Abstract
Mainly because of the high sensitivity of the isocyanate group to atmospheric moisture, it is blocked with a blocking agent for many applications and thus kept as inert as possible at room temperature (Zhang et al. in Langmuir ACS J Surf Colloids 37:12705, 2021, Meier-Westhues in Polyurethane: Lacke, Kleb-und Dichtstoffe, Vincentz Network, Hannover, p 36, 2007). When exposed to temperature, the protective group unblocks, and the isocyanate group reacts with the reactant that was previously present together with the blocked isocyanate (Meier-Westhues in Polyurethane: Lacke, Kleb- und Dichtstoffe, Vincentz Network, Hannover, p 36, 2007, Goldschmidt and Streitberger in BASF handbook on basics of coating technology, Vincentz Network, Hannover, pp 96–99, 2007). Many of the substances which are used today as blocking agents have toxicological concerns or unblock at high temperatures, which limits their application areas (Guillem Parra et al. in Blocked isocyanate polyurethane compositions using a new blocking agent, method of manufacture and uses thereof: European patent application, 2019, https://data.epo.org/publication-server/document?iDocId=6506253&iFormat=0). In this work, vanillin is presented as an effective deblocking agent at low temperatures and is compared with similar structures. The process of deblocking from an hexamethylene diisocyanate trimer (HDI-trimer) is followed by means of IR-spectroscopic measurements at different temperatures and thermogravimetric analysis. Temperature-dependent oscillation measurements using a rheometer are suitable for measuring onset temperatures and for qualitatively tracking the unblocking process. The combination of the results is used to draw conclusions about the existing deblocking mechanism. The comparatively low deblocking temperature of vanillin enables the formulation of an HDI-trimer modified with sulfonate groups and blocked with vanillin, which is dispersed in the aqueous phase and then reacted with OH-functional binders. Deblocking and subsequent reaction with the binder are followed by means of IR spectroscopy, and the mechanical properties of the coating films are examined. Vanillin is therefore suitable as a toxicologically harmless blocking agent for isocyanates and enables the production of crosslinkers for one-component water-based coatings (Arya et al. Adv Tradit Med (ADTM) 21:1, 2021).
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Isocyanates are highly reactive compounds that primarily undergo addition reactions.6,7 Of particular industrial interest is the reaction with OH-functional compounds to form urethanes and the reaction with amines to form urea derivates.3,8,9 However, isocyanates also react with water at room temperature via the unstable intermediate carbamic acid with CO2 splitting to form primary amines, which in turn can react with other isocyanate groups.10,11 This reaction is utilized in the production of polyurethane foams.6,9 For many applications, the reaction of isocyanates with water, especially in the form of atmospheric moisture, is a hindrance and has a detrimental effect on storage stability. Mainly for this reason, isocyanates are provided with a blocking agent, which prevents undesired side reactions of the isocyanate group and deblocks when exposed to temperature.2 The blocking agent is removed with the oven air and releases the highly reactive functional group to subsequently react with the reaction partner, for example an OH-functional polyester, to form a urethane. In this way, it is possible to formulate single-component systems that are almost inert at room temperature and react with each other only when exposed to higher temperatures.3,12 In addition to these advantages, the majority of isocyanates are toxicologically less hazardous after blocking them.4,13
However, the use of water as a solvent matrix is usually not possible for one of the following reasons: Most blocking agents only deblock quantitatively at temperatures above 140 °C, so that when water is used as a solvent, it has already completely evaporated, and thus, on the one hand, the evaporation of the blocking agent is greatly impeded, and, on the other hand, the flow of the coating can no longer be guaranteed and an unacceptable surface structure is formed. In the case of quantitative deblocking at lower temperatures, released isocyanate reacts with water instead of the OH-containing binder. In this case, too, durable polymer networks cannot be formed.
Vanillin, which is mainly investigated in this work as a blocking agent, is toxicologically completely harmless, available in large quantities and has such a deblocking temperature profile that, in combination with OH-containing dispersions, aqueous one-component systems can be formulated. During baking, the vanillin deblocks shortly after evaporation of the water and makes the urethane-forming isocyanate group available for only reacting with the OH-functional binder. Due to the temperature profile of the vanillin deblocking, the self-crosslinking aqueous isocyanate systems opens a new field of application for the coatings industry.
The choice of blocking agent depends primarily on the deblocking temperature, but also other factors such as compatibility in the system or the toxicological properties of the blocking agent itself, since it is present in the oven-air alongside solvents and must be removed, have also be taken into account when selecting the blocking agent.14 The deblocking temperatures given in the literature are not to be understood as absolute values, since they vary depending on the measurement method.15 Frequently used methods for determining the deblocking temperature are IR-spectroscopy, DSC- or TGA-measurements, and NMR-spectroscopy.12,16 In addition to the influence of the measurement method on the determined deblocking temperature, there are other important influencing factors: For example, lower deblocking temperatures are measured if the deblocking reaction is carried out in a solvent matrix in which the blocking agent is highly soluble. Furthermore, hydrogen bond-accepting solvents lower the deblocking temperature because recombination of the isocyanate with the blocking agent is impeded during deblocking process.17,18,19,20 For these reasons, deblocking temperatures given in the literature should only be regarded as guide values. Nevertheless, to get an overview of common blocking agents and their deblocking temperature range, a short selection is shown in Table 1.
It is often not clear from the temperatures given whether an initial deblocking temperature is meant or according to which criteria the deblocking temperature is determined.15,25 For industrial use, it is usually not the initial deblocking temperature that is decisive, but how much blocking agent remains in the binder–solvent matrix after a certain baking time at a defined temperature. For this reason, the deblocking of three different blocking agents is investigated in this work after 30 min of baking time at different temperatures gravimetrically and by means of IR spectroscopy in the presence of solvents and compared with a TGA measurement with a small sample quantity and no solvent. For this purpose, vanillin, 4-hydroxybenzaldehyde (HBA), and 4-(hydroxymethyl)benzaldehyde (HMBA) are each reacted with Desmodur® ultra N 3600, a hexamethylene diisocyanate trimer (HDI-trimer), and the deblocking is investigated by different methods. Figure 1 exemplifies the reaction of the HDI-trimer with vanillin.
In addition to classical methods such as IR spectroscopy and thermogravimetric analysis (TGA), temperature-dependent oscillation measurements are taken by using a rheometer to monitor deblocking processes.
