Abstract
Antifouling properties of unmodified kraft lignin for potential use in marine coatings were investigated. The study was based on preliminary findings that pointed toward lignin’s efficacy against seawater organisms during laboratory tests. Coatings were formulated that contained lignin as a filler and had a pigment volume concentration above the critical pigment volume concentration. This ensured direct interaction between lignin and seawater organisms, as the lignin particles remained incompletely wetted by the binder. Moreover, all formulations were waterborne to mitigate the release of volatile organic compounds. Despite the initial promise, the antifouling performance of the formulated lignin coatings during field experiments at the CoaST Maritime Test Center was limited, and the anticipated mechanism must be reconsidered. Additionally, it was found that high lignin concentrations, while facilitating organism interaction, compromised the coating's mechanical properties. Nevertheless, the waterborne coating formulation introduced here might provide a foundation for other researchers to further investigate lignin’s potential as a bio-based pigment or a filler in coatings.
Similar content being viewed by others
Introduction
With the escalating concern for environmental sustainability, the coatings industry has increasingly been seeking solutions that are not only high performance but also environmentally friendly. This demand necessitates a shift from traditionally used petrochemical-based raw materials to more sustainable, bio-based alternatives. In the quest for such materials, lignin has emerged as a promising candidate. Lignin is among the primary components of vascular plants and ranks second after polysaccharides in natural abundance.1 An amount of 98 wt.% of the worldwide extracted lignin is utilized as a renewable combustible. The remaining 2 wt.% are used in various applications, also in coatings.2 Lignin possesses numerous appealing industrial properties, including abundant natural availability, low-cost supply, and biodegradability. Its antiradical, UV absorption and antimicrobial characteristics, which primarily stem from the phenolic groups present in lignin, are particularly attractive for specialized applications.3 Compounds containing phenol groups have the ability to act as micro biocides that target cell membranes. These compounds can penetrate the cell wall and react with cellular proteins and protoplasm, leading to the inhibition of enzymes such as oxidoreductase and the general protein metabolism of the cells. Depending on the concentration of phenol, these compounds can either restrict the growth and multiplication of microbiota or destroy the cells. Increasing the hydrophilicity of phenol-based materials enhances their biocidal effect.4
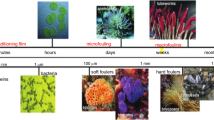
Persistent biofilm growth on surfaces like ship hulls presents significant industrial challenges.4 With maritime trade volumes reaching 11 billion tons in 2019,5 sustainable antifouling strategies are crucial to improve fuel efficiency, reduce maintenance costs, and minimize environmental harm.6 Lignin as a sustainable and abundant natural resource in combination with its antimicrobial properties could make it a promising candidate for use in antifouling coatings. However, little research has been conducted on the use of lignin as an antifouling agent in coatings. Furthermore, the integration of lignin into coatings poses a significant challenge due to its limited solubility. As an example, Dastpak et al.7 employed a 10 wt.% solution of lignin in organic solvents, but even with this concentration, it was necessary to centrifuge the mixture to eliminate insoluble residues. This substantial use of solvents undermines the pursuit of sustainability, making a product with potentially sustainable attributes paradoxically unsustainable. Therefore, the objective of this study was to utilize a commercially available kraft lignin powder and disclose a method to incorporate it into waterborne coatings, eliminating the need for organic solvents. Additionally, the potential application of these coatings as antifouling coatings was evaluated, leveraging the inherent antimicrobial properties of lignin. Considering that commonly used ablative or self-polishing binder technology is not available as waterborne yet, the coatings were formulated with a PVC above the CPVC to ensure that the phenolic groups of the lignin particles can directly interact with fouling organisms.
Material and methods
Raw materials
The raw materials that were used for the study are shown in Table 1. All raw materials were commercially available and were used without further modification.
