Abstract
Cultivation-based and DNA-based methods for determining the bacterial load and the composition of the bacterial spectrum have been successfully established for media in electrodip painting, and used for the detailed analysis of the contamination situation in an E-coating system of an automobile plant in Germany. Dominating representatives of the genus Microbacterium spp., the orders Burkholderiales and Pseudomonadales, the family Cytophagaceae and the genera Corynebacterium spp., Sphingomonas spp., and Stenotrophomonas spp. were used for inactivation experiments. Different pulsed electric field (PEF) parameters were studied for an effective and target-directed inactivation of defined bacterial suspensions containing mixtures of Gram-positive as well as Gram-negative bacteria, but also single species suspensions in adequate liquids. PEF treatment with pulse durations longer than 1.0 µs effectively killed bacteria even in low conductivity media, regardless of whether the pulses were unipolar or bipolar, indicating that the choice of pulse shape does not limit the design of the PEF system. Model calculations showed that for efficient treatment in bypass mode, a high treatment flow rate is required rather than a high inactivation efficiency of the PEF treatment. By using specific treatment parameters, such as bipolar pulses of 50 k Vcm−1 and a treatment energy of 40 J mL−1, a significant reduction in both Gram-negative and Gram-positive bacteria (> 2 log10 reduction) can be achieved while minimizing electrode corrosion and coating degradation. PEF treatment proves to be an effective alternative to the use of biocides in an E-coating system and can help maintain a bacteriostatic environment in the system by operating at different points, in transfer flow or bypass mode, ensuring biocide-free operation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As electrophoretic coating (E-coating) technology continues to improve, new waterborne paints have been developed that help to make processes more environmentally friendly. Due to lower process temperatures, the widespread use of such water-based paint systems and the inevitable introduction of contaminants, tanks are susceptible to microbial growth.1,2,3 Biofilms in recirculating water systems and deionized water tanks can also be a source of bacterial contamination.4,5,6 Disinfection measures, paint properties and the problem of contamination must be coordinated as part of a hygiene concept.7
In both anodic dip painting (ADP) and cathodic dip painting (CDP), the parts to be coated first pass through various preparation processes that require large quantities of demineralized water.2,8 As the water is pumped in and cascades through the circuit, the tanks in the E-coating line and the rinse water become contaminated, leading to a significant bacterial contamination over time. In the past, the paint mixtures killed these bacteria during the dipping process, but due to changing environmental requirements and the near-complete substitution of organic solvents and heavy metals, paints are becoming more and more microbiologically sensitive.4 An increased bacterial load can lead to a reduction in the quality of the coating and an increase in water consumption coupled with a reduction in the service life of the E-coating system due to more frequent cleaning.9 In the worst case, the entire contents of the E-coating dip tank can be lost. Therefore, targeted disinfection measures are necessary to limit the uncontrolled bacteria proliferation.
The regular way to reduce bacterial proliferation is to use certain chemical biocides in the pretreatment and E-coating dip tanks. This is done at regular intervals and on demand. However, not every disinfectant is up to the task; moreover, some products are completely incompatible with individual paint mixtures or cleaning baths.10 For this reason, careful selection and dosing of biocides are necessary to prevent coating failure. In addition, there is a risk that biocide-resistant bacteria will emerge as a result of the extensive use of biocides.1,7 This would render the biocides useless, with unpredictable consequences for maintaining trouble-free operation. In addition, the development of novel biocides is restricted by legislation and the rigorous approval process.10,11,12 In the future, legislative restrictions on biocide concentrations are expected to increasingly limit the use of biocides. It is therefore essential to search for alternative methods to ensure sustainable operation of the paint shops while acknowledging environmental considerations.
An alternative method for the reduction of bacterial contamination in liquids is the pulsed electric field (PEF) treatment. PEF treatment is a physical method wherein the cell membrane undergoes permeabilization due to an applied external field.13,14 The external field induces high electric field strengths across the cell membrane, resulting in forming of aqueous pores into the lipid bilayer.15 This phenomenon, termed electroporation, finds applications in various fields, such as medicine, genetics, food production, and biotechnology.16,17 In principle, the increased permeability of cell membranes facilitates the molecular exchange between cytoplasm and external medium across the lipid membrane. In the last two decades, PEF technology has demonstrated its feasibility at pilot and industrial scale for the effective extraction of sugar from sugar beet, for the improvement of wine production and enhancement of potato chips processing.18,19,20,21 Recently, it was demonstrated that PEF treatment enables the cascade processing of microalgae biomass and offers a promising option for energy-efficient extraction of lipids, proteins, and pigments, such as c-phycocyanin from cyanobacteria.22,23,24,25,26,27,28,29
PEF treatment as a bacterial decontamination method for liquids has been proven to extend the shelf life of liquid foods such as fruit juices, milk, and soups, while ensuring the chemical stability of the flavors and vitamins of the treated foods.30,31,32,33,34 A significant progress for PEF treatment was achieved by demonstrating the use of this method as an alternative disinfection technique to prevent the dissemination of antibiotic-resistant bacteria from hospital wastewater.35,36,37,38 Once these pathogens enter surface waters, the spread of bacteria is irreversible and the risk of contamination of water resources increases with the diversity of harmful bacteria.23 Bacterial inactivation of more than 5 log10 was achieved by a combination of heating to 55 °C and PEF treatment.37 As with industrial fluids, wastewater contains different mixtures of bacteria and the fluid properties, such as conductivity and temperature, can vary, requiring a high degree of flexibility in the technology. It was also shown that a large variety of undesirable microorganisms, among them Gram-negative, Gram-positive bacteria, and fungi, were effectively reduced by PEF treatment.31
The aim of this study within the framework of an R&D project was to develop resource-efficient water management and plant concepts by integrating PEF technology, with the objective of maintaining a bacteriostatic milieu in the system that guarantees biocide-free and trouble-free operation.39 As part of the study, PEF treatment was used for the first time to decontaminate E-coating fluids. This purely physical and automated process can significantly reduce the use of chemicals and is effective even for turbid liquids with chemically sensitive components, such as cathodic dip painting.
Materials and methods
State of the art: analysis of bacterial contamination in an automotive paint shop
For this study we used bacteria strains identified along the E-coating process sequence in a paint shop from the automotive industry, as reported in DiWaL Report 2021, BMBF Grant.39 For a better understanding of the sites identified as sources of contamination, a simplified schematic of the process sequence is shown in Fig. 1. The process is divided in three main zones: pretreatment (VBH-07 to VBH-11), E-coating (KTL-01), and post rinse zone (KTL-02 to KTL-05). From the entire pretreatment process this scheme shows only the phosphating (VBH-07) and cleaning stages (VBH-08 to VBH-11). In the study (DiWaL Report 2021), 11 pretreatment dip tanks (VBH-01 to VBH-11), the E-coating dip tank (KTL-01), and the four post rinse stages (KTL-02 to KTL-05) were investigated. Bacteria of the family Burkholderiaceae, Sphingomonadaceae, and Micrococcaceae were identified in the pretreatment zone, whereas only Burkholderiaceae spp. was found in the post rinse zone.39 The last tank (deionized water rinsing tank VBH-11) in the pretreatment zone is prone to bacterial contamination, particularly from dripping water from car bodies and from deionized water inlet. This tank was identified as the main source of bacterial contamination of the E-coating tank, with bacterial concentrations of ~ 103 cfu mL−1. According to this study, no bacteria could be cultivated in samples from the E-coating tank, KTL-01, or in the two subsequent post rinse tanks (KTL-02 and KTL-03), i.e., no CFU could be determined. While no bacterial DNA could be isolated in KTL-01, DNA was extracted from the samples of the two subsequent stages. This resulted in a very narrow spectrum of bacterial colonization, predominantly belonging to the order Burkholderiales. In contrast, a very high bacterial load with over 105 cfu mL−1 was found in the post rinse tanks KTL-04 and KTL-05, which was supposed to be the second main contamination source of the E-coating tank, due to the permeate backflow.
