818a0.
Ferapontova, E. E., Olsen, E. M., & Gothelf, K. V. (2008). An RNA aptamer-based electrochemical biosensor for detection of theophylline in serum. Journal of the American Chemical Society, 130, 4256–4258. doi:10.1021/ja711326b.
Hasanzadeh, M. (2009). Cobalt hydroxide nanoparticles modified glassy carbon electrode as a biosensor. In: Karim-Nezhad, G., Shadjou, N., Khalilzadeh, B., Saghatforoush, L. A., Abnosi, M. H., Ershad, S. (eds), for electrooxidation and determination of some amino acids. Analytical Biochemistry, 389, 130-137, doi: 10.1016/j.ab.2009.03.024.
Hasanzadeh, M., Shadjou, N., Chen, S. T., & Sheykhzadeh, P. (2012). MCM-41-NH2 as an advanced nanocatalyst for electrooxidation and determination of amino acids. Catalysis Communications, 19, 21–27. doi:10.1016/j.catcom.2011.12.007.
Hasanzadeh, M., Shadjou, N., & Omidinia, E. (2013). Mesoporous silica (MCM-41)-Fe2O3 as a novel magnetic nanosensor for determination of trace amounts of amino acids. Colloids and Surfaces B: Biointerfaces, 108, 52–59. doi:10.1016/j.colsurfb.2013.02.015.
Hendriksz, C. J., & Walter, J. H. (2004). Update on phenylketonuria. Current Paediatrics, 14, 400–406. doi:10.1016/j.cupe.2004.05.003.
Herne, T. M., & Tarlov, M. J. (1997). Characterization of DNA probes immobilized on gold surfaces. Journal of the American Chemical Society, 119, 8916–8920. doi:10.1021/ja9719586.
Kang, Y., Feng, K. J., Chen, J. W., Jiang, J. H., Shen, G. L., & Yu, R. Q. (2008). Electrochemical detection of thrombin by sandwich approach using antibody and aptamer. Bioelectrochemistry, 73, 76–81. doi:10.1016/j.bioelechem.2008.04.024.
Khezrian, S., Salimi, A., Teymourian, H., & Hallaj, R. (2013). Label-free electrochemical IgE aptasensor based on covalent attachment of aptamer onto multiwalled carbon nanotubes/ionic liquid/chitosan nanocomposite modified electrode. Biosensors and Bioelectronics, 43, 218–225. doi:10.1016/j.bios.2012.12.006.
Kitagawa, T., Smith, B. A., & Brown, E. S. (1975). Gas-liquid chromatography of phenylalanine and its metabolites in serum and urine of various hyperphenylalaninemic subjects, their relatives, and controls. Clinical Chemistry, 21, 735–740.
Kohlmeier, M. (2003). Nutrient metabolism (pp. 314–321). London: Academic.
Levicky, R., Herne, T. M., Tarlov, M. J., & Satija, S. K. (1998). Using self-assembly to control the structure of DNA monolayers on gold: a neutron reflectivity study. Journal of the American Chemical Society, 120, 9787–9792. doi:10.1021/ja981897r.
Mahan, L. K., Escott-Stump, S. (2008). Food & nutrition therapy, 12th ed., Saunders, Philadelphia. 1141-1169.
Wu, Z. S., Zheng, F., Shen, G. L., & Yu, R. Q. (2009). A hairpin aptamer-based electrochemical biosensing platform for the sensitive detection of proteins. Biomaterials, 30, 2950–2955. doi:10.1016/j.biomaterials.2009.02.017.
Yao, C., Qi, Y., Zhao, Y., Xiang, Y., Chen, Q., & Fu, W. (2009). Aptamer-based piezoelectric quartz crystal microbalance biosensor array for the quantification of IgE. Biosensors and Bioelectronics, 24, 2499–2503. doi:10.1016/j.bios.2008.12.036.
Zayats, M., Huang, Y., Gill, R., Ma, C., & Willner, I. (2006). Label-free and reagentless aptamer-based sensors for small molecules. Journal of the American Chemical Society, 128, 13666–13667. doi:10.1021/ja0651456.
Yao, W., Wang, L., Wang, H., Zhang, X., & Li, L. (2009). An aptamer-based electrochemiluminescent biosensor for ATP detection. Biosensors and Bioelectronics, 24, 3269–3274. doi:10.1016/j.bios.2009.04.016.
Matsubara, Y., Heininger, J. A., & Lin, Y. Y. (1984). Improved diagnosis of classical vs atypical phenylketonuria by liquid chromatography. Clinical Chemistry, 30, 278–280.
Naghib, S. M., Rabiee, M., Omidinia, E., & Khoshkenara, P. (2012). Investigation of a biosensor based on phenylalanine dehydrogenase immobilized on a polymer-blend film for phenylketonuria diagnosis. Electroanalysis, 24, 407–417. doi:10.1002/elan.201100391.
Naghib, S. M., Rabiee, M., Omidinia, E., Khoshkenara, P., & Zeini, D. (2012). Biofunctionalization of dextran-based polymeric film surface through enzyme immobilization for phenylalanine determination. International Journal of Electrochemical Science, 7, 120–135.
Saghatforoush, L. A., Hasanzadeh, M., Shadjou, N., & Khalilzadeh, B. (2011). Deposition of new thia-containing Schiff-base iron (III) complexes onto carbon nanotube-modified glassy carbon electrodes as a biosensor for electrooxidation and determination of amino acids. Electrochimica Acta, 56, 1051–1061. doi:10.1016/j.electacta.2010.10.031.
Schuett, V. E. (2002). Low protein food list for PKU (2nd ed.). USA: National PKU News.
Steel, A. B., Herne, T. M., & Tarlov, M. (1998). Electrochemical quantitation of DNA immobilized on gold. Journal Analytical Chemistry, 70, 4670–4677. doi:10.1021/ac980037q.
Acknowledgments
We gratefully acknowledge the support of this work by the Pasteur Institute of Iran and Drug Applied Research Center, Tabriz University of Medical Sciences. We especially thank Ali Khaneh-Zar and Hamid Shahbaz-Mohammadi.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Omidinia, E., Shadjou, N. & Hasanzadeh, M. Aptamer-based Biosensor for Detection of Phenylalanine at Physiological pH. Appl Biochem Biotechnol 172, 2070–2080 (2014). https://doi.org/10.1007/s12010-013-0656-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0656-6