Coating formulation
Slurries of lignin, Cu2O, and CaCO3 were produced by adding the respective dry powder under stirring (Dispermat™ LD50, Pendraulik Maschinen und Apparate GmbH, Springe, Germany) in a mixture of water, dispersing agent, and defoamer. The slurry formulations are shown in Table 2. Raw materials were introduced in the sequence shown, with continuous stirring throughout the process. After introducing the raw materials, glass beads (polished soda lime glass beads, 2.0–2.4 mm, Netzsch-Feinmahltechnik GmbH, Selb, Germany) were added to the slurries, corresponding to half of the original slurry weight. Every mixture was stirred at 4000 rpm for approximately 30 min to achieve a fineness of grain of < 20 μm, measured with a Grindometer (PG-021-050, 0–50 μm, Thierry Präzisionslackiertechnik GmbH, Stuttgart, Germany). The plastic dissolver plate used in the experiment had a diameter of 50 mm, which was approximately half the diameter of the container.
After preparation of the pigment slurries, two types of coatings were produced: a waterborne polyurethane (PU) coating as well as a waterborne epoxy (EP) coating. The formulations for the coatings are presented in Tables 3 and 4, respectively. The components were added in the sequence indicated, maintaining continuous stirring. However, for the lignin–epoxy coating, the curing agent was added before the slurry to avoid agglomerates. The total solid content of the coatings was adjusted individually to ensure a processable viscosity during production and application. Each sample was stirred at 3500 rpm for approximately 2 min and then physically defoamed using a Speed Mixer™ (DAC400 FVZ, Hauschild GmbH & Co. KG, Hamm, Germany) at 1000 rpm for 1 min.
Sample preparation
De-greased steel plates (200 mm \(\times\) 100 mm \(\times\) 1 mm) and acrylic plates (200 mm \(\times\) 100 mm \(\times\) 4 mm) were used as coating substrates. The antifouling samples were applied with a brush onto the acrylic panels, while the samples for mechanical testing were applied onto the steel plates using a 200-μm gap applicator frame (Ser. No. 45736, BYK-Gardner GmbH, Geretsried, Germany). The coatings were applied to match a dry film thickness of 70 µm. All coatings were allowed to cure for at least 1 week at ambient temperature. Application happened as soon as the curing agent had been added to the formulation.
Coating properties
Mechanical properties and appearance
The adhesion of the coatings was tested with a cross-cut tester (CC3000-1, 1-mm cutting distance, MTV Messtechnik oHG, Erftstadt, Germany) based on the standard DIN EN ISO 2409,8 including a tape tear-off. The ductility of the coatings was tested via Erichsen cupping (Erichsen Model 202, Erichsen GmbH & Co. KG, Hemer, Germany), based on the standard DIN EN ISO 20482.9 The gloss of the coated samples was tested with a gloss meter (micro-TRI-gloss μ, BYK Gardner GmbH, Geretsried, Germany) at the 60° angle, based on the standard DIN EN ISO 2813.10 The surface hardness of the coated samples was tested by pendulum damping (König Pendulum Hardness Tester, BYK-Gardner GmbH, Geretsried, Germany), based on the standard DIN EN ISO 1522.11
Rheology
Due to variations in solid content among the prepared coatings, they did not qualify to accurately test the effect of lignin on the rheology compared to the other pigments. As a result, exemplary mixtures of Bayhydrol® A 145 with a solid content of 45 wt.% were prepared similarly as described earlier. The formulations are shown in Table 5. No curing agent was added for the rheology measurements, and the rheology was measured approximately 24 h after the manufacturing with a rheometer (RT-20 Rotovisco, cone/plate measuring system C 35/1 Ti 97005, Thermo Fisher Scientific, Karlsruhe, Germany). The measurement was performed at 20 °C with a calibrated gap of 54 μm. The shear rate ramp testing procedure was divided into a first segment with increasing shear rate from 0 to 200 s−1 and a second segment with decreasing shear rate from 200 to 0 s−1. The duration of each segment was 120 s, the sampling interval was 1.2 s/pt.