Schematic outline of a car body E-coating line (based on a technical drawing from the BMW Group). The simplified process line shows the pretreatment zone, starting with the phosphating in VBH-07 dip tank followed by dip rinse tanks (VBH-08, VBH-09), passivation (VBH-10) and deionized water rinsing (VBH-11), E-coating dip tank (KTL-01), and the spray rinse zone with four stages (KTL-02 to KTL-05). The car bodies flow is from VBH-07 to KTL-05. In the post rinse zone, the backflow of the permeate is from KTL-05 to KTL-01 tank (light blue arrows). The blue arrows indicate the inlets for the deionized water and the red arrows indicate the inlets for the chemical additives
Identification and cultivation of bacteria
Bacterial population in the 200 mL water samples of the different tank systems was investigated by conducting PCR-DGGE analyses targeting the V1–V3 region of the Eubacteria 16S rRNA genes with an amplicon size of 509 bp (base pair) after initial membrane filtration (0.2 µm Nucleopore) and DNA extraction using a commercial kit system (FastDNA™ SPIN Kit for Soil, MP Biomedicals, Santa Ana, USA). Each PCR reaction included a 2.5 µL buffer (Extra Buffer, 15 mM MgCl2, VWR Life Science, Germany), 0.5 µL of deoxyribonucleotide triphosphate (DNT, 10 µM, VWR Life Science, Germany), 0.25 µL of each primer (40 nM, Eurofins Genomics, Ebersberg, Germany). The primers sequences according to Muyzer et al. with GC 27F (GC-Clamp) 5´-AGAGTTTGATCCTGGCTCAG-3´ containing a GC-clamp (i.e., 5´-CGC CCG CCG CGC CCC GCG CCC GTC CCG CCG CCG CCC CCG CCC C-3´) and 518R 5´-ATTACCGCGGCTGCTGG-3´, 0.125 µL of Taq polymerase (TEMPase Hot Start DNA Polymerase, VWR Life Sciences, Germany), and 1 ng µL−1 template (10 ng DNA per sample) were used, and the volume was adjusted to 25 µL by adding sterile PCR-water (DNase- and RNase-free water, MP Biomedicals, Santa Ana, USA).40 The temperature profile of PCR thermocycler (C1000 Touch, Bio-Rad, Feldkirch, Germany) consists of 3 min at 95 °C followed by 30 times at 95 °C for 30 s, 56 °C for 1 min, and 72 °C for 2 min. The prokaryotic 16S rDNA PCR amplicons were controlled by a 1% agarose gel electrophoresis (Serva, Heidelberg, Germany), together with reference bacterial 16S rRNA gene amplicons, using SYBR Gold (Invitrogen, Karlsruhe, Germany) fluorochrome for DNA band visualization with F1 Lumi-Imager (Roche Diagnostics, Mannheim, Germany).
The denaturing gradient gel electrophoreses (DGGE) gel was made with a linear urea gradient from 40–70%. Reference markers [Escherichia coli (DSM 1103), Pseudomonas aeruginosa (DSM 1117), Enterococcus faecalis (DSM 20478), Enterococcus faecium (DSM 20477), Staphylococcus aureus (DSM 2569), and Stenotrophomonas maltophilia (SMK279a)] were generated and used as a ladder to enable meaningful comparisons across different DGGE gels. A maximum of 15 µL of the PCR samples, containing equal amounts of PCR product, were loaded onto the gel. The running time of the gel was 17 h at 70 V and 56 °C. The DGGE gel was analyzed via an F1 Lumi-Imager workstation (Roche Diagnostics, Mannheim, Germany) using the Lumi-Imager software (LumiAnalyst 3.1). The excised DNA band samples from the DGGE were given to Eurofins Genomics (Ebersberg, Germany) for sequencing using MiSeq, with 2 × 300 bp targeting the V1–V3 16S rRNA gene region. The sequences were aligned with public rDNA data bank for taxonomic identification.41,42
In the cultivation approach, bacterial quantification was carried out by determining the colony-forming units (CFU) on two agar nutrient media in order to be able to detect the widest possible spectrum of bacteria. For this purpose, a nutrient-rich medium (LB broth, Luria/Miller, Roth, Karlsruhe, Germany) and a nutrient-reduced mineral medium (R2A-Agar, Merck, Germany) were used. As is known, only a fraction of the bacteria present can be cultivated on synthetic culture media.
Sampling and preparation of bacterial model suspensions
Bacteria of the families Burkholderiaceae, Sphingomonadaceae, and Micrococcaceae were selected. The selection was made based on the concentration of bacteria and the bacteria identified as sources of bacterial contamination along the E-coating process. From these families the representative bacterial species, Sphingomonas gimenis, Gram-negative, Microbacterium maritypicum, Gram-positive, and Burkholderia cepacia, Gram-negative, were isolated and used to inoculate model media. Prior to inoculation into the sample, bacteria were cultivated for 24 h at 30 °C on LB medium, on shaker, to reach a bacterial concentration around 108 cfu mL−1.
Model suspensions were used to test the bacterial inactivation efficiency of PEF treatment of representative bacteria in different process samples and growth media. One reason for using model suspensions is that some of the process samples from the paint shops, identified as a source of bacterial contamination, have a relatively low bacterial concentration below 103 cfu mL−1, which is not sufficient to accurately study inactivation rates above 3 log10 units. Secondly, systematic studies on PEF parameter dependency with scattering microbial composition, for example due to different sampling points and times, are not expedient. Samples were collected from the pretreatment zone (VBH-11 tank) and the post rinse zone (KTL-02 to KTL-05), along with several deionized water tanks used to compensate for any water loss. Native samples from VBH-11 and KTL-04 tanks were stored in a refrigerator at 4 °C and tempered at room temperature before PEF treatment. To prepare the model suspensions, liquid samples from paint shops were each enriched with one of the representative bacterial species up to a concentration of 106 cfu mL−1 (see Table 1). Prior to enrichment with externally cultured bacteria, the sample matrices were sterile filtered through a 0.2 µm pore syringe filter (VWR Life Science, Germany). In addition, bacterial suspensions in LB medium were prepared using the same procedure. For the preparation of model suspensions, representative bacteria, cultivated as described before, were centrifuged at 10,000 × g for 5 min (Biofuge Pico, Heraeus, Germany) to remove the growth medium. The resulting bacteria pellet was resuspended in model media or LB medium. Table 1 shows the model suspensions from selected tanks that were identified as sources of bacterial contamination and the isolated bacteria used to enrich the model media.
