Abstract
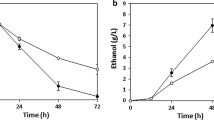
Scheffersomyces stipitis was cultivated in an optimized, controlled fed-batch fermentation for production of ethanol from glucose–xylose mixture. Effect of feed medium composition was investigated on sugar utilization and ethanol production. Studying influence of specific cell growth rate on ethanol fermentation performance showed the carbon flow towards ethanol synthesis decreased with increasing cell growth rate. The optimum specific growth rate to achieve efficient ethanol production performance from a glucose-xylose mixture existed at 0.1 h−1. With these optimized feed medium and cell growth rate, a kinetic model has been utilized to avoid overflow metabolism as well as to ensure a balanced feeding of nutrient substrate in fed-batch system. Fed-batch culture with feeding profile designed based on the model resulted in high titer, yield, and productivity of ethanol compared with batch cultures. The maximal ethanol concentration was 40.7 g/L. The yield and productivity of ethanol production in the optimized fed-batch culture was 1.3 and 2 times higher than those in batch culture. Thus, higher efficiency ethanol production was achieved in this study through fed-batch process optimization. This strategy may contribute to an improvement of ethanol fermentation from lignocellulosic biomass by S. stipitis on the industrial scale.





Similar content being viewed by others
References
Agbogbo, F. K., & Coward-Kelly, G. (2008). Cellulosic ethanol production using the naturally occurring xylose-fermenting yeast, Pichia stipitis. Biotechnology Letters, 30, 1515–1524.
Agbogbo, F. K., Coward-Kelly, G., Torry-Smith, M., Wenger, K., & Jeffries, T. W. (2007). The effect of initial cell concentration on xylose fermentation by Pichia stipitis. Applied Biochemistry and Biotechnology, 137, 653–662.
Andersson, L., Stranberg, L., Haggstrom, L., & Enfors, S. O. (1994). Modeling of high cell density fed batch cultivation. FEMS Microbiology Reviews, 14, 39–44.
Bäcklund, E., Reeks, D., Markland, K., Weir, N., Bowering, L., & Larsson, G. (2008). Fed-batch design for periplasmic product retention in Escherichia coli. Journal of Biotechnology, 135, 358–365.
Canilha, L., Carvalho, W., Felipe, M. G. A., Batista, J. A. S., & Giulietti, M. (2010). Ethanol production from sugarcane bagasse hydrolysate using Pichia stipitis. Applied Biochemistry and Biotechnology, 161, 84–92.
Chandel, A. K., Narasu, M. L., Chandrasekhar, G., Manikyam, A., & Rao, L. V. (2009). Use of Saccharum spontaneum (wild sugarcane) as biomaterial for cell immobilization and modulated ethanol production by thermotolerant Saccharomyces cerevisiae. Biores Technol, 100, 2404–2410.
Chandel, A. K., Singh, O. V., Rao, L. V., Chandrasekhar, G., & Narasu, M. L. (2011). Bioconversion of novel substrate Saccharum spontaneum, a weedy material, into ethanol by Pichia stipitis NCIM3498. Biores Technol, 102, 1709–1714.
Cho, D. H., Shin, S.-J., Bae, Y., Park, C., & Kim, Y. H. (2011). Ethanol production from acid hydrolysates based on the construction and demolition wood waste using Pichia stipitis. Biores Technol, 102, 4439–4443.
Cho, Y. H., Song, J. Y., Kim, K. M., Kim, M. K., Lee, I. Y., Kim, S. B., Kim, H. S., Han, N. S., Lee, B. H., & Kim, B. S. (2010). Production of nattokinase by batch and fed-batch culture of Bacillus subtilis. New Biotechnology, 27, 341–346.
Chu, B. C., & Lee, H. (2007). Genetic improvement of Saccharomyces cerevisiae for xylose fermentation. Biotechnology Advances, 25, 425–441.
Dellweg, H., Rizzi, M., Methner, H., & Debus, D. (1984). Xylose fermentation by yeasts: Comparison of Pachysolen tannophilus and Pichia stipitis. Biotechnology Letters, 6, 395–400.
Ferreira, A. D., Mussatto, S. I., Cadete, R. M., Rosa, C. A., & Silva, S. S. (2011). Ethanol production by a new pentose-fermenting yeast strain, Scheffersomyces stipitis UFMG-IMH 43.2, isolated from the Brazilian forest. Yeast, 28, 547–554.
Gırio, F., Fonseca, C., Carvalheiro, F., Duarte, L. C., Marques, S., & Bogel-Łukasik, R. (2010). Hemicelluloses for fuel ethanol: A review. Bioresource Technology, 101, 4775–4800.
Gutiérrez-Rivera, B., Waliszewski-Kubiak, K., Carvajal-Zarrabal, O., & Aguilar-Uscanga, M. G. (2012). Conversion efficiency of glucose/xylose mixtures for ethanol production using Saccharomyces cerevisiae ITV01 and Pichia stipitis NRRL Y-7124. Chem Technol and Biotechnol, 87, 263–270.
Hahn-Hägerdal, B., Karhumaa, K., Fonseca, C., Spencer-Martins, I., & Gorwa-Grauslund, M. F. (2007). Towards industrial pentose-fermenting yeast strains. Applied Microbiology and Biotechnology, 74, 937–953.