Antifouling properties
Early fouling screening experiments
A fresh surface seawater sample of approximately 200 mL was collected at the coastline near Taarbæk, Denmark (55.795544° N, 12.590399° E) on July 1, 2021. After collection, solutions with a concentration of 0.01 vol.% of kraft lignin, Cu2O, and CaCO3 were created. Additionally, solutions of kraft lignin with concentrations of 0.01 vol.%, 0.05 vol.%, and 0.10 vol.% were prepared using another seawater sample from July 7, 2021. A volume of 2 mL of each mixture was plated with a disposable pipette on count agar Petri dishes (Servo plate®, Sabouraud-2 wt.% glucose-agar, containing 10-g/L peptone, 20-g/L d-glucose, 17-g/L agar, Servoprax GmbH, Wesel, Germany) in a threefold determination. Afterwards, the Petri dishes were placed in a laboratory fume hood at room temperature (20 °C ± 2 °C) under a plant growing lamp (W6000K, blue light LED, 450 nm, 1180 lm, 12-h interval, Tomshine Inc., Guangdong, P. R. China). The area of coverage of growth on the plates was observed over a period of 10 consecutive days.
Field immersion
The coatings were immersed at the CoaST Maritime Test Center (CMTC) in Hundested Harbor, Denmark, located in the Baltic Sea. Two replicates of each formulation were attached to aluminum frames and submerged to a depth of 50–100 cm for a duration of 6 weeks, from June 3, 2021, to July 15, 2021. The average water conditions during this period were as follows: a temperature of 19 ± 2 °C, a salinity of 1.6 ± 0.1%, and a pH value of 7.7 ± 0.2. The coatings were examined every week, and images were captured. Later, these images were analyzed to determine the percentage of coverage of slime, algae, and barnacles. The area of coverage was calculated by overlaying a grid comprising 100 rectangular, single spaces onto each photo and visually estimating the coverage percentage. The maximum possible percentage of observed fouling in this study was 300%, where each fouling organism covered 100% of the surface of the sample in stacked layers. The samples with CaCO3 acted as negative control, and the samples with Cu2O acted as positive control.
Results and discussion
Early fouling screening
The early fouling screening methodology was inspired from standard testing methods such as ISO 22196:200712 or JISZ 2801:2000.13 The new approach was chosen due to limitations inherent in laboratory-based screening methods, which often yield results differing significantly from field tests. Traditional laboratory-based screenings are typically conducted on a limited range of organisms, which stands in contrast with actual sea conditions where thousands of different species may be present.14 This discrepancy makes laboratory-based screenings less representative of the actual conditions encountered during field tests. To address this issue, actual seawater samples were employed in the new screening method. This strategy brings the testing environment closer to real-world conditions, offering a second key advantage: simplicity. However, this approach also presents certain drawbacks. By using real seawater, tests involve an undefined batch, containing an unpredictable amount and variety of organisms.
Figure 1 illustrates the progression of microbial coverage, providing insights into the antimicrobial properties of kraft lignin, Cu2O, and CaCO3. The ability to inhibit growth was analyzed in the laboratory by directly mixing the respective pigment with a seawater sample at a concentration of 0.01 vol.%. Cuprous oxide completely inhibited the growth of microorganisms while agar plates inoculated with the CaCO3 sample were entirely covered with microorganisms after 10 days at room temperature. The tested kraft lignin showed a slight inhibitory effect against microorganisms, slowing their progression in the first 3–4 days. Afterwards, the microbial coverage of the lignin sample increased parallel to the curve of the CaCO3 sample.
Figure 2 presents the impact of kraft lignin concentration on microbial proliferation. A distinct relationship is observed wherein an increase in lignin concentration enhances antimicrobial effectiveness, a finding consistent with other studies.15,16 However, a key point of consideration is that the results of the first study, displayed in Fig. 1, should not be directly compared to the results of the second study, presented in Fig. 2, due to the usage of different seawater samples in each case. This differentiation becomes evident upon examining the curves of the 0.01 vol.% kraft lignin sample from both investigations. Microbial growth is significantly faster during the second study. Though not standardized, the experiments still offer practical insights into the antibacterial potential of the materials under investigation within an experimental batch and potentially also between different seawater samples if an internal standard is used. This, however, must be examined in future—more extended—experiments.
