Abstract
Sol–gel based coatings are used to protect metals from corrosion. They offer a barrier to the electrolyte penetration, but they do not provide active corrosion protection. Therefore, corrosion inhibitors are often added to sol–gel formulations to improve the overall corrosion behavior. Sol–gel-based coatings typically require relatively high temperatures to be properly cured, which supposes high energy consumptions and might damage some of the precursors of the formulation, including corrosion inhibitors incorporated to improve the coating’s properties. In this study, the effect of diethylenetriamine (DETA) as a curing agent, and yttrium 4-hydroxy cinnamate [Y-(4OHCin)3] as corrosion inhibitor, on the chemistry and corrosion performance of a hybrid silica-epoxy formulation are investigated. Solid nuclear magnetic resonance and attenuated total reflectance Fourier transform infrared spectroscopy are carried out to analyze the influence of the curing agent and the corrosion inhibitor on the chemical structure of the coating. The corrosion performance is assessed by means of electrochemical impedance spectroscopy, and the results are evaluated considering the chemical study and the interaction between the different compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traditional methods to prevent the corrosion of carbon steel have involved chromates, which have been widely used as corrosion inhibitors and as a major component in conversion coatings. However, chromates are toxic and carcinogenic and should be substituted.1
Hybrid organic–inorganic sol–gel-based coatings are currently of great interest as conversion coating replacements. Their inorganic moiety provides excellent barrier properties and very good adhesion to the metal being protected, while their organic components ensure compatibility with the paint systems applied on top.2 However, barrier properties might not be enough to protect against corrosion, and the coatings eventually take up electrolyte and detach from the metal, leaving it unprotected and prone to damage. Thus, the incorporation of environmentally friendly corrosion inhibitors in the sol–gel coatings should confer the active protection needed for better overall anticorrosion properties.
Due to the need for replacement of chromates, investigations have been focused on the development of new, environmentally friendly corrosion inhibitors. Rare-earth metal (REM) salts were found to be efficient corrosion inhibitors for different metallic substrates by suppressing the cathodic electrochemical reaction involved in the corrosion process.3 On the other hand, organic corrosion inhibitors have also shown their ability to suppress the anodic reaction by forming a protective layer at the anodic sites.4 Therefore, it is expected that the combination of rare earth metals with organic compounds would result in a rare earth metal complex that could suppress both the anodic and the cathodic reactions involved in the corrosion process.5,6
Lanthanum 4-hydroxy cinnamate [La(4-OHCin)3·5H2O] and yttrium 4-hydroxy cinnamate [Y(4-OHCin)3·3H2O] (where relevant, the water molecules have not been included for simplification) have consistently shown very good corrosion protection for mild steel.5,7 These inhibitors have been proposed to act synergistically in solution to reduce both electrochemical reactions.7 These corrosion inhibitors have been directly incorporated to epoxy coatings8 and La(4-OHCin)3 has also been incorporated into hybrid organic–inorganic formulations developed by the sol–gel method,9,10 showing promising results.
In the process of coating application, the consolidation of the deposited layer is traditionally obtained by applying a thermal treatment, which contributes to eliminate the water absorbed on Si–OH and Ti–OH groups and to decrease the porosity and the surface area of the material. The thermal treatment also leads to a more densified network with a higher degree of crosslinking. Usually, the Si–OH and Ti–OH residue groups condense further to Si–O–Si and Ti–O–Ti at temperatures above 400 °C,11,12 although this treatment is not always possible due to the substrate’s limitations. In addition, the organic fractions of the coating cannot withstand such high temperatures, therefore the thermal treatment for hybrid organic–inorganic networks is frequently decreased. At lower curing temperatures, it must be assumed that part of the physically and chemically bound water, as well as organic materials that have not been hydrolyzed, will persist in the structure.9,10 In a previous work,10 panels with coatings containing different percentages of La(4-OHCin)3 were subjected to a thermal treatment of 180 °C for 1 h. To understand the processes taking place at this temperature and to study the effect that the different concentrations of La(4-OHCin)3 had on the thermal treatment and the final structure of the resulting coatings, DSC isotherms at 180 °C were recorded for coatings with and without inhibitor. It was observed that in all cases, an exothermic peak appeared from the very beginning, but with increasing concentrations of La(4-OHCin)3 in the coating, the intensity of the peak, which represents the enthalpy released by the system, decreased and the peaks became broader. This reduction in the intensity indicated that La(4-OHCin)3 was interfering with the condensation of the Si–O–Si network. This result was in accordance with the observations from 29Si solid-state NMR. High concentrations of La(4-OHCin)3 induced internal stresses in the Si–O–Si polymeric network, preventing the formation of highly dense and crosslinked networks and resulting in the formation of free volume spaces that created porosity.13
Furthermore, these type of rare earth carboxylates, such as the La(4-OHCin)3 and/or Y(4-OHCin)3 studied in this work, are temperature sensitive. Indeed, these rare earth carboxylates are organometallic complexes that may contain water as coordinated ligands but also water of crystallization.14 This has been demonstrated by TGA analysis, as the water molecules were released in different steps.14 Therefore, when applying a thermal treatment, the inhibitor’s chemical structure might change by losing some of this water present in the complex. These water molecules have a very important role in the solubility of the organometallic complexes. When one or more water molecules are released from the complex structure, the solubility of the corrosion inhibitor decreases and so does the corrosion inhibition provided by the rare earth metal carboxylate.15 If the structure of the complex is modified inside the coating, a poorer corrosion inhibition may be observed due to unavailability of the rare earth metal complex to leach out of the coating for active corrosion protection. To prevent this from happening, this work aims at decreasing the thermal treatment required for a hybrid organic–inorganic coating formulation by using amines as curing agents.