Determination of bacterial viability
The viability of bacteria after PEF treatment was determined by colony counting on agar plates (LB-Agar, Roth, Karlsruhe) after incubation for 24–48 h at 30 °C. After serially diluting of untreated and treated samples, aliquots of 200 µL were plated on agar media (CASO, casein-soja bean agar, Merck, Germany). After incubation, the numbers of CFU on agar plates were counted. The CFU was calculated as an average of CFUs of at least three plates. Bacterial inactivation (I) after PEF treatment is given as log10 reduction of viability: I = log10 (Ntotal/Nviable bacteria).
PEF treatment
Experimental determination of PEF treatment parameters required for effective inactivation of bacteria in aqueous process media and growth media were carried out using a homemade laboratory transmission line pulse generator. The transmission line pulse generator based on 50 Ω coaxial cables (RG 213, Belden, Villingen-Schwenningen, Germany) delivered square-wave pulses with pulse durations ranges from 100 ns to 10 µs and pulse amplitudes up to 25 kV, as described by Frey et al.38 Commercially available electroporation cuvettes (BTX Instrument Division, Holliston, MA, USA) with a gap size of 2.0 ± 0.1 mm and a volume of 400 μL were used in all experiments as treatment chambers. The pulse rise time of the transmission line generator was 16 ns. The pulse durations were set by the length of the coaxial cable to 0.5, 1.0, and 2.0 µs, while the pulse amplitude achieved in the electroporation cuvettes was 50.00 ± 0.35 kV cm−1. The matching condition for electroporation cuvettes, i.e., the load resistance (RL) equals the characteristic impedance (Z0) of the transmission line, 50 Ω, was set with media having a conductivity of 2.0 mS cm−1. For media with different conductivities, such as samples from the E-coating line, with conductivities between 50 and 80 µS cm−1, the load resistance was adjusted to 50 Ω by a parallel resistor. The parallel resistor with coaxial geometry consisted of an outer cylinder (ground [−]) with a radius of 33 mm and an inner conductor (HV [+]) with a radius of 15 mm, both made of stainless steel. The length of the coaxial resistor was 50 mm. The coaxial resistor was filled with electrolytes based on sodium thiosulfate solution (Na2S2O3 5H2O, Carl Roth, Germany) with the conductivity of 483 µS cm−1 to achieve parallel resistances of 52.0 Ω. The electrolyte was circulated by a peristaltic pump (40 mL min−1, Ismatec ecoline, Germany) during operation through an inlet and an outlet attached to the outer cylinder to avoid resistance changes due to Joule heating. The capacity volume of the circulated electrolyte was 5 L. The pulse repetition rate was either 1 Hz or 5 Hz. The specific PEF treatment energy (W) was set to 40 ± 2 J mL−1 and 80 ± 3 J mL−1 by varying the number of pulses (N). For this, the number of pulses, for given pulse duration (tp), conductivity of the suspension (κe), applied electric field strength (E), and required specific treatment energy (W), was calculated using equation (1).
Bipolar square-wave pulses were generated by a bidirectional pulse forming line. For this special form of transmission line, after charging the line, one end of the line is short-circuited by means of a spark gap at the temporal start of the pulse. Thus, a pulse shape is formed between the two inner conductors of the coaxial cable which, viewed in time, exhibits a polarity change in the middle of the pulse. As a result, in the further course of the pulse, a first positive half-wave is followed instantaneously by another half-wave with reversed polarity but the same amplitude. For the PEF treatment, bipolar pulses with the same specific pulse energy (40 J mL−1 and 80 J mL−1), pulse amplitude (50 kV cm−1), and pulse duration (1 µs and 2 µs, were considered as the sum of the two half-waves, tp = t[+] + t[−],) as the unipolar pulses were generated. Pulse parameters for treating the model suspension with unipolar and bipolar pulses are given in Table 2.
Results and discussion
Operation modes
In addition to defining the parameter range for PEF treatment, a key task was to find the way of operation and the sites of integration of the PEF technology into the ongoing E-coating process, considering inactivation requirements and ensuring trouble-free operation. The required inactivation performance affects the technical requirements of the PEF system, in particular the specific treatment energy and flow rate, which in turn influence the required performance of the pulse generator. The choice for the site of operation is designed to protect the E-coating dip tank from bacterial contamination and growth, thus maintaining its bacteriostatic state. Contaminating bacteria are introduced in various ways: directly into the E-coating dip tank by dripping water from the car bodies during transfer from pretreatment (VBH-11 tank), by the supply of deionized water used to compensate for water evaporation, and by the backflow from the permeate rinse tanks used to recycle water and paint (tank KTL-02 to KTL-05). In this context, two operating modes were defined to calculate the required inactivation performance of the PEF treatment: (I) in “transfer-flow” (Fig. 2a), to prevent the direct contamination by bacterial transfer from a source of contamination (e.g., deionized water, or backflow from permeate rinse tanks) to the E-coating dip tank, and (II) in “bypass”(Fig. 2b), to reduce the bacterial load in a certain tank, e.g., treatment of the E-coating dip tank or the VBH-11 tank.
Schematic view of the operating modes of PEF treatment: (a) in transfer-flow, from a donor tank, the source of contamination, to the recipient tank, typically the E-coating dip tank and (b) in bypass, to reduce the bacterial load in a process tank, e.g., E-coating or VBH-11 tank. Goal of both operating modes is to keep the bacterial concentration over time (N(t)), below a certain contamination level (Nmax), to maintain the bacteriostatic state
Mathematical modeling to determine the required inactivation performance
I. PEF treatment in transfer-flow For this operating mode, the required inactivation performance of the PEF treatment (IPEF) depends on the level of bacterial contamination in the donor tank (Ndonor) and the maximum level of contamination that is acceptable for trouble-free operation (Nmax) in the receiver tank, see equation (2). The maximum bacterial load in the E-coating dip tank was determined by the paint company (PPG Deutschland Sales & Services GmbH) and paint shop operator (BMW Group) based on operating experience and should not exceed 103 cfu mL−1. On the other hand, no bacteria could be grown in the paint samples and no CFU could be detected in the E-coating dip tank (KTL-01) and the two downstream permeate rinse tanks, KTL-02 and KTL-03, while a very high bacterial load, over 105 cfu mL−1, was found in the next post-treatment zones (KTL-04 and KTL-05). By assuming that the backflow into the E-coating tank has the same bacterial concentration (Ndonor) as in KTL-04, then the required minimum inactivation performance is IPEF = log10(105·10−3) ≥ 2 log10, as calculated from equation (2). This inactivation performance is also sufficient for the PEF treatment of freshwater and deionized water feeds, as the usual contamination level of cultivable bacteria in these samples is around 103 cfu mL−1.