Coating formulation
Based on early fouling screening experiments, a coating formulation was developed that leverages the antimicrobial activity of lignin. This formulation needed to exceed the critical pigment volume concentration (CPVC) to ensure the lignin is available in contact with the organisms and not covered by a binder matrix, which would render it ineffective. This anticipated mode of action is illustrated in Fig. 3. The lignin particles in the formulations with a PVC of 5% are completely wetted by the binder and thus shielded from any possible interaction with fouling organisms. On the contrary, the lignin particles in the coatings with a PVC above the CPVC are partly exposed, allowing for an interaction between fouling organisms and lignin.
Critical pigment volume concentrations (CPVC) have been calculated for all the pigments, and the results are shown in Table 6. The calculated CPVC values were approximately 60%, which was validated by near-zero gloss values (Fig. 5d). Higher PVC values for the lignin samples resulted in cracking as shown in Fig. 4. This means that a PVC of 60% was the maximum amount of lignin that could be incorporated into the coating, which corresponded to 22 wt.% of lignin in the liquid paint with a solid content of 35 wt.% for the epoxy coatings and 29 wt.% of lignin with a solid content of 45 wt.% for the polyurethane coatings. A number is significantly higher than in other studies as, Dastpak et al.7 employing only a 10 wt.% solution of lignin in organic solvents. In comparison, the reference coating formulations with Cu2O and CaCO3 had a pigment content of 55 wt.% and 45 wt.% in the liquid paint with a solid content of 68 wt.% and 57 wt.%, respectively. The noticeable difference in solid content between the lignin-based and reference coatings can be attributed to the higher oil adsorption of lignin, as detailed in Table 6. To ensure suitable handling properties and to avoid excessive viscosity, the water content had to be increased, aiding the decision against an ablative or self-polishing coating matrix, which are only available as solventborne and would have led to significantly higher VOC levels.
For the waterborne formulations, dispersing the lignin as a slurry without binder dispersion was crucial to achieving high shear rates and proper dispersion.17 The binder was subsequently added under less intense shear conditions for homogenization. The chosen waterborne polyurethane and epoxy binder systems are rather unconventional for antifouling applications.18 Furthermore, the UV stability of epoxy binder systems is rather poor, making them a poor choice for topcoat applications.19 Nevertheless, polyurethane and epoxy coatings are widely used in many industries, and information about the incorporation of lignin into these systems is needed. In addition, the binder matrix was less crucial for testing the antifouling performance of lignin above the critical pigment volume concentration (CPVC).
Coating properties
Effect on mechanical properties
Figure 5 displays the properties of the coatings in dry state. In terms of adhesion, coatings with a 5% PVC adhere well, while those at 60% showed weaker adhesion. This highlights the significant role of the binder ratio for proper adhesion in the dried state. Lignin samples showed less adhesion compared to Cu2O and CaCO3 references at 60% PVC due to its higher oil absorption. Surface hardness is evaluated using a pendulum technique, and results show that coatings with 60% PVC demonstrate greater hardness compared to those with 5% PVC. The lignin-containing samples exhibit the highest surface hardness, likely due to superior packing density. Erichsen cupping experiments indicate that coatings with 60% PVC crack at significantly lower penetration depths than those with 5% PVC. Coatings with high lignin levels display the least ductility, likely due to the steric separation of binder molecular chains by pigment particles, leading to reduced elasticity and brittle films. In summary, the primary drawbacks in the coating properties of lignin-based coatings result from its higher oil absorption and only become apparent at unusually high PVCs. Given that standard coating formulations typically use lower PVCs, ranging from 10 to 40%,19 it is reasonable to anticipate that lignin won't negatively influence the mechanical properties of coatings under these more common conditions. However, for the specialized application of antifouling coatings that are investigated in this study, maintaining these high PVC values would still be necessary to ensure that the pigments are exposed on the surface. Alternatively, employing soluble binder systems could be considered. In this case, lignin would be dispersed at below CPVC values, and the dissolution of the binder would enable particles to surface. Currently, though, water-based soluble binders do not exist, necessitating a return to a solventborne coating system.