Amines are well known curing agents for epoxy coatings and the hybrid sol–gel formulations studied in this work have epoxy group moieties in both the organic–inorganic silicon alkoxide (GPTMS) and the organic precursor (DGEBA). It has been found that by adding an amine such as diethylenetriamine (DETA) to a hybrid silica-epoxy coating, the curing is catalyzed16,17 and therefore, lower thermal treatments are needed to densify the final hybrid polymeric network. Furthermore, by decreasing the temperature of the thermal treatment or the exposure time when curing the coatings, energy will be saved, making the coatings more economical and environmentally friendly.
Many scientists have reported the addition of amine curing agents for the crosslinking of epoxy or hybrid silica-epoxy coating systems. Davis et al.16 have shown by NMR and ATR-FTIR analysis that by incorporating a stoichiometric amount of DETA into a TEOS-GPTMS matrix an optimum crosslinking is achieved, however, no corrosion characterization was performed in this study. The coatings were cured at RT for 24 h and when required, the coatings were subjected to an additional 1 h at 150 °C. In a later study,18 a silica-epoxy matrix including DGEBA in the formulation showed a highly reticulated structure of films, resulting in a barrier layer with high corrosion resistance and a durability of several weeks in aggressive environment. However, as pointed out by the authors,18 further improvement of the structural properties, as well as the synthesis conditions, was still needed to achieve longer durability. Another work from the same authors19 showed an improved corrosion resistance (studied through electrochemical impedance spectroscopy) after 330 days of immersion in a 3.5 wt% NaCl solution. These works show excellent and promising corrosion results in the field. However, these two works show coatings synthesis at (70 °C) for long times (minimum of 4 h) in tetrahydrofuran (THF) and the authors do not mention the pot-life of the coating solutions prior to their deposition. In addition, long curing times were needed (60 °C for 48 h and 100 °C for 3 h). These are believed to be crucial factors for a later industrialization of the coatings. Recently, Yang et al.20 performed a room temperature (RT) hybrid epoxy-silica synthesis with TEOS and GPTMS, and the curing agent DETA. They found that the amount of DETA influenced the thermal stability of the coating by affecting the production of amorphous silica in the hybrid coating and concluded that when the ratio DETA-GPTMS was 1, the prepared hybrid coating had optimum corrosion protection. However, the resulting coatings also needed long times to be cured since they were allowed to dry in an oven at 50 °C for 1 week. In addition, they showed brittleness when compared to traditional epoxy coatings.
This review shows the need to further study and optimize the synthesis of silica-epoxy hybrid coatings with improved corrosion protection. Furthermore, the coating process in this work aims at investigating coatings synthesized with a low energy consumption by using lower temperature for synthesis and lower curing steps in the coating production. In addition, even if the effect of amines in epoxy coatings is well known, to the authors’ best knowledge, they have not been studied when incorporated in a sol–gel matrix containing rare earth carboxylates as corrosion inhibitors.
This paper shows the different steps needed to produce a coating formulation with the incorporation of corrosion inhibitor, with DETA as curing agent. Each step is accompanied by detailed characterization to investigate the impact of each coating component on the final properties. In a first attempt, both La(4-OHCin)3 and Y(4-OHCin)3 were added to the hybrid network. However, La(4-OHCin)3 provoked an unwanted reaction, producing a white precipitate at the bottom of the solution. It was therefore disregarded for further study, as it could not provide a homogeneous coating. On the other side, Y(4-OHCin)3 could be easily incorporated in the coating formulation and applied to the substrate.
The analytical techniques used for the characterization of the different coating formulations were EIS to determine corrosion resistance and 29Si MAS and 13C CP MAS NMR and ATR-FTIR to investigate structural changes, while the viscosity and the thickness of the coatings were also measured.
Materials and methods
Materials
The solvent n-propanol (>99%) was obtained from Scharlab, S.L. (Sentmenant, Spain). The silicon alkoxide, tetraethyl orthosilicate (TEOS, 98%), was purchased from Acros Organics (Geel, Belgium). The hybrid organic–inorganic silicon alkoxide (epoxide functionlized) 3-(glycidyloxypropyl)trimethoxy silane (GPTMS, 98%), and the organic precursors, poly(Bisphenol A-co-epichlorohydrin) glycidyl end-capped (average Mn ~ 355), and diethylenetriamine (DETA, 99%), were purchased from Sigma-Aldrich (Merck, Darmstadt, Germany). All these reagents were used as received. Sulfuric acid (95–97%) was acquired from Sharlab (Sentmenat, Barcelona, Spain) and used to prepare a 0.1 M solution, which acted as the acid catalyst for the sol–gel reaction taking place. Yttrium 4-hydroxycinnamate, Y(4-OHCin)3, was synthesized as previously reported.14
S355 J2 + N carbon steel was provided by Arcelor Mittal (Spain) and had the following chemical composition: 0.154% C, 1.430% Mn, 0.011% P, 0.04% Ni, 0.02% Si, 0.041% Al, 0.03% Cr, 0.02% other and, 98.25% Fe. The surface of the carbon steel was subjected to an abrasive SiC grinding paper, grade P1200 (600 grit) to obtain a consistent finish.
