Abstract
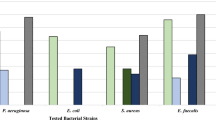
The present study describes the exploitation of microbial biodiversity from Western Ghats of Kerala for screening of bioactives having β-lactamase inhibitory activities. A total of 700 pure cultures were isolated and were screened for antibacterial activity against a β-lactam resistant Bacillus cereus strain (PL 10) isolated from the same niche. Bioactive extracts made from 45 isolates showed inhibitory activities against PL 10, of which two strains showed inhibition of extended spectrum β-lactamase (ESBL) producing Klebsiella ESBL1101 and three strains inhibited methicillin-resistant Staphylococcus aureus (MRSA) strain MRSA831. All these five strains showed wide spectrum antimicrobial activity against various fungi and bacteria. These five cultures were identified by 16S rRNA sequencing and biochemical tests and the preliminary characterizations of their bioactive extracts were carried out. This study suggests the potential of bioactives from two inhibitor–producer strains, NII 167 and NII 1054, for being developed as inhibitors against wide spectrum β-lactam resistant strains.


Similar content being viewed by others
References
Baltz, R. H. (2007). Antimicrobials from actinomycetes: back to the future. Microbe, 2, 125–131.
Chaudhary, U., & Aggarwal, R. (2004). Extended spectrum β-lactamases (ESBL)—an emerging threat to clinical therapeutics. Indian Journal of Medical Microbiology, 22, 75–80.
Sirot, D. (1995). Extended spectrum plasmid mediated β-lactamases. Journal of Antimicrobial Chemotherapy, 36, 19–34.
Reading, C., & Cole, M. (1977). Clavulanic acid: a β-lactamase-inhibiting β-lactam from Streptomyces clavuligerus. Antimicrobial Agents and Chemotherapy, 11, 852–857.
English, A. R., Retsema, J. A., Girard, A. E., Lynch, J. E., & Barth, W. E. (1978). CP-45,899 a β-lactamase inhibitor that extends the antibacterial spectrum of β-lactams: initial bacteriological characterization. Antimicrobial Agents and Chemotherapy, 14, 414–419.
Fisher, J., Belasco, J. G., Charnas, R. L., Khosla, S., & Knowles, J. R. (1980). β-Lactamase inactivation by mechanism-based reagents. Philosophical Transactions of the Royal Society B: Biological Sciences, 289, 309–319.
Drawz, S. M., & Bonomo, R. A. (2010). Three decades of β-lactamase inhibitors. Clinical Microbiology Reviews, 23, 160–201.
Bassetti, M., Righi, E., & Viscoli, C. (2008). Novel β-lactam antibiotics and inhibitor combinations. Expert Opinion on Investigational Drugs, 17, 285–296.
Thomson, J. M., Distler, A. M., & Bonomo, R. A. (2007). Overcoming resistance to β-lactamase inhibitors: comparing sulbactam to novel inhibitors against clavulanate resistant SHV enzymes with substitutions at Ambler position 244. Biochemistry, 46, 11361–11368.
Ceylan, O., Okmen, G., & Ugur, A. (2008). Isolation of soil Streptomyces as source antibiotics active against antibiotic-resistant bacteria. EurAsian Journal of BioSciences, 2, 73–82.
Stein, T. (2005). Bacillus subtilis antibiotics: structures, syntheses and specific functions. Molecular Microbiology, 56, 845–857.
He, L., Chen, W., & Liu, Y. (2006). Production and partial characterization of bacteriocin-like peptides by Bacillus licheniformis ZJU12. Research in Microbiology, 161, 321–326.
Lisboa, M. P., Bonatto, D., Bizani, D., Henriques, J. A., & Brandelli, A. (2006). Characterization of a bacteriocin-like substance produced by Bacillus amyloliquefaciens isolated from the Brazilian Atlantic forest. International Microbiology, 9, 111–118.
Sharma, N., Kapoor, G., & Neopaney, B. (2006). Characterization of a new bacteriocin produced from a novel isolated strain of Bacillus lentus NG121. Antoine Van Leeuwenhoek, 89, 337–343.
Kumar, N., Singh, R. K., Mishra, S. K., Singh, A. K., & Pachouri, U. C. (2010). Isolation and screening of soil Actinomycetes as source of antibiotics active against bacteria. International Journal of Microbiology Research, 2, 12–16.