Urethane cleavage of the 4-(hydroxymethyl)benzaldehyde-isocyanate adduct is expected to occur at significantly higher temperatures compared to the other aldehydes used, because the urethane bond is not present directly on the aromatic but on a methyl group. The methyl group, which acts as an electron donor, strengthens the urethane bond between the isocyanate and the blocking agent, thus increasing the deblocking temperature. If the urethane bond is linked to aromatic structures, these act as electron-withdrawing agents and thus weaken the urethane bond, so that the deblocking temperatures to be expected for 4-hydroxybenzaldehyde and vanillin are estimated to be significant lower.26,27,28
The exchange of substituents on the aromatic ring can also shift the deblocking temperature in both directions: The decisive factor is the electronic effect on the urethane bond.25,27 In this work, the focus is on vanillin as a blocking agent, since it has a good property profile in terms of deblocking, is toxicologically harmless, and is commercially available in large quantities at low prices.5
Two mechanisms for deblocking or in case of using an OH-functional nucleophilic component transurethanization are found in the literature, both are recognized.16,29 In the first mechanism, elimination of the urethane bond takes place first, in which the blocking agent is split off and evaporates and then free isocyanate is formed. This reacts with the nucleophilic second component by means of an addition reaction to form the final product.30 This mechanism is described as the "elimination–addition–mechanism" and is cited, for example, by Wicks et al.16 as they were able to detect free isocyanate by IR spectroscopy after deblocking. The second mechanism is SN2-analogous, such that the nucleophilic component attacks the carbon atom of the urethane, and the blocking agent is cleaved almost simultaneously, without free isocyanate being present during the process. This mechanism is called the “addition–elimination–mechanism” and was observed, for example, by Mühlebach22 in phenolic blocked isocyanates. Using vanillin as an example, both possible mechanisms are shown schematically in Fig. 2.
IR and gravimetric measurements under real baking conditions for coatings show that temperatures below 140 °C for 30 min are necessary to remove more than 80% of the vanillin from the system and the initial deblocking temperature is around 100 °C.
In this context, it is even more interesting to disperse a vanillin-blocked HDI-trimer into the aqueous phase to act as a crosslinker in a one-component system with an OH-functional binder under the effect of temperature with vanillin deblocking. Thus, the deblocking reaction of the vanillin starts shortly after the water has evaporated from the binder. Small amounts of a co-solvent remaining in the matrix then allow the reaction of the deblocked isocyanate with the OH-functional binder to form a crosslinked coating film. Since the polarity differences between the nonpolar vanillin-HDI-trimer and water are too large, a partial modification with different aromatic sulfonates was carried out to increase the compatibility. A 30% modification with 4-hydroxybenzene sulfonate in combination with the use of a surfactant (sodium hexadecyl sulfate) and co-solvent succeeds in the preparation of a novel isocyanate crosslinker dispersible in water. The synthesis of sulfonate-modified vanillin-HDI-trimer, including molar reaction ratios, is shown in Fig. 3.
This product subsequently acts as a crosslinker with an average functionality of F = 2.33 when reacted with commercially available water-based OH-functional binders. The resulting coating films exhibit excellent mechanical properties as well as good optical properties and chemical resistances.
Thus, the vanillin-blocked isocyanate described in this work combines several advantages: The blocking agent is toxicologically harmless and commercially available in large quantities, the deblocking temperature is comparatively low, and it can be used almost VOC-free in aqueous systems without having to sacrifice the advantages of polyurethanes in the coating film.
Raw materials
HDI-trimer Desmodur® ultra N 3600 and water-based binders Bayhydrol® A 2470, Bayhydrol® A 2227/1 and Bayhydrol® U 2757 were kindly provided by Covestro Deutschland AG. Vanillin (≥ 99.0%), dimethyl sulfoxide (≥ 99.5%), molecular sieves (4Å), ethyl acetate (≥ 99.5%), and 1-methoxy-2-propyl acetate (≥ 99.0%) were purchased from Carl Roth. 4-(Hydroxymethyl)benzaldehyde (≥ 98.0%) has been bought from BLDpharm as a reference standard; for blocking agent experiments a self-synthesized product has been used. 4-Hydroxybenzaldehyde (≥ 99.0%), terephthalaldehyde (≥ 99.0%), and ethanol (absolute, per analysis) were purchased from Merck. Disodium 4,5-dihydroxybenzene-1,3-disulfonate monohydrate (Tiron) (> 98.0%), disodium 3-hydroxy-2,7-naphthalenedisulfonate (> 85.0%), sodium 4-hydroxybenzenesulfonate (> 98.0%), and sodium hexadecyl sulfate (> 98.0%) were purchased from Tokyo Chemical Industry (TCI). Deuterated solvents for NMR spectroscopy were purchased from Armar (Europa). All solvents were dried by using 4Å molecular sieves; Tiron was dried by warming in the oven. Not explicitly mentioned other educts have been bought from Aldrich Chemicals and were used without further purification.
All coatings have been applied by a squeegee on steel panels (DX51D + z), aluminum samples (type Al99,5), or glass plates.
Analytical methods
1H-NMR spectra were measured on a Bruker AVANCE NEO 400 spectrometer at 400 MHz. Deuterated dimethyl sulfoxide or acetone has been used as solvents and internal standards.
IR spectra of liquid samples were measured with the Bruker Vertex 70 FTIR-spectrometer (4000–375 cm−1) with a resolution of 4 cm−1; solid samples and coatings are measured on a Bruker Lumos FTIR-Microscope. All spectra are baseline-corrected and normalized on the C–N-stretch signal of the isocyanurate at 765 cm−1 as this signal does not change during deblocking or crosslinking reaction. If spectra are normalized on other peaks, this is indicated in results and discussion part. To assess deblocking, 50% dimethyl sulfoxide containing samples of aldehyde-isocyanate trimers were applied on aluminum panels with 120-µm wet film thickness and are measured by ATR before and immediately after the 30-min baking process at different temperatures.
Thermogravimetric analysis was performed by using Netzsch TG 209 F1 Libra®. The sample weights of dried aldehyde-isocyanate-trimers are between 5.0–5.7 mg, and Tzero aluminum pans without lids have been used. Heating rates were carried out with a value of ten degrees per minute.
For the thermogravimetric tests under real conditions, the blocked HDI-trimer samples, which are 50% in dimethyl sulfoxide, were applied to aluminum sheets by squeegee with 120-µm wet film thickness and baked for 30 min at different temperatures. Since the solvent content and chemical composition are known, the percentage mass loss of the blocking agent can be calculated. In the gravimetric determination of one gram solvent-containing vanillin-trimer sample under real baking conditions, only 0.178 g of vanillin can be detected in the case of completely deblocking vanillin, provided that the solid content has been set exactly. Depending on the synthesis, it could be shown that the solid content varies by 3%. However, with incomplete, or in the case of the HMBA-trimer, very low mass loss during baking, the method, which is very interesting for practical applications, exhibits higher measurement inaccuracies due to the synthesis-related measuring uncertainty of the solid content and weighing accuracy.