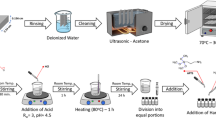
Synthesis of the sols
The synthesis of the sols was carried out by mixing in a Schott bottle a measured quantity of TEOS and GPTMS in n-propanol with the slow addition of 0.1 M sulfuric acid. This mixture was heated for 2 h at 50 °C. After that, P-DGEBA was added to the Schott bottle and the new mixture was heated an additional hour at 50 °C under stirring conditions. After the 3 h heating, the solution was then left stirring at room temperature for 24 h and subsequently, the corrosion inhibitor Y(4-OHCin)3 (when in formulation) was incorporated into the solution.
Coating preparation
Four different coatings were obtained (Si-0.5D, Si-1D, Si-1.5D, and Si-1D_Y) with different amine-H:epoxy group ratios, corresponding to 0.5, 1 and 1.5, and with addition or not of Y(4-OHCin)3.
DETA was diluted in n-propanol, added to the mixtures, and left stirring for 10 min before dip-coating the substrate into the sol. The coating formulation was then applied to the substrate by means of a home-made dip coater with controlled speed (4, 6, 14, 21, 29 and 36 cm/min were used to obtain the desired thickness and final properties).
Coated panels were then thermally cured for 1 h at 120 °C.
Characterization
The viscosity of the sols was measured by using the LOVIS 2000 M/ME viscometer, equipped with different capillaries. A capillary with an internal diameter of 1.8 mm was used for this study, which allows viscosity measurements in the range of 2.5 to 30 mPa.s.
The thickness of the coatings was measured on soda lime glass using a Dektak 150 contact profilometer. The coatings were scratched after the thermal treatment, just before their measurement. Five scans were taken across a 50 mm length scratch. The coating thickness was determined by measuring the height of the step.
29Si nuclear magnetic resonance (NMR) spectra were obtained with a Bruker Avance III 300WB spectrometer at 7.04 T (59.62 MHz for 29Si NMR). Magic angle spinning (MAS) was performed at a spinning rate of 12000 Hz. Spectra were acquired with 1H decoupling and referenced externally to tetra-methoxy-silane, TMS. The parameters used for the spectra acquisition were: 300 ppm bandwidth, 4000 scans for the Si coatings, 5 s of relaxation time, and 1024 acquisition points. The analyzed powder was obtained from sols molded as bulk material left for gelling for 24 h and treated at the same temperature and time to the equivalent coatings (120 °C for 1 h). A fine powder was obtained after crushing them with a mortar and pestle.
To quantitatively study the effect of DETA and Y(4-OHCin)3 on the coating formulations, a fit and peak deconvolution of the 29Si NMR T signal with the DMFit program21 was conducted while Q results were not taken into consideration due to the broader and less intense peaks observed in the spectra.
The degree of polycondensation, of GPTMS \(\tau\) (T) and TEOS \(\tau\) (Q) were calculated following equations (1) and (2):
where the building units are represented by the commonly used \({T}_{i}^{j}\) and \({Q}_{i}^{j}\) notation. This common notation is further explained in a previous work of the authors.10
13C cross polarization (CP) MAS solid NMR experiments were performed to understand the effect of DETA and Y(4-OHCin)3 on the organic part of the coatings and to estimate how the curing agent and the inhibitor affect the chemical structure of the coatings. The analyzed powder was obtained in the same manner as for the 29Si NMR measurements.
13C CP MAS spectra were obtained with a Bruker Avance III 300WB spectrometer at 7.04 T (75.47 MHz for 13C NMR). Magic angle spinning (MAS) was performed at a spinning rate of 12000 Hz. Spectra were acquired with 1H decoupling and were referenced externally to glycine. The parameters used for the spectra acquisition were: 300 ppm bandwidth, 4000 scans, 1 s relaxation time, and 2048 acquisition points.
Attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy was carried out using a PerkinElmer Frontier spectrometer. Spectra were acquired using a diamond attenuated total reflectance (ATR) crystal after 160 scans across the 4000–650 cm−1 wavenumber range at a resolution of 2 cm−1. Spectrum of Y(4-OHCin)3 powder was also acquired as reference.
Electrochemical impedance spectroscopy (EIS) experiments were carried out to study the corrosion behavior of the coatings over 72 h. Experiments were performed in a conventional three-electrode electrochemical cell comprising the coated/uncoated S355 J2 + N carbon steel as working electrode with a surface area of 4.91 cm2, a saturated silver/silver chloride reference electrode and a titanium mesh as the counter electrode. The electrolyte used was a 0.005 M NaCl solution, and the experiments were carried out at room temperature and open to air. The analysis was performed using a BioLogic VMP3 potentiostat controlled by EC Lab 11.33 software. The impedance spectra were recorded after 2 min, 2 and 5 h of immersion in the electrolyte and then every 24 h over a frequency range of 100 kHz to 10 mHz with 6 or 7 points measured per decade after monitoring the OCV for 1 min. The amplitude of the sinusoidal perturbation was 15 mV (root mean square, RMS). At least three repetitions of each sample were performed to confirm the reproducibility of the results.