Prabavathy, V. R., Mathivanan, N., & Murugesan, K. (2006). Control of blast and sheath blight diseases of rice using antifungal metabolites produced by Streptomyces sp. PM5. Biological Control, 39, 313–319.
Singh, J. S., Singh, S. P., Saxena, A. K., & Rawat, Y. S. (1984). India’s silent valley and its threatened rainforest ecosystems. Environmental Conservation, 11, 223–233.
Dancer, S. J. (2004). How antibiotics can make us sick: the less obvious adverse effects of antimicrobial chemotherapy. The Lancet Infectious Diseases, 4, 611–619.
Singh, S. K., Patel, A. K., Ahmed, S. U., Nampoothiri, K. M., & Pandey, A. (2007). Identification and phylogenetic analysis of TEM gene from soil isolates. Journal of Scientific and Industrial Research, 66, 660–666.
Novick, R. P. (1962). Microiodometric assay of penicillinase. Biochemical Journal, 83, 236–240.
Nampoothiri, K. M., Rubex, R., Patel, A. K., Narayanan, S. S., Krishna, S., Das, S. M., & Pandey, A. (2008). Molecular cloning, overexpression and biochemical characterization of hypothetical β-lactamases of Mycobacterium tuberculosis H37Rv. Journal of Applied Microbiology, 105, 59–67.
Perez, C., Pauli, M., & Bazevque, P. (1990). An antibiotic assay by the agar well diffusion method. Acta Biologiae et Medicine Experimentalis, 15, 113–115.
Kutchma, A. J., Roberts, M. A., Knaebel, D. B., & Crawford, D. L. (1998). Small-scale isolation of genomic DNA from Streptomyces mycelia or spores. Biotechniques, 24, 452–456.
Pandey, B., Ghimire, P., & Agrawal, V. P. (2004). Studies on the antibacterial activity of actinomycetes isolated from the Khumbu region of Mt. Everest. A paper presented in the International Conference on the Great Himalayas: Climate, Health, Ecology, Management and Conservation, Kathmandu. January 12–15. Organized by Kathmandu University and the Aquatic Ecosystem Health and Management Society, Canada.
Kumar, A., Saini, P., & Shrivastava, J. N. (2009). Production of peptide antifungal antibiotic and biocontrol activity of Bacillus subtilis. Indian Journal of Experimental Biology, 47, 57–62.
Motta, S. A., Cannavan, S. F., Tsai, S.-M., & Brandelli, A. (2007). Characterization of a broad range antibacterial substance from a new Bacillus species isolated from Amazon basin. Archives of Microbiology, 188, 367–375.
Sutyak, K. E., Wirawan, R. E., Aroutcheva, A. A., & Chikindas, M. L. (2008). Isolation of the Bacillus subtilis antimicrobial peptide subtilosin from the dairy product-derived Bacillus amyloliquefaciens. Journal of Applied Microbiology, 104, 1067–1074.
Jack, R. W., Tagg, J. R., & Ray, B. (1995). Bacteriocins of Gram positive bacteria. Microbiological Reviews, 59, 171–200.
Korenblum, E., von der Weid, I., Santos, A. L. S., Rasado, A. S., Sebastian, G. V., Coutinho, C. M. L. M., Magalhaes, F. C. M., de Paiva, M. M., & Seldin, L. (2005). Production of antimicrobial substances by Bacillus subtilis LFE1, B. firmus H2O-1 and B. licheniformis T6-5, isolated from an oil reservoir in Brazil. Journal of Applied Microbiology, 98, 667–675.
Acknowledgements
The work was funded by CSIR, New Delhi under the Task force programme, NWP 006. The permission from Forest Department, Kerala State, India for allowing soil sample collection from Silent Valley National Park is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sowmya P. Mohandas, Sita Ravikumar, Sumi J Menachery, Gayathri Suseelan contributed equally to the work.
Rights and permissions
About this article
Cite this article
Mohandas, S.P., Ravikumar, S., Menachery, S.J. et al. Bioactives of Microbes Isolated from Western Ghat Belt of Kerala Show β-Lactamase Inhibition along with Wide Spectrum Antimicrobial Activity. Appl Biochem Biotechnol 167, 1753–1762 (2012). https://doi.org/10.1007/s12010-012-9596-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-9596-9