The rheological experiments to monitor deblocking-reaction processes and to calculate values for storage and loss modulus and to measure glass transition temperatures have been performed on a Modular Compact Rheometer MCR 102 from Anton Paar using plate–plate single-use systems with a diameter of 25 mm and a measuring gap of 0.8–1 mm. Solvents of aldehyde-blocked HDI-trimers were gently distilled off in vacuum atmosphere before oscillation measurements. These samples still may contain up to fifteen percent solvent residues, which can affect the measuring data during deblocking process. Water-based dispersions of sulfonate-modified vanillin–HDI-trimer and Bayhydrol binders have been measured including approximately 50% water as a solvent. The most accurate measurement data are achieved for samples without solvents. On the other hand, it is more difficult to evaporate the blocking agent without a solvent. The measurement setup, in which the material is between two plates, also restricts the evaporation of the blocking agent to the edge area. This means it is important to note that measured deblocking temperature of samples without solvents is significantly higher. Furthermore, values for G′ and G″ are always lower in comparison with DMA measurements of a free film because the plate–plate single-use system influences measuring data by the pliability of the lower aluminum plate dependent on the diameter of the upper plate. The diameter of 25 mm is a compromise to gain accurate data in all steps during the deblocking mechanism. Parameters for all measurements were: γ = 0.05% and ω = 10 rad/s, heating rate = 1 °C/min. Glass transition temperatures are determined in the second heating run and are read at the maximum of loss modulus G’’.
The pendulum damping values have been obtained according to DIN EN ISO 1522 using a Königspendel from Erichsen. Three measurements for each panel were taken four hours after baking. Average values are given.
The adhesion of the coating films (film thickness 40–50 µm) was determined by performing the crosscut test according to DIN EN ISO 2409 after application on steel panels. Furthermore, the resistance of the samples in the event of sudden deformation based on DIN EN ISO 6272-1:2011 has been determined. For the evaluation, a drop height of one meter was chosen and it was judged if the film is cracked and if it still adheres to the steel surface after the indirect impact of the one-kilogram weight.
Synthesis of the compounds
4-(Hydroxymethyl)benzaldehyde
The synthesis was carried out according to the method described by Loim and Kelbyscheva;31 the purification of the product was partially changed.
For synthesis, a three-necked 1000-mL flask with KPG stirrer is set under nitrogen atmosphere. Then, 250 mL ethanol, 350 mL tetrahydrofuran, and 149 mmol terephthalaldehyde are added, and the mixture is cooled down to − 5 °C using a sodium chloride–ice–mixture. Next, 37.3 mmol sodium borohydride is added in 6 portions within 30 min while strong stirring. It is ensured that the apparatus is continuously flushed with nitrogen gas to drive out nascent hydrogen gas. After 30 min, the temperature is increased to 0–2 °C and is kept for 6 h.
The mixture is neutralized to pH = 5 while stirring by using 50 mL 2 molar hydrochloric acid. Ethanol and tetrahydrofuran are removed via capillary distillation under vacuum (110–40 mbar) at room temperature. To cool the distillation bridge, ice water is used. Organic residues are washed with 200 mL deionized water twice and extracted by using 200 mL ethyl acetate. The mixture stirred for one night with 2.0 g magnesium sulfate to remove water residues before magnesium sulfate is filtered off. After that the HMBA-mixture 1 is crystallized in warm (35 °C) petroleum ether, washed twice, and crystallized after decanting petroleum ether. The yield is 99 mmol from theoretical 149 mmol (66.4%).
The product is characterized by melting point (Tmelt. = 42.5 °C), (Tmelt. BLDpharm-reference = 42.0 °C), 1H-NMR, and IR spectroscopy. Composition of the purified sample 1 estimated by 1H-NMR-measurement (1H-NMR spectra in supplementary information, Fig. S1) 91.1% 4-(Hydroxymethyl)benzaldehyde, 5.76% benzene-1,4-dicarboxycarbaldehyde, and 3.11% 1,4-benzenedimethanol. 4-(Hydroxymethyl)benzaldehyde 1H-NMR (400 MHz, DMSO, δ in ppm): 4.60 (2H, d, J = 5.7 Hz C–CH2–OH), 5.42 (1H, t, J = 5.7 Hz CH2–OH), 7.54 (2H, d, J = 8.0 Hz C=CH–CH), 7.88 (2H, d, J = 8.0 Hz C=CH–CH), 9.98 (1H, s, C–CHO). Terephthalaldehyde (benzene-1,4-dicarboxycarbaldehyde) 1H-NMR (400 MHz, DMSO, ppm, δ): 8.12 (2H, d, C=CH-CH), 10.13 (1H, s, C-CHO). 4-Benzenedimethanol 1H-NMR (400 MHz, DMSO, δ in ppm): 4.47 (2H, d, J = 5.7 Hz C–CH2–OH), 5.12 (1H, t, J = 5.7 Hz C–CH2–OH), 7.28 (2H, d, C=CH–CH).
IR (resonance in [cm−1] and type): 3350 (OH valence), 2830, 2740 (CH stretch aldehyde), 1685 (C=O aldehyde), 1608, 1577 (C=C conj. aromat.), 1430 (CH deformation aldehyde), 1299 (C–O stretch), 1010 (CH/CO wagging), 809 (CH bending).32
Desmodur ® ultra N 3600 and vanillin (vanillin-trimer)
First, 29.84 mmol vanillin and 10.00 g dried dimethyl sulfoxide, acetone, or ethyl acetate are added under nitrogen atmosphere in a 50-mL two-necked flask with a magnetic stirrer. Then, 9.97 mmol Desmodur® ultra N 3600 is added while stirring. Before adding 1000 ppm dibutyltin dilaurate (DBTDL) for catalysis, IR of the reaction mixture is measured. After that the temperature is increased to 50 °C. The reaction course is controlled using IR spectroscopy. Within 4–6 h, the reaction is completed.
IR (resonance in [cm−1] and type): 3240 (NH–COO and NH stretch), 2997 (CH3 stretch), 2920 (CH2 stretch), 2260 (N=C=O valence), 1740 amide I (C=O, urethane), 1677 amide II (C=O, urethane, isocyanurate), 1587 (C=C conj. aromat.), 1540 (NH–COO and NH bending), 1465 (CH2 deformation), 1200 (=C–O–C, urethane), 1150, 1115 (C–O–C valence), 765 (C–N stretch, isocyanurate).32,33,34
Desmodur ® ultra N 3600 and 4-hydroxybenzaldehyde (HBA-trimer)
For synthesizing HBA-blocked hexamethylene diisocyanate trimer, 32.75 mmol 4-hydroxybenzaldehyde are added with 10.00 g dried dimethyl sulfoxide or 1-methoxy-2-propyl acetate in a 50-mL two-necked flask with magnetic stirrer. Then, 10.95 mmol Desmodur® ultra N 3600 is added while stirring. At last, 1000 ppm dibutyltin dilaurate (DBTDL) is added for catalysis. After that the temperature is increased to 55 °C. The reaction course is controlled using IR spectroscopy. Within 4–6 h, the reaction is completed.