II. PEF Treatment in bypass Contrary to the operation mode in the transfer-flow, the required inactivation efficiency of the PEF treatment in operation in the bypass (Fig. 2b) is determined by several process parameters. One of the most important parameters is the volumetric flow rate \(\emptyset = \dot{V}\) of the PEF treatment system, as it influences both the temporal inactivation efficiency in the treatment tank and the design of the PEF system. A second important parameter is the growth rate (µ) of the bacteria in the treated tank. This parameter results from the bacterial reproduction rate in medium and the accumulation rate caused by dripping liquids from car bodies or backflows as well as the water inflow. The time dependency of the concentration of viable bacteria C(t), as a function of volumetric flow rate (Ø), tank volume (V0), and inactivation efficiency of the PEF treatment (IPEF) can be calculated by solving the following differential equation:
where dCµ(t) is the bacterial change due to growth and accumulation of bacteria in the tank and dCPEF(t,Ø,IPEF) is the bacterial change due to inactivation by PEF treatment. Details of the calculation can be found in the Appendix I. The solution of equation (3) gives:
where C0 is the concentration of viable bacteria in the tank at beginning of the PEF treatment. Figure 3 shows the time course of the bacteria concentration C(t) on a logarithmic scale during the operation of the PEF treatment in the bypass mode. The calculation was carried out at three different ratios of volumetric flow to tank volume (Ø/V0: 0.05 h−1, 0.10 h−1 and 0.15 h−1) and three inactivation rates of the PEF treatment (IPEF: 1 log10, 2 log10 and 3 log10). A value of 0.03 h−1 was assumed for the bacterial growth and accumulation rate (µ), based on the long-term monitoring of the bacterial growth (accumulation) rate in automotive series painting (BMW Group, DiWaL Report 2021).39 According to the model calculation the Ø/V0 ratio is the most important factor for effective bacteria reduction in the treated tank when the PEF treatment is operated in bypass mode and a bactericidal effect is to be achieved. Even more, this applies to all decontamination methods that work in bypass, even filtration, where all bacteria would be filtered out. In this case the inactivation, I, for each pass through the filter would be I > > 1, and therefore, it applies:
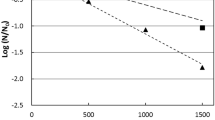
Time kinetic of bacterial concentration in the target tank during PEF treatment in bypass mode, at three distinct volume flow ratios (Ø/V0 = 0.05 h−1, 0.10 h−1 and 0.15 h−1) and three inactivation rates of PEF treatment (IPEF: 1 log10, 2 log10 and 3 log10). The initial bacterial concentration in the treatment tank is C0 = 105 cfu mL−1 and the bacterial growth and accumulation rate is µ = 0.03 h−1. The bacterial concentration in the tank is reduced from 105 to 103 bacteria per mL (means 2 log10, ↑ arrow) after approx. 39 h of PEF treatment with an inactivation efficiency of 2 log10 at a volume flow rate ratio of 0.15 h−1
And from equation (4) follows:
Equation (5) shows that with a higher inactivation efficiency (e.g., IPEF > 2 log10) of the system operating in bypass mode, the resulting microbial reduction in the treated tank is only dependent on the flow rate of the decontamination system, in other words how fast the tank contents can be pumped through the decontamination system. This has a direct impact on the design and power requirements of the PEF system, as a higher flow rate requires a higher power supply to maintain the same treatment intensity.
In the next calculation example, it is assumed that bacterial load in the target tank (e.g., E-coating dip tank), noted as initial bacterial concentration C0, has reached a critical concentration (≥105 cfu ml−1) and must be reduced to less than 103 cfu ml−1 as quickly as possible, without additional chemical treatment (e.g., biocides). According to equation (4) and the processing parameters shown in Fig. 3, the bacterial concentration in the target tank can be reduced from 105 bacteria per mL to a noncritical concentration of 103 bacteria per mL (see ↑ arrow in Fig. 3) within ~ 39 h of PEF treatment with an inactivation efficiency of IPEF ≥ 2 log10 at a volume flow rate ratio Ø/V0 of 0.15 h−1.
Increasing the volumetric flow rates shortens the operating time required to reduce the number of active bacteria in the target tank to a noncritical concentration, while the inactivation performance of the PEF treatment (IPEF) plays a subordinate role, only affecting bacterial reduction in the targeted tank when it increases from 1 log10 to 2 log10. Higher inactivation rates, e.g., IPEF > 3 log10, almost do not affect the reduction of bacteria in the treated tank (Fig. 3, ▲marks) as compared to the 2 log10 PEF inactivation efficiency (Fig. 3, ○, IPEF = 2 log10). This shows once again the importance of the flow rate, as only by increasing the flow rate ratio, in this case above 0.15 h−1 (Ø ≥ 15% of the target tank volume per h), is it possible to reach the noncritical bacteria concentration in less than 39 h. It also shows the technical requirements for the PEF system. For example, to treat the E-coating dip tank, with a volume of 230 m3, the necessary bypass flow rate for this example can be calculated as follows: 0.15 h−1·230 m3 = 34.5 m3 h−1, for which already few PEF systems are available on the market.43
In summary, a bacterial inactivation efficiency of the PEF treatment of 2 log10 is sufficient to cause at least a stagnation of bacterial growth (bacteriostatic effect) in the E-coating dip tank (KTL-01), both in bypass and transfer-flow treatment mode.
Bacterial inactivation by PEF treatment
The results reflect bacterial inactivation entirely due to the impact of the PEF and not promoted by electrically induced thermal heating. The PEF treatment energies of 40 J mL−1 and 80 J mL−1 would contribute to a temperature rise of approximately 10 K and 20 K, respectively, under adiabatic conditions, i.e., no heat transfer in the environment and electrodes. In the case of a continuous flow treatment and an initial medium temperature of 25 °C, the medium temperature would rise to a maximum of 45 °C under adiabatic conditions, which is still below the inactivation temperature of bacteria (< 70 °C). The measured temperature increases in the cuvettes immediately after PEF treatment were less than 2.5 K under our experimental conditions.
In a first approach PEF treatment was performed on native samples (without bacterial enrichment) with a specific treatment energy of 150 J mL−1 and in addition 80 J mL−1 for VBH-11 samples, to test the inactivation performance at a high energy input. Above a treatment energy of 150 J mL−1, impairment of the coating was observed. Native samples from VBH-11 and KTL-04 tanks were tempered at room temperature prior PEF treatment. No bacterial enrichment was performed in this case. These samples were contaminated with a mixed bacterial population. The predominant bacterial species are as shown in Table 1. After the PEF treatment the bacterial population in the native VBH-11 samples was completely eradicated, when treated with 150 J mL−1 indicating an inactivation efficiency of more than 3 log10 (n.d.: not detected bacteria in the treated samples), while the bacterial population in the KTL-04 samples was also reduced by more than 3 log10 from 106 cfu mL−1 to 102 cfu mL−1 (Fig. 4). At the lower treatment energy of 80 J mL−1, an inactivation rate of 2 log10 was achieved with both pulse durations of 1 µs and 2 µs. No bacteria could be cultivated from the samples of the two downstream tanks of the post rinse zone (KTL-02 and KTL-03); i.e., no CFU could be detected. Although bacterial DNA could be isolated, it was not possible to grow bacteria in this medium. It is assumed that certain bacterial species are inhibited in their growth, by diluted biocides or paint components (e.g., organic solvents). This is based on the observation that the bacteria concentration in the downstream process is higher in the diluted permeate of the KTL-05 and LKTL-04 tanks than in the tanks closer to the E-coating dip tank, KTL-02 and KTL-03.
