Abstract
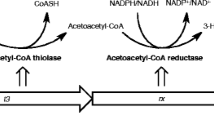
The production of ultrahigh molecular weight poly-3-hydroxybutyric acid (P3HB) from carbohydrates by recombinant Escherichia coli harboring genes from Ralstonia eutropha was evaluated. In shaken-flask experiments, E. coli XL1 Blue harboring plasmid pSK::phaCAB produced P3HB corresponding to 40 and 27 % of cell dry weight from glucose and xylose, respectively. Cultures in bioreactor using glucose as the sole carbon source at variable pH values (6.0, 6.5, or 7.0) allowed the production of P3HB with molecular weight varying between 2.0 and 2.5 MDa. These figures are significantly higher than the values often obtained by natural bacterial strains (0.5–1.0 MDa). Contrary to reports of other authors, no influence of pH was observed on the molecular weight of the polymer produced. Using xylose, P3HB with high molecular weight was also produced, indicating the possibility to produce these polymers from lignocellulosic materials.


Similar content being viewed by others
References
Anderson, A. J., & Dawes, E. A. (1990). Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiological Reviews, 54(4), 450–472.
Steinbüchel, A. (1991). In D. Byrom (Ed.), Biomaterials: novel materials from biological sources, polyhydroxyalkanoic acids (pp. 123–213). New York: Macmillan.
Slater, S. C., Voige, W. H., & Dennis, D. E. (1988). Cloning and expression in Escherichia coli of the Alcaligenes eutrophus H16 poly-beta-hydroxybutyrate biosynthetic pathway. Journal of Bacteriology, 170(10), 4431–4436.
Schubert, P., Steinbüchel, A., & Schlegel, H. G. (1988). Cloning of the Alcaligenes eutrophus genes for synthesis of poly-beta-hydroxybutyric acid (PHB) and synthesis of PHB in Escherichia coli. Journal of Bacteriology, 170(12), 5837–5847.
Peoples, O. P., & Sinskey, A. J. (1989). Poly-beta-hydroxybutyrate biosynthesis in Alcaligenes eutrophus H16. Characterization of the genes encoding beta-ketothiolase and acetoaceyl-CoA reductase. Journal of Biological Chemistry, 264(26), 15293–15297.
Schubert, P., Krüger, N., & Steinbüchel, A. (1991). Molecular analysis of the Alcaligenes eutrophus poly(3-hydroxybutyrate) biosynthesis operon: identification of the N terminus of poly(3-hydroxybutyrate) synthase and the identification of the promoter. Journal of Bacteriology, 173(1), 168–175.
Baptist, J.N. (1962). Process for preparing poly-beta-hydroxybutyric acid. US Patent 3,036,959.
Byrom, D. (1990). In E. A. Dawes (Ed.), Novel biodegradable microbial polymers, industrial production of copolymers from Alcaligenes eutrophus (pp. 113–117). Dordrecht: Kluwer Academic.
Holmes, P. A. (1985). Applications of PHB—microbially produced biodegradable thermoplastic. Physics in Technology, 16(1), 32–36.
Liebergesell, M., Hustede, E., Timm, A., Steinbüchel, A., Fuller, R. C., Lenz, R. W., et al. (1991). Formation of poly (3-hydroxyalkanoic acids) by phototrophic and chemolithotrophic bacteria. Archives of Microbiology, 155(5), 415–421.
Kim, G. J., Yun, K. Y., Bae, K. S., & Rhee, Y. H. (1992). Accumulation of copolyesters consisting of 3-hydroxybutyrate and 3-hydroxyvalerate by Alcaligenes sp. SH-69 in batch culture. Biotechnology Letters, 14(1), 27–32.
Aoyagi, Y., Doi, Y., & Iwata, T. (2003). Mechanical properties and highly ordered structure of ultra-high-molecular-weight poly[(R)-3-hydroxybutyrate] films: effect of annealing and two-step drawing. Polymer Degradation and Stability, 79(2), 209–216.
Iwata, T., Tsunoda, K., Aoyagi, Y., Kusaka, S., Yonezawa, N., & Doi, Y. (2003). Mechanical properties of uniaxially cold-drawn films of poly([R]-3-hydroxybutyrate). Polymer Degradation and Stability, 79(2), 217–224.
Iwata, T., Aoyagi, Y., Fujita, M., Yamane, H., Doi, Y., Suzuki, Y., et al. (2004). Processing of a strong biodegradable poly[(R)-3-hydroxybutyrate] fiber and a new fiber structure revealed by micro-beam X-ray diffraction with synchrotron radiation. Macromolecular Rapid Communications, 25(11), 1100–1104.