IR (resonance in [cm−1] and type): 3300 (NH–COO and NH stretch), 2937 (CH2 stretch), 2860 (CHx stretch), 2260 (N=C=O valence), 1740 amide I (C=O, urethane), 1680 amide II (C=O, urethane, isocyanurate), 1599, 1581 (C=C conj. aromat.), 1535 (NH–COO and NH bending), 1462 (CH2 deformation), 1285 (OH deformation), 1205 amide V (=C–O–C, urethane), 1155, 1100 (C–O–C valence), 759 (C–N stretch, isocyanurate).32,33,34
Desmodur ® ultra N 3600 and 4-(hydroxymethyl)benzaldehyde (HMBA-trimer)
HMBA-mixture 1 is used for synthesizing HMBA-trimer. For the mixture consisting of 91.1% 4-(hydroxymethyl)benzaldehyde (HMBA), 5.76% terephthalaldehyde, and 3.11% 1,4-phenylenedimethanol, an OH-equivalent mass of 148.43 g/mol OH is calculated from NMR data. Isocyanate equivalent weight of HDI-trimer Desmodur® ultra N 3600 is 182.6 g/mol NCO, so that reaction ratio is 1.23 g Desmodur® ultra N 3600 and 1.00 g HMBA- mixture. Next, 10.00 g ethyl acetate or dried dimethyl sulfoxide is added in a 50-mL two-necked flask with magnetic stirrer. Then, the apparatus is set under nitrogen atmosphere and 4.48 g of the HMBA-mixture is added while stirring. After the HMBA mixture is completely dissolved, 5.52 g of Desmodur® ultra N 3600 is added while stirring and IR is measured. At last, 1000 ppm of dibutyltin dilaurate (DBTDL) is added for catalysis. The clear solution is heated of to 45 °C, the reaction time is between 6–10 h, and the yield is controlled by IR spectroscopy.
IR (resonance in [cm−1] and type): 3325 (OH valence), 3260 (NH–COO and NH stretch), 2940 (CH2 stretch), 2905 (CHx stretch), 2260 (N=C=O valence), 1725 amide I (C=O, urethane), 1680 amide II (C=O, urethane, isocyanurate), 1608 (C=C conj. aromat.), 1540 (NH–COO and NH bending), 1460 (CH2 deformation), 1245 (=C–O–C, urethane), 1162, 1132 (C–O–C valence), 765 (C–N stretch, isocyanurate).32,33,34
Vanillin-Na-4-HBS-trimer
Synthesizing vanillin–sulfonate-trimer is a three-step procedure. At first, 70% of isocyanate groups of HDI-trimer Desmodur® ultra N 3600 are attached to vanillin. In a three-necked flask with KPG stirrer, 134.1 mmol vanillin is dissolved in 418 mmol dried dimethyl sulfoxide. Then, 63.7 mmol Desmodur® ultra N 3600 are added. Nitrogen gas is introduced into the sample while stirring by using a needle. The sample is heated up to 60 °C and 800 ppm dibutyltin dilaurate (DBTDL) is added for catalysis. Yield is controlled by IR spectroscopy. After a reaction time of 2–3 h, vanillin is attached to HDI-trimer and remaining isocyanate groups (30%) are linked to sodium 4-hydroxybenzenesulfonate. For the second reaction step, temperature is decreased to 40 °C and 57.4 mmol sodium 4-hydroxybenzenesulfonate is added in portions under nitrogen atmosphere while strong stirring. The second reaction step is completed after 5–7 h, which is confirmed by IR spectroscopy. The sample is highly viscous and slightly yellowish. Sulfonate groups are neutralized while strong stirring with 3.4 mmol triethylamine to pH = 7.2. Before dispersing the vanillin-Na-4-HBS-trimer in watery phase, 2.8 mmol of the surfactant sodium hexadecyl sulfate is dispersed in the sample. Furthermore, a solution of an alkylol ammonium of a polyfunctional polymer (Disperbyk-181) is added by a dosage of 0.2% on solid content to act as a wetting agent. Finally, 362 mmol deionized water is dispersed within an hour using a dissolver at 5000 rpm to set the solid content of the dispersion at 40%. Until usage the sample is stored under nitrogen atmosphere.
IR (resonance in [cm−1] and type): 3250 (NH–COO and NH stretch), 2937 (CH2 stretch), 2860 (CH3 stretch), 2260 (N=C=O valence), 1733 amide I (C=O, urethane), 1680 amide II (C=O, urethane, isocyanurate), 1577 (C=C conj. aromat.), 1535 (NH–COO and NH bending), 1436 (CH3 stretch), 1285 (OH deformation), 1198 amide V (=C–O–C, urethane), 1155, 1118 (C–O–C valence), 762 (C–N stretch, isocyanurate), 729 (CH2 rocking), 696 (NH deformation).32,33,34
Application testing
For application testing, a formulation consisting of vanillin-Na-4-HBS-trimer and different water-based binders Bayhydrol® A 2470, Bayhydrol® A 2227/1 and Bayhydrol® U 2757 is prepared. Reaction ratios are calculated based on isocyanate- and hydroxy-equivalent weights of deblocked vanillin-Na-4-HBS-trimer and solvent-free hydroxy functional dispersions.
The formulations are applied by drawing down a bar on aluminum sheets and are cured at 140 °C for 30 min to achieve a dry film thickness of 40–50 µm.
Results and discussion
In this work, vanillin is presented as a blocking agent for isocyanates, and properties such as deblocking temperature and mechanism are investigated using various analytical methods. Polyisocyanate adducts with 4-hydroxybenzaldehyde and 4-(hydroxymethyl)benzaldehyde represent two further OH-functional aldehydes whose deblocking is investigated.
It can be shown that vanillin is an excellent and toxicologically safe deblocking agent, which separates from the urethane at comparatively low temperatures. Similar properties are exhibited for 4-hydroxybenzaldehyde. The isocyanate-aldehyde adduct with 4-(hydroxymethyl)benzaldehyde does not deblock at all or only to a very small extent in the temperature range investigated.
In the second part of the work, based on the property profile of vanillin as a blocking agent, a water-dispersible blocking agent with vanillin is successfully synthesized by partial modification of the HDI-trimer with a sulfonate and used for crosslinking OH-containing water-based dispersions.
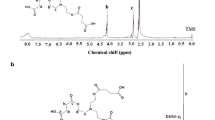
Before investigating deblocking process for aldehyde–isocyanate trimers, all synthesized products have been characterized by 1H-NMR and IR spectroscopy. 1H-NMR spectroscopy is performed to demonstrate that the aldehyde group does not react while urethane building reaction. IR spectroscopy allows to confirm urethane formation by reaction of hydroxy-aldehydes with hexamethylene diisocyanate trimer.