Bacterial inactivation in native samples from VBH-11 and KTL-04 tanks after PEF treatment with unipolar square-wave pulses (1 µs and 2 µs) using a specific treatment energy of 150 J mL−1 and 80 J·mL−1 (at 50 kV cm−1) adjusted by the number of pulses. The conductivities of the native samples are 0.05 mS cm−1 (VBH-11) and 0.08 mS cm−1 (KTL-04) as listed in Table 2. Each experiment was repeated at least twice in triplicate. The used bacterial suspensions contained a mixture of previously identified and enriched Gram-positive and Gram-negative bacteria
Bacterial inactivation by PEF treatment with unipolar and bipolar pulses
Our second approach aimed to decrease the specific treatment energy and the pulse duration while ensuring a bacterial inactivation efficiency above 2 log10, as required by the model calculation. Shorter pulses are expected to be less prone to electrode charge accumulation, thereby reducing paint degradation by minimizing electrochemical reactions. For comparison, we applied square-wave pulses with pulse durations of 1 µs and 2 µs to model suspensions enriched with selected bacteria, as described in Table 1. The PEF treatment procedure is detailed in Table 2. Although short pulses (≤ 1 µs) were likely to prevent paint degradation, we decided to test the higher energy input of 80 J·mL-1 with longer unipolar pulses of 2 µs to compare inactivation efficiency as this protocol was expected to provide the highest inactivation rate. Figure 5a shows the results of PEF inactivation of sterile filtered samples from the VBH-11 tank enriched with B. cepacia (gray bars), M. maritypicum (blue bars), and S. gimenis (green bars) as a function of specific treatment energy and pulse duration. Figure 5b shows the results of PEF inactivation of model suspensions prepared with samples from the KTL-04 tank inoculated with B. cepacia. For comparison, the bacterial inactivation was also tested in the nonselective LB medium enriched with each of these bacterial strains (gray bars with white stripes). The lowest inactivation, around 2 log10, was achieved with Gram-negative bacteria (B. cepacia and S. gimenis) when treated in VBH-11 model medium with lower specific treatment energies (40 J·mL-1). For all other PEF treatments, the inactivation rate was greater than 2 log10. Contrary to expectations the highest inactivation was achieved for the Gram-positive bacteria M. maritypicum, when treated in the model suspension based on VBH-11 samples (blue bars). In general, bacterial inactivation was higher in model media than in LB media. We believe that this is again due to residual biocides or other chemical compounds that inhibit bacterial growth or have bactericidal properties, especially in model media from KTL-04 tanks.
Inactivation efficiency as a function of treatment energy and pulse duration for PEF treatment with unipolar square-wave pulses. (a) Inactivation of B. cepacia (gray bars), M. maritypicum (blue bars), and S. gimenis (green bars) inoculated in sterile filtered samples from VBH-11 tank and for comparison bacteria in LB medium (gray diagonal strips); (b) Inactivation of B. cepacian in sterile filtered samples from KTL-04 tank and for comparison bacteria in LB medium (diagonal strips). All experiments were repeated at least twice, each time in triplicate (Color figure online)
PEF treatment with unipolar pulses leads to buildup of charges on the electrodes and partial electrolysis of the solution. Ions such as hydroxide (OH−) or hydrogen cations (H+) are forming by water dissociation during the pulse application.44,45 This may result in coagulation of the coating, in corrosion of the electrode and in introduction of small particles of electrode material in the liquid. Thus, the E-coating process is affected. The use of bipolar pulses has the advantage over unipolar pulses, that the ions formed during the first half-wave are neutralized by the polarity reversal during the second half-wave.39 This prevents charge buildup on the electrodes and limits side effects such as coating deposition on the treatment electrodes and coagulation.39,45,46 However, a requirement for successful bacterial inactivation is a complete charging of the cell membrane, which according to our previous results, Frey et al. can only be achieved with longer pulses (> 0.5 µs) at small cell diameters (Ø bacteria: ~ 1 µm) and low conductivity (< 0.5 mS cm−1).38 This study has shown that bipolar pulses with a half-wave of less than 0.6 µs result in poor inactivation performance despite the same treatment. With the short bipolar pulse of 1 µs (2 × 0.5 µs) used in our study, the exposure time of the electric field of the half-waves (0.5 µs) is below this time limit, so that a poor inactivation efficiency was to be expected. Even with this disadvantage, short bipolar pulse of 1 µs (2 × 0.5 µs) duration were also tested, as this treatment cause less electrochemical reactions at the electrodes, thus preventing damage to the CDP or ADP.45 As the model calculations have shown, the required inactivation rates should be higher than 2 log10, which can be achieved by increasing the specific energy input. Although the strategy followed in this approach was to reduce the specific energy input to 40 J ml−1 in order to improve the efficiency of the method, we tested for 1 µs (2 × 0.5 µs) bipolar pulses also higher energy inputs (80 J ml−1). It was therefore necessary to test the bacterial inactivation efficiency of PEF treatment with bipolar pulses and compare it with the inactivation achieved by treatments with unipolar pulses of the same pulse length.
Figure 6 illustrates the inactivation efficiency of PEF treatment with bipolar pulses as a function of the specific treatment energy and the pulse duration, which is given as twice the half-wave width (e.g., 2 × 0.5 µs). Figure 6a illustrates the inactivation rates of B. cepacia (gray bars), M. maritypicum (blue bars), and S. gimenis (green bars) in VBH-11 model media. Meanwhile, Fig. 6b shows the inactivation rates of B. cepacia in KTL-04 model media. It can be observed that inactivation using bipolar pulses is less effective compared to unipolar pulses of the same duration and amplitude. Moreover, short bipolar pulses (half wave ≤ 0.5 µs) are ineffective, for instance, to reduce the M. maritypicum Gram-positive bacteria by 1 log10 (90%) and therefore not recommended for use in industrial applications. To ensure an inactivation efficiency of the PEF treatment, IPEF ≥ 2 log10, as required by the model calculation for all operation mode, bipolar pulses of a pulse duration longer than 2 µs (i.e., 1.0 µs for each half-wave) are required.