Effect on rheological properties
The influence of kraft lignin, Cu2O, and CaCO3 on the rheological behavior of dispersions is investigated by incorporating them into exemplary binder mixtures. The applied shear rate ramp testing procedure provides insight into the viscosity of the mixtures at resting (0 s−1) and high shear stress situations (200 s−1). The viscosity curves presented in Fig. 6 demonstrate the pseudoplastic characteristics of the measured mixtures. Pseudoplasticity is distinguished from thixotropy by the lack of hysteresis between the sol and gel curves.17 Both rheological behaviors are defined by a relatively high resting viscosity that decreases with increasing shear rate.6 This is a property that is relevant for adequate storage stability of coatings in general.
The samples with a PVC of 5% demonstrated higher viscosity values than the 60% PVC samples, which is due to the experiment's design to maintain constant solid content. Samples with a higher PVC consist mainly of water and pigments, while those with a lower PVC balance the solid content with higher proportions of binder and hence are more viscous. The dispersion particles of the binder provide more resistance due to their molecular interactions and associations, resulting in a higher viscosity across the entire shear rate range tested.
At the same PVC, the lignin-containing mixtures generally exhibit higher viscosity than the compositions with other pigments. This might be attributed to the higher oil absorption number of lignin but also its chemical structure. The significant proportion of OH groups in lignin might enable the formation of a shear-dependent network via hydrogen bonding, resulting in the increased pseudoplastic behavior of the lignin-pigmented samples.
Antifouling performance during field immersion
The investigation of marine biofouling-prevention properties for the tested coatings took place over 6 weeks during the summer at the CoaST Maritime Test Center (CMTC). The progress of organism colonization on the polyurethane coatings is illustrated in Fig. 7 and is also representative of the epoxy-based coatings, given the lack of significant differences observed between the two coating types. The stacked bars showcase the fouling coverage of the panel with the greatest coverage among the two tested panels for each respective coating.
In the case of Cu2O coatings, only those with a PVC concentration of 60% demonstrated success in preventing macrofouling for a duration of up to 5 weeks; however, after the 6th week, growth of algae was observed. On the contrary, the Cu2O coatings with a PVC of 5 vol.% did not effectively prevent fouling. The reasons for this are twofold. Firstly, the release rate of the coating is proportional to the copper concentration—a lower concentration results in a diminished release rate.20 Secondly, the PVC level was considerably below the CPVC, which restricted water penetration, and in turn, limited the dissolution and subsequent sufficient release of Cu2O.
Coatings containing lignin did not demonstrate significant antifouling properties at either of the tested concentrations. The observed growth rates were similar to the CaCO3 coatings, which acted as negative control. It might be possible that the used lignin inhibited early slime formation as predicted by the screening experiment but remained undetected by the human evaluator due to the dark brown color of the coatings and the length of the weekly observation interval. Nevertheless, it can be concluded that inhibition did not happen at a level that would be relevant for any practical application. The exposed contact area of lignin particles might have been too small to show a significant effect against marine biofouling. On the contrary, during the laboratory experiments, the surface of lignin particles in contact with seawater and seawater organisms was significantly higher. Thus, future studies might want to focus on different release mechanisms for lignin, e.g., by using degraded lignin with lower molecular weight that allows for diffusion through the coating matrix. It might also be worthwhile to test ablative or self-polishing binder systems; however, it must be carefully evaluated if potentially increased VOC levels are worth the reduced release of toxic biocides, such as copper to the environment.