All 1H-NMR and IR spectra of 4-(hydroxymethyl)benzaldehyde and isocyanate-aldehyde-trimers are in supplementary material, Figs. S3–S9.
At first, the deblocking of aldehyde-HDI-trimers, containing dimethyl sulfoxide as a solvent, is investigated by ATR after 30-min baking at different temperatures. Figure 4 illustrates changes in IR spectra after 30-min baking of vanillin-trimer at different temperatures:
In the spectra, the urethane/isocyanurate group can be detected at 1740 cm−1 and 1677 cm−1 with the corresponding amide II or N–H signal at 1535 cm−1 caused by N–H-bending vibration of the urethane groups. An amide II signal is visible, if at least one proton is bound to the nitrogen of the amide.34
The presence of vanillin in the sample can be detected by the signal at 1608 cm−1, caused by the conjugated aromatic C=C-vibration and by C–O–C-valence peaks at 1150 cm−1 and 1115 cm−1.32,35
The sample, which is cured at 100 °C, shows significant urethane peaks at 1740 cm−1, 1535 cm−1 (N–H-bending) and 1200 cm−1 (=C–O–C, urethane). Furthermore, C–O–C-valence peaks (1150 cm−1 and 1115 cm−1) and conjugated C=C-vibration signal (1608 cm−1) of vanillin appear, so that it can be assumed that, if at all, only small amounts of vanillin deblock at a temperature of 100 °C.
The intensities of the urethane-specific bands decrease slightly at the 120 °C sample, so that it can be assumed that parts of the vanillin already deblock at this temperature.
The spectrum of the sample baked at 140 °C shows that urethane cleavage has occurred almost completely, as the urethane bands have been almost completely degraded. In addition, it can be shown that the blocking agent vanillin has also almost completely evaporated, as the C–O–C bands (1150 cm−1 and 1115 cm−1) and the aromatic ring vibration (1608 cm−1) are no longer detectable. However, a new amide I signal is visible at 1638 cm−1, which indicates the formation of N,N-di-substituted amides.34 Furthermore, the amide II or N–H vibration peak is shifted to 1560 cm−1, which only allows the conclusion that the chemical environment of the proton has changed during curing process. Possibly this can be explained by a low absorption of atmospheric moisture and subsequent reaction with the isocyanate released after urethane cleavage to form a linked amide structure.
It is also interesting that free isocyanate at 2260 cm−1 is detectable after and during urethane cleavage, respectively, so that the elimination–addition mechanism for the deblocking of vanillin from the HDI-trimer can be demonstrated.
The deblocking reaction of the HBA-trimer is almost analogous to the reaction presented for vanillin trimer so that the influence of the missing methoxy group is not significant in terms of stabilizing or destabilizing the urethane bond. IR spectra of deblocking process for HBA trimer are in supplementary information, Fig. S10.
In contrast to that, an additional CH2 group between the aromatic ring and the urethane, like 4-(hydroxymethyl)benzaldehyde has, shows a significant influence on the deblocking temperature.
Figure 5 shows ATR spectra of HDI-trimer samples blocked with 4-(hydroxymethyl)benzaldehyde and baked at 100 °C, 140 °C and 170 °C for 30 min.
When 4-(hydroxymethyl)benzaldehyde is used as a blocking agent for HDI-trimer, no significant changes in the spectra can be detected by IR spectroscopy up to a temperature of 170 °C.
The urethane bands at 1725 cm−1 (amide I, C=O, urethane), 1540 cm−1 (amide II, N–H-bending), and 1245 cm−1 (C=O–C, urethane) are still present at 170 °C with almost the same high intensity. This is also true for the band at 1608 cm−1, which represents the conjugated aromatic C=C vibration of HMBA, and for the C–O–C valence vibrations at 1162 cm−1 and 1132 cm−1, so there is no indication that the blocking agent has split off or is evaporated. Also, the formation of new bands that would indicate structural changes cannot be considered, so the 4-(hydroxymethyl)benzaldehyde is not deblocked in significant amounts.
Thus, compared to vanillin, the deblocking temperature is shown to be increased when the urethane bond is not directly attached to the aromatic ring. A single CH2-group acting as an electron donor strengthens the urethane bond so much that almost no deblocking is observed up to 170 °C. If this is absent, as it is the case with vanillin, deblocking begins already below 120 °C, because the withdrawing effect of the aromatic ring decreases the urethane bond.14,25
To verify these results, TGA measurements were taken with nearly solvent-free trimer samples. In comparison, the evaporation of the blocking agent was additionally investigated under real baking conditions in the presence of solvents to show the influence of the solvent on the deblocking temperature and speed.
The results of the TGA measurements are shown in Fig. 6.
Overall, these results confirm the data from the IR measurements. Again, the 4-(hydroxymethyl)benzaldehyde deblocks only to a very small extent, if at all, up to the end of the measurement range at 250 °C. For the vanillin- and HBA-blocked HDI-trimer samples, only about 30% of the blocking agent is evaporated up to a temperature of 175 °C, and complete deblocking is not observed until around 250 °C.
This is due to two reasons: Deblocking occurs with a delay because the temperature rise of the sample is relatively rapid at a rate of 10 °C per minute, and urethane cleavage and subsequent evaporation of the blocking agent do not occur spontaneously. Furthermore, no solvent is present that would solvate the cleaved blocking agent and thus shift the reaction equilibrium during urethane cleavage toward deblocking. For these reasons, deblocking, or more precisely evaporation of the blocking agent, is delayed in the TGA measurements.
The influence of the solvent and the method are clarified when the results of the thermogravimetric analyses under real baking conditions are compared with the TGA measurements of the solvent-free samples in a diagram, as in the following for the HDI-trimer blocked with vanillin, as shown in Fig. 7.
Although the experimental procedure is only comparable to a limited extent, the evaporation of the blocking agent proceeds more rapidly and at lower temperatures in the presence of solvent. For example, at 140 °C about 70% of vanillin has already been released into the oven air. After 30 min at 170 °C, almost no vanillin is detectable. In further tests, in which solvent-free blocked HDI-trimers were also baked for 30 min at various temperatures, similar mass losses as in the TGA samples were found (Figs. 7 and 8).
Thermogravimetric analyses under real baking conditions also show that the mass loss of the HDI-trimers blocked by HMBA is very low, even at high temperatures. It should be noted here that the error bars cover a larger range, since the relative error becomes significantly higher if there is low mass loss but constant solid accuracy.
TGA results for HBA-trimer are in supplementary information, Fig. S11.
Thus, IR spectra show a new band formation at 1638 cm−1 during deblocking reaction and the amide II band is shifted to 1560 cm−1 (Fig. 4); both indicate structural changes. In conjunction with obtaining apparently crosslinked, non-tacky and hard samples after baking, it was decided to provide further information on the deblocking reaction by performing temperature-dependent oscillation measurements with a rheometer.