Inactivation efficiency as a function of treatment energy and pulse duration for PEF treatment with bipolar square-wave pulses. (a) Inactivation of B. cepacia (gray bars), M. maritypicum (blue bars), and S. gimenis (green bars) inoculated in sterile filtered samples from VBH-11 tank; (b) Inactivation of B. cepacian in sterile filtered samples from KTL-04 tank. All experiments were repeated at least twice, each time in triplicate (Color figure online)
Although it was expected that Gram-positive bacteria would be more robust to PEF treatment than Gram-negative bacteria, we observed that by selecting appropriate treatment parameters, inactivation in the range of 1 log10 – 2 log10 rate reductions can be achieved even with low specific treatment energies (e.g., 40 J mL−1).47,48,49 From the results in Fig. 5, the inactivation rates in the model media are higher than in LB medium. It is assumed that this is due to residual biocides or other bactericidal components (e.g., organic solvents) in these samples, which could affect the survival rate of bacteria after PEF treatment. But the fact that the bacterial inactivation rate in LB medium is greater than 2 log10 indicates that PEF treatment can provide the required inactivation efficiency even in biocide-free E-coating systems. However, in a biocide-free operation, the absence of biocides would affect the growth and accumulation rate of bacteria (µ) in the E-coating tank. The low value of µ = 0.03 h−1 used in our calculations is related to the intensive use of biocides and organic solvent residues in paints, which have an antibacterial effect. The actual growth factor could not be estimated for this type of operation as the E-coating system was never operated in a biocide-free mode. Therefore, at higher bacterial growth rates, an antibacterial effect of PEF treatment in bypass mode can only be achieved by increasing the treatment flow rate. Consequently, a coating bath maintenance method based on PEF treatments of critical tanks and backflow circuits should consider each coating system individually. Extrapolation of the results is only possible to a limited extent, and it is recommended to devise tailored solutions. If the E-coating system shows significant contamination, it is necessary to inspect the hygiene standards in place. The presence of high levels of bacteria indicates inadequate cleaning procedures and poor hygiene standards. PEF treatments with specific treatment energy of 40 J mL−1 can inactivate both Gram-negative and Gram-positive bacteria selected from this specific E-coating line to a degree high enough (> 2 log10) to ensure a bacteriostatic state in the system. Therefore, PEF treatment can be effective as a biocide-free bath maintenance method for the treatment of contamination hotspots in E-coating lines for high-throughput car body paint shops.
Conclusions
The results of this study prove that PEF treatment with pulse durations longer than 1.0 µs resulted in efficient bacterial inactivation even in media with low conductivity, regardless of the pulse shape, unipolar or bipolar. Thus, the design of the PEF system is not limited by the choice of pulse shape. The model calculations show that the bypass treatment mode requires a high treatment flow to be efficient: Ø ≥ 15% of the treated tank volume per h. For such applications, the development of a suitable pulse generator based on solid states switches capable of treating high volume flow rates (> 10,000 L h−1) is required. By using appropriate treatment parameters, such as bipolar pulses of 40 kV cm−1 to 50 kV cm−1 and a treatment energy of 40 J mL−1, a 2 log10 reduction in both Gram-negative and Gram-positive bacteria can be achieved while limiting electrode corrosion and coating degradation. In summary, PEF treatment is an effective alternative to biocide application that can be used in an E-coating system to maintain the bacteriostatic status of the system by operating at different spots, in transfer-flow or bypass mode, ensuring biocide-free operation.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and the Appendix. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request. Source data are provided with this paper: https://doi.org/10.2314/KXP:1778016480
References
Schwarzentruber, P, “Mikrobiologische Lack-Analyse in Echtzeit.” Farbe Lack, 108 24 (2002)
Gühring, IK, Mikrobieller Befall von Elektrotauchlack in der Automobilindustrie. University of Stuttgart, Stuttgart (2000)
Schumacher, J, Gebicke, W, Neue Konzepte zum Wassermanagement im Lackierbetrieb von PKW-Karossen. DWA Deutsche Vereinigung für Wasserwirtschaft Abwasser und Abfall e.V, Hennef, Deutschland (2013)
Streitberger, H-J (ed.) Automotive Paints and Coatings, 2nd edn. Wiley-VCH-Verl, Weinheim (2008)
Schmalz, DR, Grajecki, U, “Keine Chance für Bakterien: Desinfektion von ETL-Bädern, Ionenaustauschern und Membranen.” J. Oberfl. Technol., 50 20–23. https://doi.org/10.1007/BF03252557 (2010)
Mattila-Sandholm, T, Wirtanen, G, “Biofilm Formation in the Industry: A Review.” Food Rev. Int., 8 573–603. https://doi.org/10.1080/87559129209540953 (1992)
Di Maiuta, N, Schwarzentruber, P, Dow, CS, “Enhancement of the Antimicrobial Performance of Biocidal Formulations Used for the Preservation of White Mineral Dispersions.” Appl. Microbiol. Biotechnol., 89 429–439. https://doi.org/10.1007/s00253-010-2884-9 (2011)
Fröhlich, G, Halbartschlager, J, “Resource‐Efficient Use of Water in High‐Volume Car Body Painting Plants.” Chem. Ing. Tech., 91 1417–1433. https://doi.org/10.1002/cite.201900051 (2019)
Preiß, P, Eva Bohem, M, Gusbeth, C, Sack, M, Herzog, D, Schwartz, T, Dekold, S, Poboss, N, Lang-Koetz, C, Frey, W, “Less Chemicals and More Power: Pulsed Electric Field-Treatment for Reduction of Microorganisms: A Biocide-Free Bath Maintenance Method in Pre-treatment of Dip Coating Plants for High-Volume Car Body Painting Plants.” In: Weißgraeber, P, Heieck, F, Ackermann, C (eds.) Advances in Automotive Production Technology–Theory and Application, p. 311. Springer, Berlin Heidelberg (2021)
Schulte, S, Dissertation. Universität Duisburg-Essen, Duisburg (2003)
European Parliament, Council of the European Union, Regulation (EU) No 528/2012 concerning the making available on the market and use of biocidal products OJ L 167, p. 1–123 (27.6.2012). http://data.europa.eu/eli/reg/2012/528/oj
Rushton, L, Sass, A, Baldwin, A, Dowson, CG, Donoghue, D, Mahenthiralingam, E, “Key Role for Efflux in the Preservative Susceptibility and Adaptive Resistance of Burkholderia cepacia Complex Bacteria.” Antimicrob. Agents Chemother., 57 2972–2980. https://doi.org/10.1128/aac.00140-13 (2013)