Results and discussion
Effect of DETA and Y(4-OHCin)3 on the thickness of the coatings
The DETA molecule has 13 hydrogens, 5 of which are bonded to a nitrogen and therefore are active and can react to open one epoxy ring.16 Considering that in these formulations there are 2 molecules containing epoxy rings, GPTMS (1 epoxy group) and P-DGEBA (2 epoxy groups) and taking into account their molar ratios shown in Table 1, the amine-H:epoxy group ratios studied for formulations Si-0.5D, Si-1D and Si-1.5D, are 0.5:1, 1:1 and 1.5:1, respectively.
The sol viscosity was measured 24 h after the sol synthesis, before the addition of the curing agent (DETA). This sol had a density of 1.03 ± 0.01 g/cm3 and a dynamic viscosity of 13.4 ± 1.1 mPa.s at 25 °C. The corrosion inhibitor 5 wt% of Y(4-OHCin)3 was incorporated into the stoichiometric matrix (Si-1D_Y) with the aim of improving the corrosion performance of the coating. This concentration of the corrosion inhibitor quickly dissolved in the formulation giving place to a transparent sol. To obtain a coating with a thickness comparable to the coating control (Si-1D), the viscosity of this sol was adjusted to 13 mPa.s by adding more n-propanol to the formulation before the addition of DETA. The viscosity after the addition of the curing agent, DETA, was not measured due to the rapid increase in viscosity that it had on the formulation, which could block the viscometer.
The thicknesses of the derived coatings with different concentrations of DETA are shown in Fig. 1. The thickness of the coating increases with the withdrawal speed during coating deposition by dipping. Formulations Si-1D and Si-1D_Y are very similar as expected considering their similar viscosity values. These last two formulations resulted in the thickest coatings, while the Si-1.5D formulation gave the thinnest coating. The addition of amine with ratios above and below 1 (0.5 and 1.5, respectively) relative to the epoxy have both the effect of decreasing the thickness of the coatings.
The coatings derived from these formulations were deposited onto the carbon steel substrate S355 by dipping at a withdrawal speed of 14 cm/min, with a resulting thickness of around 6 ± 0.5 µm for formulations Si-0.5D and Si-1D and 5 ± 0.7 µm for formulation Si-1.5D.
Effect of DETA and Y(4-OHCin)3 on the corrosion properties of the formulations
Figure 2 shows the electrochemical impedance spectra of the four coating formulations under study and the steel surface images after the electrochemical analysis of the coatings. The main objective of this experiment is to investigate the effect that the curing agent DETA and the corrosion inhibitor Y(4-OHCin)3 have on the barrier properties of the coatings. Comparing Figs. 2a and b, (amine-H:epoxy group ratios of 0.5:1 and 1:1 respectively), it is observed that when there are not enough hydrogen atoms to react with all the epoxy groups present in the formulation (0.5:1), the modulus of the initial low frequency impedance is one order of magnitude lower (107 vs 108 Ohm cm2) than when the amine to epoxy ratio is stoichiometric (1:1). The coating Si-0.5D has only been exposed to the solution for 72 h whereas coating Si-1D was exposed for 168 h, after which the modulus of the impedance is still higher than for coating Si-0.5D after 72 h.
On the other hand, when a higher ratio than stoichiometric of amine to epoxy is used (1.5:1, Si-1.5D, Fig. 2c), although the initial performance is similar to the stoichiometric coating (coating Si-1D), coating Si-1.5D seems to be taking up water faster, as the modulus of impedance in the middle frequency region decreases quicker than in coatings Si.0.5D and Si-1D with time. This is expected due to the hydrophilic nature of the unreacted amino groups in the hybrid coating that plays a role in absorbing moisture. Both groups collectively contribute to the coating's moisture absorption, consequently diminishing its effectiveness in corrosion protection.20 By comparing the images of the coating surfaces after the electrochemical analysis (Fig. 2), it can be observed that coating Si-0.5D (Fig. 2e) has several pits after 72 h of exposure, whereas the surface of coating Si-1D only shows 2 black protuberances on the surface that seem to be the result of the coating taking up water (Fig. 2f). On the other hand, in Fig. 2g (Si-1.5D), the deposition of corrosion product coming from the surface of the steel can be observed.
When Y(4-OHCin)3 was added to the stoichiometric formulation (Si-1D_Y, Fig. 2d), the spectrum shows initial barrier properties with low frequency impedances of 108 Ohm cm2. This behavior is similar to the control coating (Si-1D). However, by comparing the middle frequency range of both spectra, which is related to the predisposition of a coating to absorb water,22 the coating with Y(4-OHCin)3 takes up water faster.