Hong, J. (1986). Optimal substrate feeding policy for a fed batch fermentation with substrate and product inhibition kinetics. Biotechnology and Bioengineering, 28, 1421–1431.
Huang, H., Ridgway, D., Gu, T., & Moo-Young, M. (2004). Enhanced amylase production by Bacillus subtilis using a dual exponential feeding strategy. Bioproc Biosyst Eng, 27, 63–69.
Jeffries, T. W. (2006). Engineering yeasts for xylose metabolism. Current Opinion in Biotechnology, 17, 320–326.
Jeffries, T. W., & Van Vleet, J. R. (2009). Pichia stipitis genomics, transcriptomics, and gene clusters. FEMS Yeast Research, 9, 793–807.
Krahulec, S., Kratzer, R., Longus, K., & Nidetzky, B. (2012). Comparison of Scheffersomyces stipitis strains CBS 5773 and CBS 6054 with regard to their xylose metabolism: implications for xylose fermentation. Microbiologyopen, 1, 64–70.
Kwon, E. Y., Kim, K. M., Kim, M. K., Lee, I. Y., & Kim, B. S. (2011). Production of nattokinase by high cell density fed-batch culture of Bacillus subtilis. Bioproc Biosyst Eng, 34, 789–793.
Ladisch, M. R., & Dyck, K. (1979). Dehydration of ethanol: New approach gives positive energy balance. Science, 205, 898–900.
Lee, J., Rodrigues, R. C., & Jeffries, T. W. (2009). Simultaneous saccharification and ethanol fermentation of oxalic acid pretreated corncob assessed with response surface. Biores Technol, 100, 6307–6311.
Lin, T. H., Huang, C. F., Guo, G. L., Hwang, W. S., & Huang, S. L. (2012). Pilot-scale ethanol production from rice straw hydrolysates using xylose-fermenting Pichia stipitis. Biores Technol, 116, 314–319.
Nor, Z. M., Tamer, M. I., Scharer, J. M., Moo-Young, M., & Jervis, E. J. (2001). Automated fed-batch culture of Kluyveromyces fragilis based on a novel method for on-line estimation of cell specific growth rate. Biochemical Engineering Journal, 9, 221–231.
Pacheco Chavez, R. A., Tavares, L. C., Teixeira, A., Carvalho, J., Converti, A., & Sato, S. (2004). Influence of the nitrogen source on the productions of a-amylase and glucoamylase by a new Trichoderma sp. from soluble starch. Chem Biochem Eng, 18, 403–407.
Prior, B. A., Kilian, S. G., & du Preez, J. C. (1989). Fermentation of D-xylose by the yeasts Candida shehatae and Pichia stipitis. Proc Biochem, 2, 21–32.
Riesenberg, D. (1991). High-cell density cultivation of Escherichia coli. Current Opinion in Biotechnology, 2, 380–384.
Scordia, D., Cosentino, S. L., Lee, J.-W., & Jeffries, T. W. (2012). Bioconversion of giant reed (Arundo donax L.) hemicellulose hydrolysate to ethanol by Scheffersomyces stipitis CBS6054. Biomass and Bioen, 39, 296–305.
Silva, J. P., Mussatto, S. I., & Roberto, I. C. (2010). The influence of initial xylose concentration, agitation, and aeration on ethanol production by Pichia stipitis from rice straw hemicellulosic hydrolysate. Applied Biochemistry and Biotechnology, 162, 1306–1315.
Slininger, P. J., Dien, B. S., Gorsich, S. W., & Liu, Z. L. (2006). Nitrogen source and mineral optimization enhance D-xylose conversion to ethanol by the yeast Pichia stipitis NRRL Y-7124. Applied Microbiology and Biotechnology, 72, 1285–1296.
Slininger, P. J., Gorsich, S. W., & Liu, Z. L. (2009). Culture nutrition and physiology impact the inhibitor tolerance of the yeast Pichia stipitis NRRL Y-7124. Biotechnology and Bioengineering, 102, 778–790.
Unrean, P., & Nguyen, N. H. (2012). Rational optimization of culture conditions for the most efficient ethanol production in Scheffersomyces stipitis using design of experiments. Biotech Prog, 28, 1119–1125.
Unrean, P., & Nguyen, N. H. (2012). Metabolic pathway analysis of Scheffersomyces (Pichia) stipitis: Effect of oxygen availability on ethanol synthesis and flux distributions. Applied Microbiology and Biotechnology, 94, 1387–1398.
Arslan, Y., & Eken-Saraçoğlu, N. (2010). Effects of pretreatment methods for hazelnut shell hydrolysate fermentation with Pichia stipitis to ethanol. Biores Technol, 101, 8664–8670.
Yong, Q., Li, X., Yuan, Y., Lai, C., Zhang, N., Chu, Q., Xu, Y., & Yu, S. (2012). An improved process of ethanol production from hemicellulose: Bioconversion of undetoxified hemicellulosic hydrolyzate from steam-exploded corn stover with a domesticated Pichia stipitis. Applied Biochemistry and Biotechnology, 167, 2330–2340.
Acknowledgment
This work has been supported by Thailand Research Fund (TRF) Grant for New Researcher.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Unrean, P., Nguyen, N.H. Optimized Fed-Batch Fermentation of Scheffersomyces stipitis for Efficient Production of Ethanol from Hexoses and Pentoses. Appl Biochem Biotechnol 169, 1895–1909 (2013). https://doi.org/10.1007/s12010-013-0100-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0100-y