Kotnik, T, Pucihar, G, Miklavcic, D, “Induced Transmembrane Voltage and its Correlation with Electroporation-Mediated Molecular Transport.” J. Membr. Biol., 236 3–13. https://doi.org/10.1007/s00232-010-9279-9 (2010)
Kotnik, T, Rems, L, Tarek, M, Miklavčič, D, “Membrane Electroporation and Electropermeabilization: Mechanisms and Models.” Ann. Rev. Biophys., 48 63–91 (2019)
Neumann, E, Rosenheck, K, “Permeability Changes Induced by Electric Impulses in Vesicular Membranes.” J. Membr. Biol., 10 279–290. https://doi.org/10.1007/BF01867861 (1972)
Kotnik, T, Frey, W, Sack, M, Haberl Meglič, S, Peterka, M, Miklavčič, D, “Electroporation-Based Applications in Biotechnology.” Trends Biotechnol., 33 480–488. https://doi.org/10.1016/j.tibtech.2015.06.002 (2015)
Frey, W, Gusbeth, C, Sakugawa, T, Sack, M, Mueller, G, Sigler, J et al. Environmental Applications, Food and Biomass Processing by Pulsed Electric Fields. In: Hidenori Akiyama und Richard Heller (Hg.): Bioelectrics. Tokyo: Springer Japan, S. 389–476 (2017)
Sack, M, Sigler, J, Eing, C, Stukenbrock, L, Stangle, R, Wolf, A, Muller, G, “Operation of an Electroporation Device for Grape Mash.” IEEE Trans. Plasma Sci., 38 1928–1934. https://doi.org/10.1109/TPS.2010.2050073 (2010)
Sack, M, Sigler, J, Frenzel, S, Eing, C, Arnold, J, Michelberger, T, Frey, W, Attmann, F, Stukenbrock, L, Müller, G, “Research on Industrial-Scale Electroporation Devices Fostering the Extraction of Substances from Biological Tissue.” Food Eng. Rev., 2 147–156. https://doi.org/10.1007/s12393-010-9017-1 (2010)
Schultheiss, C, Bluhm, H, Mayer, H-G, Kern, M, Michelberger, T, Witte, G, “Processing of Sugar Beets with Pulsed-Electric Fields.” IEEE Trans. Plasma Sci., 30 1547–1551. https://doi.org/10.1109/TPS.2002.804212 (2002)
Fauster, T, Ostermeier, R, Scheibelberger, R, Jäger, H, “Pulsed Electric Field (PEF) Application in the Potato Industry.” In: Knoerzer, K, Muthukumarappan, K (eds.) Innovative Food Processing Technologies, p. 253. Elsevier, Amsterdam (2021)
Akaberi, S, Krust, D, Müller, G, Frey, W, Gusbeth, C, “Impact of Incubation Conditions on Protein and C-Phycocyanin Recovery from Arthrospira Platensis Post-Pulsed Electric Field Treatment.” Bioresour. Technol., 306 123099. https://doi.org/10.1016/j.biortech.2020.123099 (2020)
Akaberi, S, Gusbeth, C, Silve, A, Senthilnathan, DS, Navarro-López, E, Molina-Grima, E, Müller, G, Frey, W, “Effect of Pulsed Electric Field Treatment on Enzymatic Hydrolysis of Proteins of Scenedesmus almeriensis.” Algal Res., 43 101656. https://doi.org/10.1016/j.algal.2019.101656 (2019)
Eing, C, Bonnet, S, Pacher, M, Puchta, H, Frey, W, “Effects of Nanosecond Pulsed Electric Field Exposure on Arabidopsis thaliana.” IEEE Trans. Dielect. Electr. Insul., 16 1322–1328. https://doi.org/10.1109/TDEI.2009.5293945 (2009)
Eing, C, Goettel, M, Straessner, R, Gusbeth, C, Frey, W, “Pulsed Electric Field Treatment of Microalgae—Benefits for Microalgae Biomass Processing.” IEEE Trans. Plasma Sci., 41 2901–2907. https://doi.org/10.1109/TPS.2013.2274805 (2013)
Goettel, M, Eing, C, Gusbeth, C, Straessner, R, Frey, W, “Pulsed Electric Field Assisted Extraction of Intracellular Valuables from Microalgae.” Algal Res., 2 401–408. https://doi.org/10.1016/j.algal.2013.07.004 (2013)
Jaeschke, DP, Mercali, GD, Marczak, LDF, Müller, G, Frey, W, Gusbeth, C, “Extraction of Valuable Compounds from Arthrospira platensis Using Pulsed Electric Field Treatment.” Bioresour. Technol., 283 207–212. https://doi.org/10.1016/j.biortech.2019.03.035 (2019)
Krust, D, Gusbeth, C, Müller, ASK, Scherer, D, Müller, G, Frey, W, Nick, P, Bioelectrochemistry. Elsevier, Amsterdam, Netherlands (2022)
Silve, A, Papachristou, I, Wüstner, R, Sträßner, R, Schirmer, M, Leber, K, Guo, B, Interrante, L, Posten, C, Frey, W, “Extraction of Lipids from Wet Microalga Auxenochlorella protothecoides using Pulsed Electric Field Treatment and Ethanol-Hexane Blends.” Algal Res., 29 212–222. https://doi.org/10.1016/j.algal.2017.11.016 (2018)
Hamilton, W, Sale, A, “Effects of High Electric Fields on Microorganisms: II. Mechanism of Action of the Lethal Effect.” Biochim. Biophys. Acta (BBA) Gen. Subj., 148 789–800. https://doi.org/10.1016/0304-4165(67)90053-0 (1967)
Rivas, A, Rodrigo, D, Martínez, A, Barbosa-Cánovas, GV, Rodrigo, M, “Effect of PEF and Heat Pasteurization on the Physical–Chemical Characteristics of Blended Orange and Carrot Juice.” LWT Food Sci. Technol., 39 1163–1170. https://doi.org/10.1016/j.lwt.2005.07.002 (2006)
Sharma, P, Bremer, P, Oey, I, Everett, DW, “Bacterial Inactivation in Whole Milk Using Pulsed Electric Field Processing.” Int. Dairy J., 35 49–56. https://doi.org/10.1016/j.idairyj.2013.10.005 (2014)
Zhou, J, Hung, Y-C, Xie, X, “Making Waves: Pathogen Inactivation by Electric Field Treatment: From Liquid Food to Drinking Water.” Water Res., 207 117817. https://doi.org/10.1016/j.watres.2021.117817 (2021)
Grimi, N, Lebovka, NI, Vorobiev, E, Vaxelaire, J, “Effect of a Pulsed Electric Field Treatment on Expression Behavior and Juice Quality of Chardonnay Grape.” Food Biophys., 4 191–198. https://doi.org/10.1007/s11483-009-9117-8 (2009)
Rieder, A, Schwartz, T, Schön-Hölz, K, Marten, S-M, Süss, J, Gusbeth, C, Kohnen, W, Swoboda, W, Obst, U, Frey, W, “Molecular Monitoring of Inactivation Efficiencies of Bacteria During Pulsed Electric Field Treatment of Clinical Wastewater.” J. Appl. Microbiol., 105 2035–2045. https://doi.org/10.1111/j.1365-2672.2008.03972.x (2008)
Gusbeth, C, Frey, W, Volkmann, H, Schwartz, T, Bluhm, H, “Pulsed Electric Field Treatment for Bacteria Reduction and Its Impact on Hospital Wastewater.” Chemosphere, 75 228–233. https://doi.org/10.1016/j.chemosphere.2008.11.066 (2009)
Gusbeth, CA, Frey, W, Schwartz, T, Rieder, A, “Critical Comparison Between the Pulsed Electric Field and Thermal Decontamination Methods of Hospital Wastewater.” Acta Phys. Pol. A, 115 1092–1094. https://doi.org/10.12693/APhysPolA.115.1092 (2009)
Frey, W, Gusbeth, C, Schwartz, T, “Inactivation of Pseudomonas putida by Pulsed Electric Field Treatment: A Study on the Correlation of Treatment Parameters and Inactivation Efficiency in the Short-Pulse Range.” J. Membr. Biol., 246 769–781. https://doi.org/10.1007/s00232-013-9547-6 (2013)
Frey, W, Gusbeth, C, Schwartz, T, Krolla, P, Preiss, P, Lang-Koetz, C, Technische Informationsbibliothek (TIB), Hannover. https://doi.org/10.2314/KXP:1778016480 (2020)