Bengtsson, S., Pisco, A. R., Johansson, P., Lemos, P. C., & Reis, M. A. (2010). Molecular weight and thermal properties of polyhydroxyalkanoates produced from fermented sugar molasses by open mixed cultures. Journal of Biotechnology, 147(3–4), 172–179.
Kabe, T., Tsuge, T., Kasuya, K., Takemura, A., Hikima, T., Takata, M., et al. (2012). Physical and structural effects of adding ultrahigh-molecular-weight poly [(R)-3-hydroxybutyrate] to wild-type poly [(R)-3-hydroxybutyrate]. Macromolecules, 45(4), 1858–1865.
Kusaka, S., Abe, H., Lee, S. Y., & Doi, Y. (1997). Molecular mass of poly[(R)-3-hydroxybutyric acid] produced in a recombinant Escherichia coli. Applied Microbiology and Biotechnology, 47(2), 140–143.
Kusaka, S., Iwata, T., & Doi, Y. (1999). Properties and biodegradability of ultra-high-molecular-weight poly[(R)-3-hydroxybutyrate] produced by a recombinant Escherichia coli. International Journal of Biological Macromolecules, 25(1–3), 87–94.
Sim, S. J., Snell, K. D., Hogan, S. A., Stubbe, J., Rha, C., & Sinskey, A. J. (1997). PHA synthase activity controls the molecular weight and polydispersity of polyhydroxybutyrate in vivo. Nature Biotechnology, 15(1), 63–67.
Gerngross, T. U., & Martin, D. P. (1995). Enzyme-catalyzed synthesis of poly[(R)-(-)-3-hydroxybutyrate]: formation of macroscopic granules in vitro. Proceedings of the National Academy of Sciences, 92(14), 6279–6283.
Choi, J. I., & Lee, S. Y. (2004). High level production of supra molecular weight poly(3-hydroxybutyrate) by metabolically engineered Escherichia coli. Biotechnology and Bioprocess Engineering, 9(3), 196–200.
Agus, J., Kahar, P., Hyakutake, M., Tomizawa, S., Abe, H., Tsuge, T., et al. (2010). Unusual change in molecular weight of polyhydroxyalkanoate (PHA) during cultivation of PHA-accumulating Escherichia coli. Polymer Degradation and Stability, 95(12), 2250–2254.
Tomizawa, S., Hyakutake, M., Saito, Y., Agus, J., Mizuno, K., Abe, H., et al. (2011). Molecular weight change of polyhydroxyalkanoate (PHA) caused by the PhaC subunit of PHA synthase from Bacillus cereus YB-4 in recombinant Escherichia coli. Biomacromolecules, 12(7), 2660–2666.
Agus, J., Kahar, P., Abe, H., Doi, Y., & Tsuge, T. (2006). Molecular weight characterization of poly [(R)-3-hydroxybutyrate] synthesized by genetically engineered strains of Escherichia coli. Polymer Degradation and Stability, 91(5), 1138–1146.
Wei, X. X., Shi, Z. Y., Yuan, M. Q., & Chen, G. Q. (2009). Effect of anaerobic promoters on the microaerobic production of polyhydroxybutyrate (PHB) in recombinant Escherichia coli. Applied Microbiology and Biotechnology, 82(4), 703–712.
Gomez, J. G. C., Méndez, B. S., Nikel, P. I., Pettinari, M. J., Prieto, M. A., & Silva, L. F. (2012). Making green polymers even greener: towards sustainable production of polyhydroxyalkanoates from agroindustrial by-products. In M. Petre (Ed.), Advances in applied biotechnology (pp. 41–61). Manhattan: InTech.
Nduko, J. M., Suzuki, W., Matsumoto, K., Kobayashi, H., Ooi, T., Fukuoka, A., et al. (2012). Polyhydroxyalkanoates production from cellulose hydrolysate in Escherichia coli LS5218 with superior resistance to 5-hydroxymethylfurfural. Journal of Bioscience and Bioengineering, 113(1), 70–72.
Fonseca, G. G., Fonseca, G. G., de Arruda-Caulkins, J. C., & Vasconcellos, A. R. (2008). Production and characterization of poly-(3-hydroxybutyrate) from recombinant Escherichia coli grown on cheap renewable carbon substrates. Waste Management & Research, 26(6), 546–552.
Menon, V., & Rao, M. (2012). Trends in bioconversion of lignocellulose: biofuels, platform chemicals & biorefinery concept. Progress in Energy and Combustion Science, 38(4), 522–550.