Another method for comparing the proportion of defects or disbonded areas of the coatings under study is the breakpoint frequency method. This method provides information on the state of the coating during the EIS measurement. The breakpoint frequency is the frequency at which the slope of the impedance modulus changes from −1 to 0 (from capacitive to resistive) and it is associated with a phase angle of −45°.23 The higher the breakpoint frequency, the more compromised the coating is. For the control coating (Si-1D), the breakpoint changes from 1 Hz (0 h) to 100 Hz (168 h). On the other hand, for the coating containing the corrosion inhibitor (Si-1D_Y), the breakpoint varies from 1 Hz (0 h) to 1000 Hz (168 h), which means that the coating containing the inhibitor has a bigger area containing defects than the control coating. This is confirmed by comparing the images of the coated surfaces after the EIS study (Fig. 2), where it is observed that the control coating derived from formulation Si-1D has two defects, whereas the coating with Y(4-OHCin)3 (Si-1D_Y) has several defects all around the area immersed in the electrolyte. In a previous work of the authors,10 it was observed that there is an optimum concentration of rare earth metal carboxylate as corrosion inhibitor.
The literature reports that amines can react with rare earth metals forming an amine-Ln complex, displacing the other ligands and water molecules that previously could be coordinated to the rare earth metal.24,25 It is believed that the addition of DETA into the Si-1D_Y coating displaces the 4-OHCin groups, and as this is an anodic inhibitor, if it is not added in optimum concentrations, it promotes pitting.26 However, the addition of higher concentrations of Y(4-OHCin)3 was not possible as the resultant solution was not stable and it was not possible to obtain a homogeneous coating out of it.
Effect of DETA and Y(4-OHCin)3 on the chemistry of the formulations
To understand the effect of the different ratios of amine H:epoxy groups on the chemical structure of the coatings, NMR and ATR-FTIR analyses were carried out. Coatings were examined by ATR-FTIR after deposition on the carbon steel surface to inspect the ring opening reaction from the epoxy group. Figure 3 shows a diagram of the expected ring opening mechanism with DETA as well as the expected final chemical structure.
As observed in Fig. 4b, the band at around 910 cm−1 corresponding to the C–O stretch9 from the epoxy ring diminishes in intensity with increasing ratios of amine H: epoxy, as expected. When comparing the ATR-FTIR spectra of Si-1D_Y with the one of Y(4-OHCin)3 (Fig. 4a), the main feature to notice is the appearance of a small band at 1631 cm−1 that is not present in the Si-1D spectrum. This small peak is characteristic of the C=C stretch from the carboxylate group present in the corrosion inhibitor. However, at wavenumbers of around 1380 cm−1 the symmetric stretch band of the carboxylate group usually appears, as observed in the spectrum of the Y(4-OHcin)3 taken as a reference. This peak is completely missing in the spectrum of the coating containing the inhibitor, which suggests a reaction taking place between the amine and the yttrium carboxylate24,25 in agreement with the results of the electrochemical data shown above.
29Si MAS and 13C CP MAS solid-state NMR were carried out to understand the effect that DETA and the thermal treatment, as well as the addition of Y(4-OHCin)3, had on the organic and inorganic structure of the coatings. In this case, the formulations were prepared in the same way as explained in the experimental procedure; however, the samples were not applied by dipping, but were set as blocks. Two blocks for each formulation were prepared; one was left to gel for 24 h at room temperature and the other one was thermally treated at 120 °C for 1 h (same procedure as for the coatings on the substrate). The blocks treated for 24 h at room temperature had a volume of around 45 mL, while those treated for 1 h at 120 °C had a smaller volume (~5 mL) to ensure all the material was thermally treated for the 1 h.
Figures 5a and b show the 13C CP MAS solid-state NMR spectra from the bulk materials treated at RT and at 120 °C respectively, with Fig. 6 showing the peak signal assignments of these spectra for the main molecules present in the three formulations under study. The peaks were normalized with respect to the peak at ~ 42 ppm which corresponds to the carbon of the –CH3 group from the P-DGEBA molecule, as it is believed that the intensity of these peaks will remain unchanged due to the relatively stable –CH3 group.
The peaks broaden when the material has been subjected to the 120 °C thermal treatment. This is a typical behavior for highly crosslinked networks.16 Focusing on the spectra acquired for the bulk materials at room temperature (Fig. 5a), it can be observed that at low amine H:epoxy ratios (0.5:1, formulation Si-0.5D), the signals corresponding to the epoxy ring (carbon 3 and carbon 4) are still present, however they disappear at the stoichiometric ratio (1:1, formulation Si-1D). The spectrum for the material with higher amine-H:epoxy ratios (1.5:1, formulation Si-1.5D) is very similar to the stoichiometric one, the epoxy ring carbons are not present but in contrast, two peaks at around 50 and 55 ppm can be observed, which are thought to be related to unreacted DETA, which usually appears at chemical shifts of around 41 and 51 ppm.16 They appear slightly shifted due to the reaction taking place between the –NH groups and the epoxy rings. At higher chemical shifts the aromatic carbons are observed (120–160 ppm).
