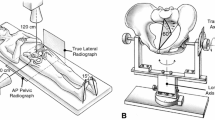
Abstract
The risk of dislocation after THA reportedly is minimized if the acetabular implant is oriented at 45° inclination and 15° anteversion with respect to the anterior pelvic plane. This reference plane now is used in computer-assisted protocols. However, this static approach may lead to postoperative instability because the dynamic variations of the pelvis influence effective cup orientation and are not taken into account in this approach. We propose an ultrasound tool to register the preoperative dynamics of the pelvis for THA planning during computer-assisted surgery. To assess this pelvic behavior and its consequences on implant orientation, we tested a new 2.5-dimensional ultrasound-based approach. The pelvic flexion was registered in sitting, standing, and supine positions in 20 subjects. The mean values were −25.2° ± 5.8° (standard deviation), 2.4° ± 5.1°, and 6.8° ± 3.5°, respectively. The mean functional anteversion varied by 26° and the mean functional inclination by 12° depending on the pelvic flexion. We therefore recommend including dynamic pelvic behavior to minimize dislocation risk. The notion of a safe zone should be revisited and extended to include changes with activity.






Similar content being viewed by others
References
Chen E, Goertz W, Lill CA. Implant position calculation for acetabular cup placement considering pelvic lateral tilt and inclination. Comput Aided Surg. 2006;11:309–316.
Dardenne G, Cano JD, Hamitouche C, Stindel E, Roux C. A new optimization approach for the calibration of an ultrasound probe using a 3D optical localizer. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:3048–3051.
DiGioia AM, Hafez MA, Jaramaz B, Levison TJ, Moody JE. Functional pelvic orientation measured from lateral standing and sitting radiographs. Clin Orthop Relat Res. 2006;453:272–276.
Eddine TA, Migaud H, Chantelot C, Cotten A, Fontaine C, Duquennoy A. Variations of pelvic anteversion in the lying and standing positions: analysis of 24 control subjects and implications for CT measurement of position of a prosthetic cup. Surg Radiol Anat. 2001;23:105–110.
Jaramaz B, DiGioia AM 3rd, Blackwell M, Nikou C. Computer assisted measurement of cup placement in total hip replacement. Clin Orthop Relat Res. 1998;354:70–81.
Kluess D, Martin H, Mittelmeier W, Schmitz KP, Bader R. Influence of femoral head size on impingement, dislocation and stress distribution in total hip replacement. Med Eng Phys. 2007;29:465–471.
Lazennec JY. Relations hanche rachis: conséquences fonctionnelles applications aux arthroplasties totales de hanche. Le Complexe Lombo-Pelvien: De L’anatomie à la Pathologie. Montpellier, France: Sauramps Medical; 2005.
Lazennec JY, Charlot N, Gorin M, Roger B, Arafati N, Bissery A, Saillant G. Hip-spine relationship: a radio-anatomical study for optimization in acetabular implant cup positioning. Surg Radiol Anat. 2004;26:136–144.
Legaye J, Duval-Beaupère G, Hecquet J, Marty C. Pelvic incidence: a fundamental pelvic parameter for three-dimensional regulation of spinal sagittal curves. Eur Spine J. 1998;7:99–103.
Lembeck B, Mueller O, Reize P, Wuelker N. Pelvic tilt makes acetabular cup navigation inaccurate. Acta Orthop. 2005;76:517–523.
Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip replacement arthroplasties. J Bone Joint Surg Am. 1978;60:217–220.
Mayr E, Kessler O, Prassl A, Rachbauer F, Krismer M, Nogler M. The frontal pelvic plane provides a valid reference system for implantation of the acetabular cup: spatial orientation of the pelvis in different positions. Acta Orthop. 2005;76:848–853.
McCollum DE, Gray WJ. Dislocation after total hip arthroplasty: causes and prevention. Clin Orthop Relat Res. 1990;261:159–170.
Mercier L, Langø T, Lindseth F, Collins DL. A review of calibration techniques for freehand 3-D ultrasound systems. Ultrasound Med Biol. 2005;31:143–165.
Murray DW. The definition and measurement of acetabular orientation. J Bone Joint Surg Br. 1993;75:228–232.
Nishihara S, Sugano N, Nishii T, Ohzono K, Yoshikawa H. Measurements of pelvic flexion angle using three-dimensional computed tomography. Clin Orthop Relat Res. 2003;411:140–151.
Prager RW, Rohling RN, Gee AH, Berman L. Rapid calibration for 3-D freehand ultrasound. Ultrasound Med Biol. 1998;24:855–869.
Sanchez-Sotelo J, Berry DJ. Epidemiology of instability after total hip replacement. Orthop Clin North Am. 2001;32:543–552, vii.
Acknowledgment
We thank the members of the laboratory for their contributions in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
One or more of the authors (GD) have received funding from the French National Agency of Research, Réseau National des Technologies pour la Santé.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
Appendix 1: Fully Automatic Calibration of an Ultrasound Probe
Appendix 1: Fully Automatic Calibration of an Ultrasound Probe
The calibration step of an US probe allows us to obtain the 3-D position of any point located on the 2-D ultrasonic (US) image using a 3-D localizer. This is therefore a crucial step for our application. Several methods can be found in the literature [14], however, some criteria are very important for clinical uses: accuracy, simplicity, robustness and rapidity. This appendix presents a fully automatic method based on a special phantom and on simulated data which can satisfy these constraints.
We observed that for the calibration method based on a plane [17], the US image didn’t change for some motions which are needed for a correct calibration. This is the case for the translations of the probe along the plane, the rotation about the normal axis of the plane and the rotation about an axis contained in the plane and in the US image (Probe axis) (Fig. 7).
The simulation can therefore be used instead of carrying out manually those motions. To do that, we suppose that the US probe and the plane are fixed. The position and orientation of the US probe is recorded thanks to 3D localizer: this is our 3D initial virtual reference. In order to simulate the previous described motions, the transformations representing these motions are applied mathematically to this initial virtual reference. These four motions are therefore taken into account for the estimation of the calibration without any human interventions.
However, two remaining motions are needed to estimate correctly the calibration parameters: the translations of the probe on the normal axis of the plane and the rotation around the parallel vector (Fig. 8).
These motions introduce modifications on the US image and these modifications must therefore be estimated and applied to the initial image in order to simulate these two remaining movements. To do that, the scale factor must be first calculated and the rotation centre around the parallel vector must be visible on the US image. A phantom allowing us to realize it and to simulate all motions has been therefore developed (Fig. 9).
It consists of three nylon wires which form a simple triangle, where the base wire represents the surface of a virtual plane and the two others wires allow us to determine the scale factors and the rotation centre. The probe is fixed and aligned on this triangle during the procedure. Each wire has a known position in the space according to the rigid body attached on the phantom.
To evaluate the accuracy, 20 results were obtained by repeating the calibration procedure 20 times. A single point phantom was used to perform this evaluation. The accuracy was assessed by measuring the distance between this point and the positions of the 3D points obtained with the calibration results (Table 2).
Our novel approach of US calibration is therefore based on mathematical simulation instead of manual positioning of the probe avoiding thus user intervention. In addition to potentially reducing the calibration time, the simulation provides accurate datasets using only one US image. The results of this fully automatic method show that it is not only easy and fast to perform but also accurate.
About this article
Cite this article
Dardenne, G., Dusseau, S., Hamitouche, C. et al. Toward a Dynamic Approach of THA Planning Based on Ultrasound. Clin Orthop Relat Res 467, 901–908 (2009). https://doi.org/10.1007/s11999-008-0408-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-008-0408-z