Muyzer, G, de Waal, EC, Uitterlinden, AG, “Profiling of Complex Microbial Populations by Denaturing Gradient Gel Electrophoresis Analysis of Polymerase Chain Reaction-Amplified Genes Coding for 16S rRNA.” Appl. Environ. Microbiol., 59 695–700. https://doi.org/10.1128/aem.59.3.695-700.1993 (1993)
Rogall, ET, Jacob, S, Triebskorn, R, Schwartz, T, “The Impact of the Anti-Diabetic Drug Metformin on the Intestinal Microbiome of Larval Brown Trout (Salmo trutta f. fario).” Environ. Sci. Eur., 32 1–14. https://doi.org/10.1186/s12302-020-00341-6 (2020)
Jungfer, C, Friedrich, F, Varela Villarreal, J, Brändle, K, Gross, H-J, Obst, U, Schwartz, T, “Drinking Water Biofilms on Copper and Stainless Steel Exhibit Specific Molecular Responses Towards Different Disinfection Regimes at Waterworks.” Biofouling, 28 891–907. https://doi.org/10.1080/08927014.2013.813936 (2013)
Gaudreau, M, Hawkey, T, Petry, J, Kempkes, M, Scaleup of PEF Systems for Food and Waste Streams. Diversified Technologies Inc., Bedford, MA, USA (2006)
Morren, J, Roodenburg, B, de Haan, SW, “Electrochemical Reactions and Electrode Corrosion in Pulsed Electric Field (PEF) Treatment Chambers.” Innov. Food Sci. Emerg. Technol., 4 285–295. https://doi.org/10.1016/S1466-8564(03)00041-9 (2003)
Roodenburg, B, Morren, J, Berg, HE, de Haan, SW, “Metal Release in a Stainless Steel Pulsed Electric Field (PEF) System: Part I. Effect of Different Pulse Shapes; Theory and Experimental Method.” Innov. Food Sci. Emerg. Technol., 6 327–336. https://doi.org/10.1016/j.ifset.2005.04.006 (2005)
Kotnik, T, Miklavcic, D, Mir, LM, Bioelectrochemistry. Elsevier, Amsterdam, Netherlands (2001)
García, D, Gómez, N, Mañas, P, Condón, S, Raso, J, Pagán, R, “Occurrence of Sublethal Injury after Pulsed Electric Fields Depending on the Micro‐organism, the Treatment Medium PH and the Intensity of the Treatment Investigated.” J. Appl. Microbiol., 99 94–104. https://doi.org/10.1111/j.1365-2672.2005.02611.x (2005)
Trevors, JT, Chassy, BM, Dower, WJ, Blaschek, HP, “Electrotransformation of Bacteria by Plasmid DNA.” In: Guide to Electroporation and Electrofusion, p. 265. Elsevier, Amsterdam (1992)
Barbosa-Canovas, GV, Pierson, MD, Zhang, QH, Schaffner, DW, “Pulsed Electric Fields.” J. Food Sci., 65 65–79. https://doi.org/10.1111/j.1750-3841.2000.tb00619.x (2000)
Acknowledgments
This study was carried out in the joint project DiWaL, funded by the German Federal Ministry of Education and Research (BMBF) under the WAVE program, FKZ 02WAV1405C. The consortium of this project was built with following partners: BMW Group Leipzig; Eisenmann GmbH; Emil Frei GmbH & Co. KG; PPG Deutschland Business Support GmbH; Pforzheim University, Institute for industrial Ecology (INEC); KIT, Institute for Pulsed Power and Microwave Technology (IHM) and the Institute of Functional Interfaces (IFG).
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was supported by the German Federal Ministry of Education and Research (BMBF) under the WAVE program, grant number FKZ 02WAV1405C. The consortium of this project was built with following partners: BMW Group Leipzig; Eisenmann GmbH; Emil Frei GmbH & Co. KG; PPG Deutschland Business Support GmbH; Pforzheim University, Institute for industrial Ecology (INEC); Karlsruhe Institute of Technology (KIT), Institute for Pulsed Power and Microwave Technology (IHM), and the Institute of Functional Interfaces (IFG).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or nonfinancial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
I. Details for calculation of the time evolution of the bacterial concentration in the treated tank, during PEF treatment in bypass:
To calculate the evolution of the concentration of viable bacteria over time, C(t), the following assumptions and notations were used: N(t) and N(t + dt) are the number of viable bacteria in the tank at timepoint t and t + dt, respectively. Assuming that the number of viable bacteria in the tank will change by the accumulation of bacteria (given by the growth and accumulation rate, µ) and by bacterial inactivation (IPEF), due to PEF treatment, then, at timepoint t + dt the number of bacteria in the tank will change accordingly to:
where Ndead is the number of bacteria inactivated during an infinitesimal time dt in the PEF system. In addition to the meaning of equation (2) as the efficiency of the PEF treatment, IPEF also stands for the bacterial inactivation rate, which is defined as follows:
In equation (7) Ntotal is the total number of bacteria treated during dt and can be expressed as:
where dV is the infinitesimal volume, treated during an infinitesimal time dt in the PEF system. V0 represents the tank volume. The ratio dV · V0−1 determines the number of bacteria treated during dt. From equations (7) and (8) and the meaning of Ndead = Ntotal-Nviable it follows for Ndead at time t + dt:
The flow rate through the PEF system is considered to be constant and is given by:
Thus, it follows for Ndead at time t + dt:
By rearranging equation (6) and using equation (11) we obtain:
The infinitesimal change in concentration, dC(t), is obtained by dividing the equation (12) by the volume of the tank, V0, and considering that dN = N(t + dt)-N(t) = V0·dC(t) and N(t) = V0·C(t):
By integrating the equation (14), the time dependence of the concentration of viable bacteria can be calculated:
Equation (13) can also be written as follows:
Thus, the change in bacterial concentration in the tank is the difference between the changes in bacterial accumulation and growth, dCµ(t), and the bacteria inactivated by PEF treatment, dCPEF(t, Ø, IPEF), as given by equation (3). It can be written:
where
and
Finally, equation (19) can be written as follows:
II. Pulse shape of the unipolar and bipolar square pulses delivered by the home-built transmission line pulse generator:
See Fig.
Examples of measured temporal shapes of pulses obtained with a transmission line generator (a) unipolar square-wave pulses of 1 µs duration and 10 kV pulse amplitude. For an electrode gap of 2 mm, the electrical field strength was 50.00 ± 0.35 kV cm−1. (b) Bipolar square wave of 2.0 µs pulse duration, considered as the sum of the two half-wave pulses, generated by a bidirectional pulse forming line. The electric field strength in the treatment chamber was the same as for unipolar pulses
7.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gusbeth, C., Krolla, P., Bruchmann, J. et al. Bacterial decontamination of process liquids and paints in E-coating lines by pulsed electric field treatment. J Coat Technol Res 21, 1385–1398 (2024). https://doi.org/10.1007/s11998-023-00901-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-023-00901-4