On the other hand, the spectra of the bulk materials treated at 120 °C (Fig. 5b) show greater differences between the three formulations. Si-0.5D (0.5:1; amine-H:epoxy) still shows a peak corresponding to carbon 4, meaning that the epoxy ring is not fully opened in this material. Carbon 3 is not clearly seen but this could be due to the close presence of the broad peak from carbon B related to the P-DGEBA molecule. As more DETA is added, peaks corresponding to carbon 1 and carbon 2 shift toward higher chemical shifts. These carbons are attached to a silicon that is also bonded to a methoxy group (–OCH3) in the GPTMS. After hydrolysis, the silicon is bonded to a hydroxyl group (–OH), so due to the differences in the atom’s environment, these signals shift. The replacement of a methoxy group for a hydroxyl group will displace the signal of the carbon closer to the silicon toward higher chemical shifts, meaning that the formulation with the highest concentration of DETA is slightly more hydrolyzed than the other two. DETA is a weak base; therefore, it can also catalyze the hydrolysis and condensation reactions taking place in the coating. Hydrolysis reactions usually proceed by nucleophilic attack by the oxygen of the water (acid catalysis) or the oxygen from the hydroxyl groups (base catalysis). When a base catalyst is in place, the hydrolysis step typically gets progressively faster, resulting in smaller but higher branched molecules, whereas in acidic catalysis reactions, the fastest step is the first hydrolysis reaction and condensation starts before hydrolysis is complete, therefore resulting in chain-like structures in the sol. Thus, this explains why the formulation containing the highest concentration of DETA is the one with the highest hydrolysis rate.
13C CP MAS NMR enhances the 13C signals via their dipolar couplings to 1H. Therefore, quaternary carbons give lower relative intensities due to the lack of any directly attached 1H. Their relative intensity is dependent on the cross polarization of more distant protons, for which dipolar coupling is weaker. In Fig. 5, the relative intensity of carbons F and C (quaternary carbons) increases with the concentration of DETA in the formulation and with the thermal treatment given to the bulks which seems to be related to the overall more rigid structure caused by the higher crosslinking of the network.
Figure 5 also shows the 13C CP MAS NMR spectrum from the bulk material derived from formulation Si-1D with 5 wt% Y(4-OHCin)3 [Si-1D_Y]. As observed, when comparing the 13C solid-state NMR spectra from the bulk materials cured at RT and at 120 °C, the peaks broaden when the materials have been subjected to a thermal treatment, which is a typical behavior observed when polymers crosslink.16,27 The signals of the spectra are assigned to the carbons of the structures represented in Figs. 6a and b.
The main differences between formulations Si-1D and Si-1D_Y are observed in Fig. 5a (RT) in the signals observed at 24 and 26 ppm. This is thought to be related to the environment of carbon 2 that changes with the curing of the bulk materials. Carbon 2 is close to the silicon atom. As the environment of the silicon atom changes due to hydrolysis and condensation reactions, so does the carbon and this can be observed in a displacement of its chemical shift. When the inhibitor is added to the formulation, there is a shift toward higher chemical values (from 24 to 26 ppm). A substitution of a –OCH3 for a –OSi bond will not have much effect on the displacement of the peak due to the similar electronegativity values of carbon and silicon atoms; however, the replacement of the –OCH3 by –OH (hydrolysis reaction) shifts the signals toward higher chemical values. Therefore, the 13C CP MAS NMR data infers that the addition of Y(4-OHCin)3 favors the hydrolysis reaction in the bulk material cured at RT. However, after curing at 120 °C (Fig. 5b), the signals from carbon 2 for both samples are similar, suggesting that the thermal treatment helps to finalize the hydrolysis and condensation reactions in a similar way.
The epoxy ring signals at 52 ppm are still present for both formulations with and without inhibitor (even in the spectra taken after the thermal treatment at 120 °C) being more pronounced for the Si-1D_Y coating, suggesting a slightly lower degree of crosslinking. However, the one at 45 ppm is not observed in the spectrum of the formulations.
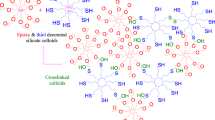
Figure 7 shows a schematic representation of the proposed structure of the final network formed.
29Si solid-state MAS NMR was also performed to study the effect of DETA concentration in the formulation on the silicon network structure of the materials. The four spectra for formulations Si-0.5D, Si-1D, Si-1.5D, and Si-1D_Y cured at 120 °C for 1 h are shown in Fig. 8. Only T signals were accounted for in the deconvolution and fitting of the peaks due to the stronger T signals and the lower signal-to noise ratio observed in the lower part of the spectra where the Q signals are present. This low Q signal is due to the higher concentration of GPTMS with respect to TEOS in the formulations, as well as the known high relaxation times required for the high molecular weight polymeric Q species to give a pronounced peak.10
As observed in Table 2, after the fitting of the T peaks and application of equations 1 and 2, the T conversion percentage has been calculated. Considering the fitting errors for each spectrum they all gave T conversions of around 90%, thus there is no evidence that the concentration of DETA in the formulation has any remarkable effect on the hydrolysis and condensation of the silicon network. It is believed that DETA does not have a deep impact on the final silicon structure of the hybrid network as it is added after the sol–gel reaction has taken place, the sols are dip-coated only 10 min after the DETA addition due to the quick increase in viscosity that this curing agent provokes, and the amine predominantly reacts with the epoxy rings present in the sol.