Dias, M. O., Cunha, M. P., Jesus, C. D., Rocha, G. J., Pradella, J. G., Rossell, C. E., et al. (2011). Second generation ethanol in Brazil: can it compete with electricity production? Bioresource Technology, 102(19), 8964–8971.
Sambrook, J., Fritsch, E. F., & Maniatis, T. (1989). Molecular cloning: a laboratory manual (2nd ed.). Cold Spring Harbor: Cold Spring Harbor Laboratory Press.
Gomez, J. G. C., Rodrigues, M. F. A., Alli, R. C. P., Torres, B. B., Bueno, C. L., Oliveira, M. S., et al. (1996). Evaluation of soil gram-negative bacteria yielding polyhydroxyalkanoic acids from carbohydrates and propionic acid. Applied Microbiology and Biotechnology, 45(6), 785–791.
Sánchez, R. J., Schripsema, J., Silva, L. F., Taciro, M. K., Pradella, J. G., & Gomez, J. G. C. (2003). Medium-chain-length polyhydroxyalkanoic acids (PHAmcl) produced by Pseudomonas putida IPT 046 from renewable sources. European Polymer Journal, 39(7), 1385–1394.
Braunegg, G., Sonnleitner, B., & Lafferty, R. M. (1978). A rapid gas chromatographic method for the determination of poly-β-hydroxybutyric acid in microbial biomass. European Journal of Applied Microbiology and Biotechnology, 6(1), 29–37.
Riis, V., & Mai, W. (1988). Gas chromatographic determination of poly-β-hydroxybutyric acid in microbial biomass after hydrochloric acid propanolysis. Journal of Chromatography, 445, 285–289.
Silva, L. F., Gomez, J. G., Oliveira, M. S., & Torres, B. B. (2000). Propionic acid metabolism and poly-3-hydroxybutyrate-co-3-hydroxyvalerate (P3HB-co-3HV) production by Burkholderia sp. Journal of Biotechnology, 76(2–3), 165–174.
Bullock, W. O., Fernández, J. M., & Short, J. M. (1987). XL1-Blue: a high efficiency plasmid transforming recA Escherichia coli strain with β-galactosidase selection. Biotechniques, 5, 376–379.
Kidwell, J., Valentin, H. E., & Dennis, D. (1995). Regulated expression of the Alcaligenes eutrophus PHA biosynthesis genes in Escherichia coli. Applied and Environmental Microbiology, 61(4), 1391–1398.
Sim, S. J., Sneel, K. D., Kim, B. W., Rha, C. K., & Sinskey, A. J. (2001). Increased poly-β-hydroxybutyrate (PHB) chain length by the modulation of PHA synthase activity in recombinant Escherichia coli. Biotechnology Letters, 23(24), 2057–2061.
Taguchi, S., Maehara, A., Takase, K., Nakahara, M., Nakumura, H., & Doi, Y. (2001). Analysis of mutational effects of a polyhydroxybutyrate (PHB) polymerase on bacterial PHB accumulation using an in vivo assay system. FEMS Microbiology Letters, 198(1), 65–71.
Taguchi, S., Nakamura, H., Hiraishi, T., Yamato, I., & Doi, Y. (2002). In vitro evolution of a polyhydroxybutyrate synthase by intragenic suppression-type mutagenesis. Journal of Biochemistry, 131(6), 801–806.
Hiroe, A., Tsuge, K., Nomura, C. T., Itaya, M., & Tsuge, T. (2012). Rearrangement of gene order in the phaCAB operon leads to effective production of ultrahigh-molecular-weight poly[(R)-3-hydroxybutyrate] in genetically engineered Escherichia coli. Applied and Environmental Microbiology, 78(9), 3177–3184.
Pettinari, M. J., Nikel, P. L., Ruiz, J. A., & Méndez, B. S. (2008). ArcA redox mutants as a source of reduced bioproducts. Journal of Molecular Microbiology and Biotechnology, 15(1), 41–47.
Simon, R., Priefer, U., & Pühler, A. (1983). A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Nature Biotechnology, 1, 784–791.
Acknowledgments
The authors wish to thank CNPq-Brazil for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bocanegra, J.K., da Cruz Pradella, J.G., da Silva, L.F. et al. Influence of pH on the Molecular Weight of Poly-3-hydroxybutyric Acid (P3HB) Produced by Recombinant Escherichia coli . Appl Biochem Biotechnol 170, 1336–1347 (2013). https://doi.org/10.1007/s12010-013-0257-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0257-4