Conclusions and outlook
The current study evaluated the potential of unmodified kraft lignin as an ingredient for antifouling coatings. The agar spread plate method with seawater offers a practical and straightforward approach for early fouling screenings, but improvements in methodology and standardization are necessary to ensure consistent and reliable results. Future research could benefit from the use of known antifouling compounds, comparing their effectiveness to provide further authentication to the study and maybe find correlation to field experiments. If the suggested extended study is conducted, several improvements should be considered. One such improvement is to transfer the used grid evaluation from a visual to a microscopic method,21,22 which could enhance the identification of microbial colonies. Bacterial-binding dyes, such as epifluorescent21 or nucleic acid dye23 in combination with biosensors24 or fluorescent microscopy,21 along with surface plasmon resonance spectroscopy,25 are well-known strategies for enumerating microorganisms. Refining the results may involve different incubation temperatures,26 nutrient compositions,27 media types (e.g., seawater-agar28 or silica gel),29 depths of seawater sampling,29 and plating methods (e.g., pour plate instead of spread plate).26 Additionally, testing antimicrobial properties under laboratory conditions using different strategies, such as flow cytometry23 or turbidity measurements30 instead of agar plating, can reduce the necessary testing time.
The anticipated mode of action for lignin in antifouling coatings needs to be reconsidered. While it was possible to formulate coatings that contained lignin with a concentration above the CPVC, exposing free lignin particles on the surface of the coating, the efficacy was too low to show any effect against biofouling. Future studies might focus on different release mechanisms for lignin. This might be easier to achieve in solventborne coating systems than in waterborne coating systems. If lignin is used in solventborne coating systems, it should be carefully considered if the potentially higher VOC levels compared to conventional biocide release coatings can be accepted.
Despite the lack of sufficient antifouling activity, the performed experiments indicated some promising alternative applications for lignin, such as a rheology additive, bio-based filler, or in-can preservative for waterborne coatings. Concluding this study, the presented waterborne coating formulation offers a stepping stone for future researchers, enabling further exploration of lignin's potential within the suggested, but also additional application domains.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Lebo, SE, Gargulak, JD, McNally, TJ, “Lignin.” Encycl. Polym. Sci. Technol., https://doi.org/10.1002/0471440264.PST179 (2002)
Behr, A, Seidensticker, T, “Einführung in die Chemie nachwachsender Rohstoffe: Vorkommen, Konversion, Verwendung.” Springer (2017).
Alzagameem, A, et al. “Antimicrobial Activity of Lignin and Lignin-Derived Cellulose and Chitosan Composites Against Selected Pathogenic and Spoilage Microorganisms.” Polymers, 11 670 (2019)
Bruns, R, Kaulen, J, Kretschik, M, Kugler, M, “R&D in Material Protection: New Biocides.” In: Paulus, W (ed.) Directory of Microbicides for the Protection of Materials. Preprint (2005)
Sirimanne, SN, Review of Maritime Transport 2019. (2019)
Streitberger, H-J, Goldschmidt, A, BASF Handbook Basics of Coating Technology. Vincentz Network, (2018)
Dastpak, A, et al., “Solubility Study of Lignin in Industrial Organic Solvents and Investigation of Electrochemical Properties of Spray-Coated Solutions.” Ind. Crops. Prod. 148 (2020)
Paints and Varnishes – Cross-Cut Test DS/EN ISO 2409:2020. https://findit.dtu.dk/en/catalog/5f731ae9d9001d00df4b7aa7
Metallic Materials – Sheet and Strip – Erichsen Cupping Test: DS/EN ISO 20482:2013. https://findit.dtu.dk/en/catalog/5bf5530dd9001d0a0b5a94a3
Paints and Varnishes – Determination of Gloss Value at 20 Degrees, 60 Degrees and 85 Degrees: DS/EN ISO 2813:2014. https://findit.dtu.dk/en/catalog/5bf55274d9001d0a0b59fc9a
Paints and Varnishes – Pendulum Damping Test: DS/EN ISO 1522:2007. https://findit.dtu.dk/en/catalog/5bf5525bd9001d0a0b59e7b5
Measurement of Antibacterial Activity on Plastics Surfaces: ISO 22196:2007
Antimicrobial Products - Test for Antimicrobial Activity: JISZ 2801:2000
Satheesh, S, Ba-Akdah, MA, Al-Sofyani, AA, “Natural Antifouling Compound Production by Microbes Associated with Marine Macroorganisms—A Review.” Electron. J. Biotechnol., 21 26–35. https://doi.org/10.1016/j.ejbt.2016.02.002 (2016)
Sláviková, E, Košíková, B, “Inhibitory Effect of Lignin by-Products of Pulping on Yeast Growth.” Folia Microbiologica, 39 241–243 (1994)
Gabov, K, Oja, T, Deguchi, T, Fallarero, A, Fardim, P, “Preparation, Characterization and Antimicrobial Application of Hybrid Cellulose-Lignin Beads.” Cellulose, 24 641–658 (2017)
Meichsner, G, Mezger, T, Schröder, J, Lackeigenschaften messen und steuern Rheologie - Grenzflächen - Kolloide. Vincentz (2003)
Yebra, DM, Kiil, S, Dam-Johansen, K, “Antifouling Technology - Past, Present and Future Steps Towards Efficient and Environmentally Friendly Antifouling Coatings.” Prog. Org. Coat., 50 75–104 (2004)
Müller, B, Poth, U, Coatings Formulation: An International Textbook. Vincentz Network, (2017). https://doi.org/10.1515/9783748600268.