Figure 8 also shows the 29Si NMR fitted data of the Si-1D_Y materials. In this case, it can be observed that Y(4-OHCin)3 influences the hydrolysis and condensation of the sol–gel reaction, as the T conversion % decreases from 90% for Si-1D to 80% with the incorporation of the yttrium complex [Si-1D_Y] (Table 2). It seems that the complex is interfering in the formation of the Si–O–Si polymeric network which agrees with the observations of the 13C CP MAS NMR data.
The solid-state NMR data indicate that Y(4-OHCin)3 is interfering in the crosslinking of the polymeric network in both the organic epoxy ring opening and in the inorganic hydrolysis and condensation reactions. This less crosslinked structure could be the reason why the barrier properties of the coating are reduced when the rare earth metal complex is included in the coating formulation. The ATR-FTIR data also suggest an interaction between the carboxylate group from the inhibitor and the coating matrix, which could interfere in the curing process and contribute to the detrimental effect on the corrosion performance of the coating (reduced barrier properties).
In a previous work of the authors,10 the addition of La(4-OHCin)3 was successfully added into a different hybrid sol–gel formulation giving optimized corrosion performance, which points out the importance of studying the effect that each inhibitor may have on different coating formulations.
Conclusions
A hybrid silica-epoxy formulation has been developed with an optimized ratio of curing agent/epoxy group. The effect of the amine on the structure of the formulation as well as on the barrier properties of the coating has been studied. Among the different formulations analyzed, the coating with the stoichiometric formulation exhibited the highest barrier properties.
The inclusion of 5 wt% of the corrosion inhibitor Y(4-OHCin)3 successfully resulted in the formation of a stable sol capable of producing a homogenous coating. However, the presence of Y(4-OHCin)3 had a detrimental effect on the barrier properties of the coating, thereby impacting the corrosion protection of carbon steel. Solid-state NMR data indicated that the rare-earth carboxylate hindered both the amine-epoxide curing reaction and the condensation reactions of the silicon network.
Data availability
Data will be made available on request.
References
Twite, RL, Bierwagen, GP, “Review of Alternatives to Chromate for Corrosion Protection of Aluminum Aerospace Alloys.” Prog. Org. Coat., 33 (2) 91–100. https://doi.org/10.1016/S0300-9440(98)00015-0 (1998)
Balgude, D, Sabnis, A, “Sol–Gel Derived Hybrid Coatings as an Environment Friendly Surface Treatment for Corrosion Protection of Metals and Their Alloys.” J. Solgel Sci. Technol., 64 (1) 124–134. https://doi.org/10.1007/s10971-012-2838-z (2012)
Hinton, BRW, “Corrosion Inhibition with Rare Earth Metal Salts.” J. Alloys Compd., 180 (1–2) 15–25. https://doi.org/10.1016/0925-8388(92)90359-H (1992)
Sanyal, B, “Organic Compounds as Corrosion Inhibitors in Different Environments: A Review.” Prog. Org. Coat., 9 (2) 165–236. https://doi.org/10.1016/0033-0655(81)80009-X (1981)
Forsyth, M, Seter, M, Hinton, B, Deacon, G, Junk, P, “New ‘Green’ Corrosion Inhibitors Based on Rare Earth Compounds.” Aust. J. Chem., 64 (6) 812–819. https://doi.org/10.1071/CH11092 (2011)
Forsyth, M, Wilson, K, Behrsing, T, Forsyth, C, Deacon, GB, Phanasgoankar, A, “Effectiveness of Rare-Earth Metal Compounds as Corrosion Inhibitors for Steel.” Corrosion, 58 953–960 (2002)
Blin, F, Leary, SG, Wilson, K, Deacon, GB, Junk, PC, Forsyth, M, “Corrosion Mitigation of Mild Steel by New Rare Earth Cinnamate Compounds.” J. Appl. Electrochem., 34 591–599 (2004)
Peng, Y, et al. “Leaching Behavior and Corrosion Inhibition of a Rare Earth Carboxylate Incorporated Epoxy Coating System.” ACS Appl. Mater. Interfaces, 11 (39) 36154–36168. https://doi.org/10.1021/acsami.9b13722 (2019)
Suarez Vega, A, Agustín-Sáenz, C, Brusciotti, F, Somers, A, Forsyth, M, “Effect of Lanthanum 4-Hydroxy Cinnamate on the Polymerisation, Condensation and Thermal Stability of Hybrid Sol–Gel Formulations.” J. Solgel Sci. Technol., 96 (1) 91–107. https://doi.org/10.1007/s10971-020-05315-x (2020)
Suárez-Vega, A, Agustín-Sáenz, C, O’Dell, LA, Brusciotti, F, Somers, A, Forsyth, M, “Properties of Hybrid Sol–Gel Coatings with the Incorporation of Lanthanum 4-Hydroxy Cinnamate as Corrosion Inhibitor on Carbon Steel with Different Surface Finishes.” Appl. Surf. Sci., 561 149881. https://doi.org/10.1016/j.apsusc.2021.149881 (2021)
