Abstract
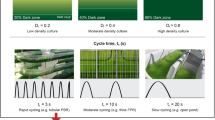
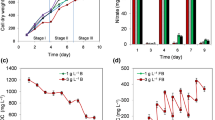
Algal growth requires optimal irradiance. In photobioreactors, optimal light requirements change during the growth cycle. At low culture densities, a high incident light intensity can cause photoinhibition, and in dense algal cultures, light penetration may be limited. Insufficient light supply in concentrated algae suspensions can create zones of dissimilar photon flux density inside the reactor, which can cause suboptimal algal growth. However, growth of dense cultures can also be impaired due to photoinhibition if cells are exposed to excessively high light intensities. In order to simultaneously maintain optimal growth and photon use efficiency, strategies for light supply must be based on cell concentrations in the culture. In this study, a lipid-producing microalgal strain, Neochloris oleoabundans, was grown in batch photobioreactors. Growth rates and biomass concentrations of cultures exposed to constant light were measured and compared with the growth kinetic parameters of cultures grown using sequentially increasing light intensities based on increasing culture densities during batch growth. Our results show that reactors operated under conditions of sequential increase in irradiance levels yield up to a 2-fold higher biomass concentration when compared with reactors grown under constant light without negatively impacting growth rates. In addition, this tailored light supply results in less overall photon use per unit mass of generated cells.





Similar content being viewed by others
References
Chisti, Y. (2007). Biodiesel from microalgae. Biotechnology Advances, 25, 294–306.
Chisti, Y. (2006). Microalgae as sustainable cell factories. Environmental Engineering and Management Journal, 5, 261–274.
Akkerman, I., Jansen, M., Rocha, J., & Wijffels, R. H. (2002). Photobiological hydrogen production: Photochemical efficiency and bioreactor design. International Journal of Hydrogen Energy, 27, 1195–1208.
Spolaore, P., Joannis-Cassan, C., Duran, E., & Isambert, A. (2006). Commercial applications of microalgae. Journal of Bioscience and Bioengineering, 101, 87–96.
Apt, K. E., & Behrens, P. W. (1999). Commercial developments in microalgal biotechnology. Journal of Phycology, 35, 215–226.
Vunjak-Novakovic, G., Kim, Y., Wu, X., Berzin, I., & Merchhuk, J. C. (2005). Air-lift bioreactors for algal growth on flue gas: Mathematical modelling and pilot-plant studies. Industrial and Engineering Chemistry Research, 44, 6154–6163.
Doucha, J., Straka, F., & Livansky, K. (2005). Utilization of fluee gases for cultivation of microalgaae (Chlorella sp.) in an outdoor open thin-layer photobioreactor. Journal of Applied Phycology, 17, 403–412.
Mehta, S. K., & Gaur, J. P. (2005). Use of algae for removing heavy metal ions from wastewater: Progress and prospects. Critical Reviews in Biotechnology, 25, 113–152.
Olaizola, M. (2003). Commercial development of microalgal biotechnology: From the test tube to the marketplace. Biomolecular Engineering, 20, 459–466.
Hu, Q., Sommerfeld, M., Jarvis, E., Ghirardi, M., Posewitz, M., Seibert, M., et al. (2008). Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant Journal, 54, 621–639.
Li, J., Xu, S., & Su, W. W. (2003). Online estimation of stirred-tank microalgal photobioreactor cultures based on dissolved oxygen measurement. Biochemical Engineering Journal, 14, 51–65.
Evers, E. G. (1991). A model for light-limited continuous cultures—Growth, shading and maintenance. Biotechnology and Bioengineering, 38, 254–259.
Ugwu, C. U., Aoyagi, H., & Uchiyama, H. (2008). Photobioreactors for mass cultivation of algae. Bioresource Technology, 99, 4021–4028.
Gordon, J. M., & Polle, J. E. W. (2007). Ultrahigh bioproductivity from algae. Applied Microbiology and Biotechnology, 76, 969–975.
Ogbonna, J. C., Yada, H., & Tanaka, H. (1995). Effect of cell movement by random mixing between the surface and bottom of photobioreactors on algal productivity. Journal of Fermentation and Bioengineering, 79, 152–157.
Ogbonna, J. C., Yada, H., & Tanaka, H. (1995). Kinetic-study on light-limited batch cultivation of photosynthetic cells. Journal of Fermentation and Bioengineering, 80, 259–264.
Eriksen, N. T. (2008). The technology of microalgal culturing. Biotechnology Letters, 30, 1525–1536.
Qiang, H., & Richmond, A. (1996). Productivity and photosynthetic efficiency of Spirulina platensis as affected by light intensity, algal density and rate of mixing in a flat plate photobioreactor. Journal of Applied Phycology, 8, 139–145.