For the HDI-trimer blocked by vanillin, a strong increase in storage (G′) and loss modulus (G″) was observed during the temperature-dependent oscillation measurement. A glass transition temperature of TG = 75 °C, which was determined in the second heating run, shows that a crosslinking reaction has taken place (Figs. 9, 10).
At 140 °C, the gel point, i.e., G′ = G″, is reached. After that, a network formation is visible by an increase of the storage modulus.
If only the deblocking reaction is present, vanillin and HDI-trimer would coexist and lead to no significant increase in storage and loss modulus. However, since this is the case and it could be shown that the blocking agent vanillin evaporates almost completely, it must be assumed that the isocyanates react with each other to form amide-containing structures. The glass transition temperature of TG = 75 °C also indicates a highly crosslinked polymer network. For example, UV-curable coatings with low amount of double bonds show TG values of 37–43 °C and with high amounts of double bonds the TG increases to values between 53 and 78 °C.36
Data for oscillation measurements of HBA-trimer are in supplementary information, Figs. S12 and S13.
For the HMBA, which deblocks only to a small amount at high temperatures, the DMTA measurements show only a slight increase in the storage and loss modulus values, and this occurs only when the sample is exposed to 170 °C for 1 h, as shown in Fig. 11.
The determined glass transition temperature of 29 °C is also significantly lower, so that only a low network density can be assumed when HMBA is used as a blocking agent (Fig. 12).
In another temperature-dependent oscillation experiment, the pure HDI-trimer, also with the same amount of DBTDL catalyst, was rheometrically crosslinked to exclude the influence of moisture on the crosslinking reaction of blocked isocyanates (Fig. 13).
Thus, no crosslinking reaction of pure DBTDL-catalyzed HDI-trimer is investigated, and it shows that humidity has no significant influence on the measurement setup. Furthermore, a glass transition temperature cannot be determined, spectra in supplementary information, Fig. S14, as the sample is tacky and not cured.
This result is interesting in that it allows conclusions to be drawn about the deblocking mechanism and the reaction of the isocyanate after or during urethane cleavage: The free electron pair of the urethane, consisting of vanillin and the HDI-trimer, nucleophilically attacks the carbonyl group of another urethane and starts an SN2-mechanism. Since the blocking agent vanillin is a good leaving group, the reaction of isocyanates occurs under vanillin evaporation to uretdiones. The reaction is shown in Fig. 14.
This explains, on the one hand, the presence of a crosslinked polymer network with only small amounts of vanillin remaining in the film and also the new amide I band (1638 cm−1) as well as the shift of the amide II band (to 1560 cm−1) observed in the IR spectrum.
Furthermore, these results show that upon deblocking the SN2 analog addition–elimination mechanism occurs, because if crosslinking occurs exclusively according to the elimination–addition mechanism (SN1), the free isocyanate of the pure HDI-trimer would also have formed uretdiones.
Thus, it can be concluded that the SN2 analogous addition–elimination mechanism can demonstrated when vanillin is used as a blocking agent. Interestingly, free isocyanate was also detected in this work by IR spectroscopy after urethane cleavage, which contradicts the SN2 mechanism.
Taking all the measurement data together, it can be assumed that both deblocking mechanisms can run side by side, but that the addition–elimination mechanism probably is preferred when using vanillin as a blocking agent for HDI-trimer.
In the second part of this work, vanillin trimer is dispersed into the aqueous phase to act as a crosslinker in a one-component system with an OH-functional binder. Since the polarity differences between vanillin trimer and water are too high, a partial modification of the trimer is necessary. The use of a surfactant such as sodium hexadecyl sulfate alone is not sufficient to disperse vanillin trimer in the aqueous phase.
Sulfonates are suitable for this purpose, so that the solubility and dispersibility of the trimer should be increased by means of dried disodium 4,5-dihydroxybenzene-1,3-disulfonate monohydrate (Tiron), disodium 3-hydroxy-2,7-naphthalenedisulfonate, or sodium 4-hydroxybenzenesulfonate as a structural modification, respectively.37,38
The solubility of disodium 3-hydroxy-2,7-naphthalenedisulfonate in other solvents than water is so low that the modification succeeds only to an insufficient extent. For this reason, disodium 3-hydroxy-2,7-naphthalenedisulfonate is not suitable for modifying HDI-trimer. Contrary to expectations, when the dried Tiron has been used, both OH groups partially reacted with the HDI-trimer, so that partial prepolymers were already formed by a 20% modification, resulting in an inhomogeneous sample with a slight orange tinge. The best results in terms of dispersibility were obtained by using sodium 4-hydroxybenzenesulfonate (Na-4-HBS) as sulfonate modification, which is used for further experiments.
In combination with a surfactant (sodium hexadecyl sulfate) and an additional wetting and dispersing additive (Disperbyk-181), a stable vanillin-Na-4-HBS-trimer-dispersion with 40% solid content could be prepared after neutralization by means of triethylamine (TEA).
IR spectra of synthesizing vanillin-Na-4-HBS-trimer is in supplementary information, Fig. S9.
For the evaluation of deblocking and subsequent reaction with the OH-functional binder, binder and hardener components have been mixed and baked according to the ratios calculated in Table 2. In addition, a test is carried out in which the Na 4-HBS-trimer dispersion is cured without the crosslinking component to check whether the deblocking reaction also succeeds after sulfonate modification. The evaluation is based on IR measurements and DMTA measurements using a rheometer.
Figure 15 presents the results of the pure trimer dispersion and the conversions with OH-functional polyacrylate dispersion Bayhydrol® A 2470 and OH-functional poly-carbonate ester-polyurethane dispersion Bayhydrol® U 2757 after baking at 140 °C for 30 min. The IR spectra of the samples before baking and of all the conversions are in the supplementary information, Figs. 15 and 16.
The IR spectra are evaluated primarily with respect to the urethane, amide I and amide II bands. Analogous to the results for the pure vanillin trimer, it can also be observed here that the urethane band at 1740 cm−1 has completely disappeared, the amide I band at 1680 cm−1 and the amide II peak at 1570 cm−1 are shifted, so that urethane cleavage can be assumed.
For the reactions with the OH-functional binder dispersions, an increase in intensity of the urethane band at 1740 cm−1 and the presence of the amide II band at about 1540 cm−1 can be observed in both samples. This confirms the formation of urethane functionality after the deblocking of vanillin and the subsequent reaction with the OH-groups of the binder dispersions.
When the polyacrylate dispersion is used, the already weaker absorbing amide II band is only weakly visible, but the oscillation of the entire urethane group at 1740 cm−1 is found to be significant.
Thus, the IR results indicate successful deblocking of the vanillin-Na-4-HBS-trimer with subsequent reaction with the OH-functional water-based binder dispersions to form crosslinked urethane-based polymer networks.