Brinker, CJ, Roth, EP, Scherer, GW, Tallant, DR, “Structural Evolution During the Gel to Glass Conversion.” J. Non-Crystal. Solids, 71 (1–3) 171–185 (1985)
Agustín-Sáenz, C, Martín-Ugarte, E, Jorcin, JB, Imbuluzqueta, G, Santa Coloma, P, Izagirre-Etxeberria, U, “Effect of Organic Precursor in Hybrid Sol–Gel Coatings for Corrosion Protection and the Application on Hot Dip Galvanised Steel.” J. Solgel Sci. Technol., 89 (1) 264–283. https://doi.org/10.1007/s10971-018-4840-6 (2019)
Cambon, JB, et al. “Effect of Cerium on Structure Modifications of a Hybrid Sol–gel Coating, Its Mechanical Properties and Anti-corrosion Behavior.” Mater. Res. Bull., 47 (11) 3170–3176. https://doi.org/10.1016/j.materresbull.2012.08.034 (2012)
Deacon, GB, Forsyth, M, Junk, PC, Leary, SG, Lee, WW, “Synthesis and Characterisation of Rare Earth Complexes Supported by Para-Substituted Cinnamate Ligands.” Z. Anorg. Allg. Chem., 635 (6–7) 833–839. https://doi.org/10.1002/zaac.200801379 (2009)
Seter, M, Hinton, B, Forsyth, M, “Understanding Speciation of Lanthanum 4-Hydroxy Cinnamate and Its Impact on the Corrosion Inhibition Mechanism for AS1020 Steel.” J. Electrochem. Soc., 159 (4) C181–C189. https://doi.org/10.1149/2.058204jes (2012)
Davis, SR, Brough, AR, Atkinson, A, “Formation of Silica/Epoxy Hybrid Network Polymers.” [Online]. Available: www.elsevier.com/locate/jnoncrysol
Trentin, A, et al. “Dual Role of Lithium on the Structure and Self-Healing Ability of PMMA-Silica Coatings on AA7075 Alloy.” ACS Appl. Mater. Interfaces, 11 (43) 40629–40641. https://doi.org/10.1021/acsami.9b13839 (2019)
Torrico, RFAO, et al. “Structure and Properties of Epoxy-Siloxane-Silica Nanocomposite Coatings for Corrosion Protection.” J. Colloid Interface Sci., 513 617–628. https://doi.org/10.1016/j.jcis.2017.11.069 (2018)
Uvida, MC, Almeida, ADA, Pulcinelli, SH, Santilli, CV, Hammer, P, “Structural Properties of Epoxy–Silica Barrier Coatings for Corrosion Protection of Reinforcing Steel.” Polymers, 14 (17) 3474. https://doi.org/10.3390/polym14173474 (2022)
Yang, S, Jia, Z, Xu, J, Hong, R, “Influence of DETA on Thermal and Corrosion Protection Properties of GPTMS-TEOS Hybrid Coatings on Q215 Steel.” Coatings, 13 (7) 1145. https://doi.org/10.3390/coatings13071145 (2023)
Massiot, D, et al. “Modelling One- and Two-Dimensional Solid-State NMR Spectra.” Magn. Reson. Chem., 40 (1) 70–76. https://doi.org/10.1002/mrc.984 (2002)
Lamaka, SV, et al. “Novel Hybrid Sol–Gel Coatings for Corrosion Protection of AZ31B Magnesium Alloy.” Electrochim. Acta, 53 (14) 4773–4783. https://doi.org/10.1016/J.ELECTACTA.2008.02.015 (2008)
Hack, HP, Scully, JR, “Defect Area Determination of Organic Coated Steels in Seawater Using the Breakpoint Frequency Method.” J. Electrochem. Soc., 138 (1) 33–40. https://doi.org/10.1149/1.2085574 (1991)
Wilfong, WC, Kail, BW, Bank, TL, Howard, BH, Gray, ML, “Recovering Rare Earth Elements from Aqueous Solution with Porous Amine-Epoxy Networks.” ACS Appl. Mater. Interfaces, 9 (21) 18283–18294. https://doi.org/10.1021/acsami.7b03859 (2017)
Xie, F, Zhang, TA, Dreisinger, D, Doyle, F, “A Critical Review on Solvent Extraction of Rare Earths from Aqueous Solutions.” Miner. Eng., 56 10–28. https://doi.org/10.1016/j.mineng.2013.10.021 (2014)
Aliofkhazraei, M, Developments in Corrosion Protection. 2014.
Perrin, FX, Ziarelli, F, Dupuis, A, “Relation Between the Corrosion Resistance and the Chemical Structure of Hybrid Sol–Gel Coatings with Interlinked Inorganic–Organic Network.” Prog. Org. Coat., 141 105532. https://doi.org/10.1016/J.PORGCOAT.2019.105532 (2020)
Acknowledgments
The authors would like to thank the Diputación Foral de Gipuzkoa through the project REANTI (Exp 066/18) and the Basque Government for the Elkartek project Frontiers-V (ref. KK2019/00077). They also recognize the Australian Research Council (ARC) through grant DP180101465. Maria Forsyth acknowledges the Ikerbasque Foundation for Science for their support through a Ikerbasque professorship.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Suárez-Vega, A., Agustín-Sáenz, C., O’Dell, L.A. et al. Effect of the curing agent DETA and its interaction with a rare earth carboxylate as corrosion inhibitor in a hybrid silica-epoxy formulation. J Coat Technol Res (2024). https://doi.org/10.1007/s11998-024-00958-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11998-024-00958-9