Kiil, S, Dam-Johansen, K, Weinell, CE, Pedersen, MS, Codolar, SA, “Mathematical Modelling of a Self-polishing Antifouling Paint Exposed to Seawater: A Parameter Study.” Inst. Chem. Eng., 80 45–52 (2002)
Kepner, RL, Pratt, JR, “Use of Fluorochromes for Direct Enumeration of Total Bacteria in Environmental Samples: Past and Present.” Microbiol. Rev., 58 603–615 (1994)
Hanks, JH, James, DF, “The Enumeration of Bacteria by the Microscopic Method.” J. Bacteriol., 39 297–305 (1940)
Lebaron, P, Parthuisot, N, Catala, P, “Comparison of Blue Nuclei Acid Dyes for Flow Cytometric Enumeration of Bacteria in Aquatic Systems.” Appl. Environ. Microbiol., 64 1725–1730 (1998)
Sapsford, KE, Shriver-Lake, LC, “Bacterial Detection Using Evanescent Wave-Based Fluorescent Biosensors.” Principles of Bacterial Detection: Biosensors, Recognition Receptors and Microsystems, 109–123 (2008). https://doi.org/10.1007/978-0-387-75113-9_6
Torun, Ö, HakkiBoyaci, I, Temür, E, Tamer, U, “Comparison of Sensing Strategies in SPR Biosensor for Rapid and Sensitive Enumeration of Bacteria.” Biosens. Bioelectron., 37 53–60 (2012)
Buck, J, Cleverdon, RC, “The Spread Plate as a Method for the Enumeration of Marine Bacteria 1, 2.” Limnol. Oceanogr., 5 78–80 (1960)
Lewin, RA, “Enumeration of Bacteria in Sea Water.” Internationale Revue der gesamten Hydrobiologie und Hydrographie, 59 (5) 611–619 (1974)
Zobell, CE, Allen, EC, “The Significance of Marine Bacteria in the Fouling of Submerged Surfaces.” J. Bacteriol., 29 239–251 (1935)
Jannasch, HW, Jones, GE, “Bacterial Populations in Sea Water as Determined by Different Methods of Enumeration 1.” Limnol. Oceanogr., 4 (2) 128–139 (1959)
Mattila, T, “Automated Turbidometry—A Method for Enumeration of Bacteria in Food Samples.” J. Food Prot., 50 640–642 (1987)
Acknowledgments
Financial support from the Hempel Foundation to CoaST (The Hempel Foundation Coatings Science and Technology Centre).
Funding
Open access funding provided by Technical University of Denmark.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Butschle, M., Schlautek, R., Kunschert, L. et al. Integration of unmodified kraft lignin powder in waterborne coatings and investigation of antifouling properties. J Coat Technol Res (2024). https://doi.org/10.1007/s11998-023-00867-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11998-023-00867-3