The results of the IR measurements can also be confirmed with the aid of the DMTA measurements. Data for pure vanillin-Na-4-HBS-trimer illustrate a strong increase of storage and loss modulus indicating deblocking and a subsequent reaction (Fig. 16).
It is not possible to make a reliable statement regarding the gel point due to measurement inaccuracies caused by the increased amount of solvent in the sample.
When crosslinking with a binder, the diagrams show first a slight increase in storage and loss modulus, which is caused by the evaporation of solvent, and then a further stronger increase, which is due to the deblocking and crosslinking reaction with the binder. In the second heating run, the glass transition temperature is read at the maximum of the loss modulus (G″). When crosslinking with the OH-functional polyacrylate dispersion (Bayhydrol® A 2470) and the polyester-polyacrylate dispersion (Bayhydrol® A 2227/1), a glass transition temperature of TG = 68 °C is measured in each case. DMTA diagrams of crosslinking reactions and second heating runs are in supplementary information, Figures S18–S21. The OH-equivalent weights of the binders are relatively close to each other at 969 g/mol OH and 1065 g/mol OH, so that—assuming a similar structure of the binders—the number of urethane bonds and thus the expected mechanical properties, which are partly reflected by the glass transition temperature, are also comparable. Results for crosslinking vanillin-Na-4-HBS-trimer with poly-carbonate ester-polyurethane dispersion are illustrated in Fig. 17.
Using the OH-functional poly-carbonate ester-polyurethane dispersion (Bayhydrol® U 2757), a much lower glass transition temperature of TG = 23 °C is determined by DMTA. Furthermore, the TG range is larger in this sample compared to other tested binders, so that the polymer network can be evaluated as more amorphous. The significantly higher OH equivalent weight with a value of 1720 g/mol OH leads to lower network densities and thus a softer film including a reduction of the glass transition temperature, which is also reflected in the measurement data (Fig. 18).
Finally, the coating films obtained after crosslinking were examined in terms of application properties: Focusing on the properties of the polymer network after crosslinking reaction, in addition to a visual assessment, the impact test and pendulum damping were carried out. Furthermore, the adhesion of the samples to steel was investigated and a rapid chemical resistance test was carried out, in which a solvent is dropped on the coating surface for 30 s and wiped off and the exposed area is then inspected. Table 3 summarizes the results of the application tests:
As shown before, even curing vanillin-Na-4-HBS-trimer results in a partly crosslinking polymer network with N,N-di-substituted amides, so that the film is not tacky and its pendulum damping is 45. The chemical resistance of the coating is significantly lower compared to conversions with an OH-functional binder. Furthermore, crystallinity of partly not crosslinked isocyanate groups could be a reason for brittleness and partly only physical dried coating film, which is indicated by results of the impact test. Films including polyacrylates and polyester-polyacrylates (Bayhydrol® A 2470 and Bayhydrol® A 2227/1) show higher values for pendulum damping and better chemical resistance, but are less flexible.
Application tests confirm the results of the DMTA measurement of the film with Bayhydrol® U 2757, which has a significantly lower glass transition temperature (TG = 23 °C). With a value of 13 in the pendulum damping test, the film can be rated as very soft, but thus also significantly more flexible, so that the film does not crack after the impact test. The chemical resistance of the film is comparable to the other samples tested.
All cured coatings including a water-based binder show an excellent adhesion with GT = 0 on steel according to DIN EN ISO 2409. The gloss of all samples is visually evaluated high, and all coating films have only minor film defects and a slight yellowish appearance.
Finally, blocking HDI-trimer with vanillin and modifying with sulfonate is suitable for dispersing the resulting vanillin-Na-4-HBS-trimer in water, and the coating films obtained by reaction with OH-functional aqueous binders show promising application properties.
Conclusions
In this work, vanillin and 4-hydroxybenzaldehyde were shown to be suitable blocking agents for isocyanates. Vanillin in particular is a toxicologically safe blocking agent that is commercially available in large quantities and deblocks at comparatively low temperatures. In addition to the influence of solvents on deblocking, the structural effects on deblocking temperatures were also illustrated using 4-(hydroxymethyl)-benzaldehyde.
Furthermore, it can be shown from measured data that both deblocking mechanisms described in the literature occur during the deblocking of vanillin.
Finally, the partial modification of the HDI-trimer with sodium 4-hydroxybenzenesulfonate offers the possibility to prepare an aqueous dispersion and to crosslink it with OH-functional, water-based binders. The obtained coating films show promising mechanical and application properties.
References
Zhang, S, Hsu, L, Toolis, A, Li, B, Zhou, J, Lin, T, Chen, Z, “Investigation of the Atmospheric Moisture Effect on the Molecular Behavior of an Isocyanate-Based Primer Surface.” Langmuir ACS J. Surf. Colloids, 37 12705 (2021)
Meier-Westhues, U, Polyurethane: Lacke, Kleb-und Dichtstoffe, Vincentz Network, Hannover, p. 36, 2007
Goldschmidt, A, Streitberger, H-J, BASF Handbook on Basics of Coating Technology, Vincentz Network, Hannover, pp. 96–99, 2007
Guillem Parra, M, Galia Clua, M, Lligadas Puig, G, Blocked Isocyanate Polyurethane Compositions Using a New Blocking Agent, Method of Manufacture and Uses Thereof: European Patent Application (2019). https://data.epo.org/publication-server/document?iDocId=6506253&iFormat=0
Arya, SS, Rookes, JE, Cahill, DM, Lenka, SK, “Vanillin: A Review on the Therapeutic Prospects of a Popular Flavouring Molecule.” Adv. Tradit. Med. (ADTM), 21 1 (2021)