Zijffers, J. W. F., Janssen, M., Tramper, J., & Wijffels, R. H. (2008). Design process of an area-efficient photobioreactor. Marine Biotechnology, 10, 404–415.
Gordon, J. M. (2002). Tailoring optical systems to optimized photobioreactors. International Journal of Hydrogen Energy, 27, 1175–1184. Pergamon-Elsevier Science Ltd.
Meireles, L. A., Guedes, A. C., Barbosa, C. R., Azevedo, J. L., Cunha, J. P., & Malcata, F. X. (2008). On-line control of light intensity in microalgal bioreactor using a novel automatic system. Enzyme and Microbial Technology, 42, 554–559.
Yoon, J. H., Shin, J. H., Ahn, E. K., & Park, T. H. (2008). High cell density culture of Anabaena variabilis with controlled light intensity and nutrient supply. Journal of Microbiology and Biotechnology, 18, 918–925.
Wijanarko, A., Dianursanti, Sendjaya, A. Y., Hermansyah, H., Witarto, A. B., & Gozan, M. (2008). Enhanced Chlorella vulgaris Buitenzorg growth by photon flux density alteration in serial photobioreactors. Biotechnology and Bioprocessing Engineering, 13, 476–482.
Li, Y. Q., Horsman, M., Wang, B., Wu, N., & Lan, C. Q. (2008). Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Applied Microbiology and Biotechnology, 81, 629–636.
Tornabene, T. G., Holzer, G., Lien, S., & Burris, N. (1983). Lipid composition of the nitrogen starved green alga Neochloris oleoabundans. Enzyme and Microbial Technology, 5, 435–440.
Hu, Q., Guterman, H., & Richmond, A. (1996). A flat inclined modular photobioreactor for outdoor mass cultivation of photoautotrophs. Biotechnology and Bioengineering, 51, 51–60.
Eaton, A. D., Clesceri, L. S., Rice, E. W., & Greenberg, A. E. (Eds.). (2005). Standard methods for the examination of water and wastewater, 2540D total suspended solids dried. Washington: American Public Health Association.
Eaton, A. D., Clesceri, L. S., Rice, E. W., & Greenberg, A. E. (Eds.). (2005). Standard methods for the examination of water and wastewater, 4500-NO − 3 I. Cadmium reduction flow injection method. Washington: American Public Health Association.
Grima, M., Fernandez, J., Fernanddez, A., & Chisti, Y. (2001). Tubular photobioreactor design for algal cultures. Journal of Biotechnology, 92, 113–131.
Luo, H. P., & Al-Dahhan, M. H. (2004). Analyzing and modeling of photobioreactors by combining first principles of physiology and hydrodynamics. Biotechnology and Bioengineering, 85, 382–393.
Ogbonna, J. C., & Tanaka, H. (2000). Light requirement and photosynthetic cell cultivation—Development of processes for efficient light utilization in photobioreactors. Journal of Applied Phycology, 12, 207–218.
Grima, E. M., Perez, J. A. S., Camacho, F. G., Sanchez, J. L. G., & Alonso, D. L. (1993). N−3 PUFA productivity in chemostat cultures of microalgae. Applied Microbiology and Biotechnology, 38, 599–605.
Barbosa, M. J., Janssen, M., Ham, N., Tramper, J., & Wijffels, R. H. (2003). Microalgae cultivation in air-lift reactors: Modeling biomass yield and growth rate as a function of mixing frequency. Biotechnology and Bioengineering, 82, 170–179.
Bassham, J. A. (1980). Energy crops (Energy Farming). In A. S. Pietro (Ed.), Biochemical and photosynthetic aspects of energy production. New York: Academic.
Steele, J. H. (1965). Notes on some theoretical problems in production ecology. In C. R. Goldman (Ed.), Primary productivity in aquatic environments. Mem. Ist. Ital. Idrobiol., 18 Suppl (pp. 385–398). Berkeley: University of California Press.
Liehr, S. K., Suidan, M. T., & Eheart, J. W. (1990). A modeling study of carbon and light limitation in algal biofilms. Biotechnology and Bioengineering, 35, 233–243.
Needoba, J. A., & Harrison, P. J. (2004). Influence of low light and a light:dark cycle on NO3− uptake, intracellular NO3− and nitrogen isotope fractionation by marine phytoplankton. Journal of Phycology, 40, 505–516.
Philips, N. J. J., & Myers, J. (1954). Growth rate of Chlorella in flashing light. Plant Physiology, 29, 152–161.
Acknowledgment
This research was supported by the Utah Science Technology and Research (USTAR) initiative’s biofuels program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wahal, S., Viamajala, S. Maximizing Algal Growth in Batch Reactors Using Sequential Change in Light Intensity. Appl Biochem Biotechnol 161, 511–522 (2010). https://doi.org/10.1007/s12010-009-8891-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8891-6