Eaves, D (ed.) Handbook of Polymer Foams. Rapra Technology Ltd, Shrewsbury, pp. 9–10, 2004
Caraculacu, AA, Coseri, S, “Isocyanates in Polyaddition Processes, Structure and Reaction Mechanisms.” Prog. Polym. Sci., 26 (5) 799–851. https://doi.org/10.1016/S0079-6700(00)00033-2 (2001)
Gibb, N, Goodman, JM, “The Formation of High-Purity Isocyanurate Through Proazaphosphatrane-Catalysed Isocyanate Cyclo-Trimerisation: Computational Insights.” Org. Biomol. Chem., 2013 (11) 90–97 (2013)
Bernardini, J, Licursi, D, Anguillesi, I, Cinelli, P, Coltelli, MB, Antonetti, C, Galletti, AMR, Lazzeri, A, “Exploitation of Arundo donax L. Hydrolysis Residue for the green synthesis of Flexible Polyurethane Foams.” BioResources, 12 (2) 3630–3655 (2017)
Ni, H, Nash, HA, Worden, JG, Soucek, MD, “Effect of Catalysts on the Reaction of an Aliphatic Isocyanate and Water.” J. Polym. Sci. Part A: Polym. Chem., 40 1677–1688 (2002)
Brock, T, Groteklaes, M, Mischke, P, European Coatings Handbook, Vincentz Network, Hannover, pp. 167, 198, 2010
Rolph, MS, Markowska, ALJ, Warriner, CN, O’Reilly, RK, “Blocked Isocyanates: From Analytical and Experimental Considerations to Non-polyurethane Applications.” Polym. Chem., 7 7351 (2016)
de Jong, H, Blocked Isocyanates and Their Production and Use, 12/19/1990, European patent number: EP0403044A2
Mohammed, IA, Sankar, G, “Synthesis, Deblocking and Cure Reaction Studies of Secondary Alcohol-Blocked Isocyanates.” High Perform. Polym., 23 535–541 (2011)
Delebecq, E, Pascault, J-P, Boutevin, B, Ganachaud, F, “On the Versatility of Urethane/Urea Bonds: Reversibility, Blocked Isocyanate, and Non-isocyanate Polyurethane.” Chem. Rev., 113 80 (2013)
Wicks, DA, Wicks, ZW, “Blocked Isocyanates III: Part A Mechanisms and Chemistry.” Prog. Org. Coat., 36 148 (1999)
Sang, S, Li, Y, Wang, K, Tang, J, “Application of Blocked Isocyanate in Preparation of Polyurethane(Urea) Elastomers.” J. Appl. Polym. Sci., 138 50582 (2021)
Nasar, AS, Raghavan, A, Kumar, VS, “Synthesis of Poly(Urethane-Imide): Effect of Solvents With and Without Basic Nitrogen Atom and Other Parameters on the Imide Formation Reaction Between Blocked-Isocyanate Prepolymers and Pyromellitic Dianhydride.” J. Macromol. Sci. Part A, 42 309 (2005)
Gnanarajan, TP, Nasar, AS, Iyer, NP, Radhakrishnan, G, “Synthesis of Poly (Urethane‐imide) Using Aromatic Secondary Amine‐Blocked Polyurethane Prepolymer.” J. Polym. Sci. A Polym. Chem., 38 4032 (2000)
Baker, JW, Gaunt, J, “3. The Mechanism of the Reaction of Aryl Isocyanates with Alcohols and Amines. Part II. The Base-Catalysed Reaction of Phenyl Isocyanate with Alcohols.” J. Chem. Soc. (1949)
Ren, F, Zhou, R, Sun, F, Ma, H, Zhou, Z, Xu, W, “Blocked Isocyanate Silane Modified Al2O3/Polyamide 6 Thermally Conductive and Electrical Insulation Composites with Outstanding Mechanical Properties.” RSC Adv., 7 29779 (2017)
Mühlebach, A, “Pyrazoles—A Novel Class of Blocking Agents for Isocyanates.” J. Polym. Sci. A Polym. Chem., 32 753 (1994)
Mischke, P, Film Formation in Modern Paint Systems, Vincentz Network, Hannover, p. 126, 2010
Alex He, Z, Blank, WJ, “Crosslinking with Malonate Blocked Isocyanates and with Melamine Resins.” J. Coat. Technol., 71 85 (1999)
Guo, S, He, J, Luo, W, Liu, F, “Research on the Thermal Decomposition Reaction Kinetics and Mechanism of Pyridinol-Blocked Isophorone Diisocyanate.” Materials (Basel, Switzerland), 9 110 (2016)
Sankar, G, Sultan Nasar, A, “Effect of Isocyanate Structure on Deblocking and Cure Reaction of N-Methylaniline-Blocked Diisocyanates and Polyisocyanates.” Eur. Polym. J., 45 911 (2009)
Lee, JM, Subramani, S, Lee, YS, Kim, JH, “Thermal Decomposition Behavior of Blocked Diisocyanates Derived from Mixture of Blocking Agents.” Macromol. Res., 13 427 (2005)
Bailey, ME, Kirss, V, Spaunburgh, RG, “Reactivity of Organic Isocyanates.” Ind. Eng. Chem., 48 794 (1956)
Essenfeld, A, Kuang-Jong, W, Tsuboi, H, "A New Formaldehyde-Free Etch Resistant Melamine Crosslinker." Polym. Mater. Sci. Eng., 17 (1997)
Quérat, E, Tighzert, L, Pascault, J-P, “Hydroxamic Acid Esters and Oximes as Isocyanate Blocking Agents.” Angew. Makromol. Chem., 219 185 (1994)
Loim, NM, Kelbyscheva, ES, “Synthesis of Dendrimers with Terminal Formyl Groups.” Russ. Chem. Bull., 53 2080 (2004)
Socrates, G, Infrared and Raman Characteristic Group Frequencies: Tables and Charts, Wiley, Chichester, pp. 122–125, 143–148 (2010)
Ming, W, Tian, M, van de Grampel, RD, Melis, F, Jia, X, Loos, J, van der Linde, R, “Low Surface Energy Polymeric Films from Solventless Liquid Oligoesters and Partially Fluorinated Isocyanates.” Macromolecules, 35 6920 (2002)
Hesse, M, Meier, H, Zeeh, B, Spectroscopic Methods in Organic Chemistry: 100 Tables. Thieme, Stuttgart, 2008
Zhao, C, Huang, C, Chen, Q, Ingram, IDV, Zeng, X, Ren, T, Xie, H, “Sustainable Aromatic Aliphatic Polyesters and Polyurethanes Prepared from Vanillin-Derived Diols via Green Catalysis.” Polymers, 12 586 (2020)
Matner, M, Casselmann, H, Zhuang, W, Achten, D, Ehlers, M, Transparent Polyurethanes with High Glass Transition Temperature Tg: United States Patent Application Publication
Suzuki, T, Yamane, M, Nishioka, T, Nukada, Y, Morita, O, “Effects of Internal Hydrophilic Groups of a Newly Developed Sustainable Anionic Surfactant on Biodegradability and Ecotoxicity.” Chemosphere, 286 131676 (2022)
Mansour, AM, Radacki, K, Shehab, OR, “Role of the Ancillary Ligand in Controlling the Lysozyme Affinity and Electronic Properties of Terpyridine fac-Re(CO)3 Complexes.” Dalton Trans. Camb. Engl., 2021 (50) 10701 (2003)
Acknowledgments
This work was supported by the German “Bundesministerium für Bildung und Forschung (BMBF)” in the framework of the program “FHProfUnt”, Grant 13FH125PX6, and Covestro Deutschland AG.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Knospe, P., Reichmann, R., Gutmann, J.S. et al. Vanillin as low-temperature isocyanate-blocking agent and its use in one-component aqueous coatings. J Coat Technol Res 20, 501–520 (2023). https://doi.org/10.1007/s11998-022-00696-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-022-00696-w