Abstract
The performance of a material in a biological environment is mediated by its surface properties and by the combination of physical and mechanical properties that are required for a specific application. The more relevant surface properties in these respects include its chemical structure (hydrophilicity and the presence of functional groups that could initiate reactions in biological systems) and the morphology (the distribution and abundance of hydrophilic/hydrophobic phases, crystalline/amorphous phases and surface topographical assets). In surface modification, one aims to tailor the surface characteristics of a material toward a specific application without detrimentally affecting the bulk properties. This strategy allows for an increase in the success of implants’ application by increasing their lifetime. In this review, several approaches to the surface modification of polymers are described, as are a number of viable applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the more frequent, expensive, and serious problems faced by human healthcare is the loss or failure of organs or tissues. The medical need for tissue and organ substitutes can arise from trauma, infections, inherited problems, age-related diseases, and organ failure.1
The field of biomaterials embraces a wide range of applications. Polymers have been extensively used as coatings in biomedical devices. They are also used as the bulk material in devices such as catheters, heart valves, and dialysis membranes. These polymers that were originally developed for a variety of industrial applications were then adapted for use as biomaterials, based on their favorable characteristics.
The performance of a material in a range of biological environments is initially dependent on its surface properties. Cell attachment and consequent cell behavior are determined by a complex set of occurrences that involve processes such as protein adsorption and intercellular signaling. Most biological reactions occur on surfaces and interfaces. Therefore, the surface of the biomaterial is a key factor determining the biological response and, therefore, the biocompatibility.2 Properties such as the wettability, chemical composition, porosity, softness/stiffness, roughness, and the surface charge of the biomaterial’s surface, are highly significant with respect to the existing physiological environment.3,4
When designing a biomaterial, these factors should be taken into consideration in optimizing those features that are related to the overall biocompatibility. Among the surface properties that can be tuned by the judicious choice and use of coating are the surface chemical structure (such as the inherent hydrophilicity), the presence of functional groups on the surface (that could be used to inter as sites for action/reaction with biological systems, in biological media), the surface morphology (the distribution and abundance of specified hydrophilic/hydrophobic regions and the presence of ordered/disordered phases), and the overall surface topography.5 For example, surface chemical properties are major factors in determining the nature and extent of any adhesion of proteins in “in vivo” systems. This behavior is directly related to subsequent cellular interactions with the coating and, therefore, to the materials’ biocompatibility. Also, it has to be recognized that if a coating is inappropriately chosen, pathological microorganisms may easily adhere to the resultant surfaces, causing infection. The incidence of such infections varies from 1% (in hip prostheses) up to 100% (for urinary tract catheters) and up to 50% in aortic vascular graft infections.6 An infection involves three major steps: microbial adhesion, microbial proliferation, and the formation of a bacterial film (biofilm). The surface modification (coating and surface deactivation) of the parent substrate has been one of the more used and studied approaches to avoid the biofilm deposition (biofouling). Surface modifications are used to tailor the surface characteristics of a material to a specific application without destructively affecting the bulk properties. This approach provides a basis for increasing the success of implants’ application by increasing their service lifetime.
Two major strategies have been used by researchers to increase the overall biocompatibility of substrates. One involves the synthesis of new materials and the other concerns the modification of the surface of pre-existing materials, for example by the use of appropriate coating technologies and pretreatments. Some of the available techniques that are used to enhance biocompatibility are described below.
Interaction of surfaces with biological systems
When a biomaterial is implanted in a living organism, several types of interaction can become established between the host tissue and the biomaterial, perhaps more specifically with its surface. These interactions, although being classified as being normal, may lead, if not properly controlled, to a failure of the implant and, in the worst case scenario, to the host’s death.
In order to understand, to predict, and to avoid such effects, it is important to recognize that, besides the effect of the implant on the host’s tissues, additional factors should be considered. Other very important considerations include the events occurring at the biomaterial–tissue interface, the effect of the host on the material, and the consequences that such interaction might bring about. Several instances of biomaterial–tissue interactions have been reported and are listed in Table 1. The most significant will be further described in this article.
Immediately after implantation of a biomaterial into a biological environment, adsorption of lower molecular weight proteins onto its surface begins. Among these proteins are usually albumin and immunoglobulins (antibodies involved in physiological defense mechanisms). Proteins adsorption to a surface is a highly complex and dynamic process. Figure 1 gives an example of the dynamics of this process. Over time, the initially adsorbed proteins are displaced by others that are larger and less motile, presenting a more pronounced affinity for the material’s surface. This phenomenon is commonly known as the Vroman effect.7,8
Representation of the Vroman effect. After exposure to the physiological medium, small, and abundant proteins (protein A) adsorb and interact with the polymeric surface. These weakly surface-active proteins are subsequently displaced by other larger proteins that give a greater affinity with the coated material (adapted from Palacio et al.9)
At the end of this process, a film that is composed of proteins and extracellular matrix (ECM) material coats the surface. This film is commonly referred to as the “conditioning layer”. The logic behind this terminology is that the nature of these proteins is a major factor of interactions with any biological environment, including blood, of the attachment of posterior cells and of their behavior (such as the attachment and activation of neutrophils, macrophages, and other inflammatory cells) and bacterial adhesion.10
Blood–material interactions
When a medical device is brought into contact with blood, several responses can occur at the blood/biomaterial interface. One of the more common and severe events is that of blood coagulation at the material surface, ending up with the development of thromboemboli. This is a highly intricate process which begins with the adsorption of proteins onto the surface followed by the interaction with blood cells, namely with platelets.11,12 Once attached to the surface, platelets aggregate and become active. This activation is translated in the release of several molecules which act as a signalization system activating additional platelets. At this stage, a series of complex and succeeding reactions take place (coagulation cascade). During this process, thrombin (a protease) converts the soluble fibrinogen into insoluble fibrin strands. These strands are then crosslinked to form a blood clot or thrombus. Figure 2 represents this process.
The resultant thrombus may become unattached and get carried by the blood stream finally resulting in blocking a blood vessel (thromboembolism). This process usually occurs in the lungs, brain, gastrointestinal tract, kidneys, or legs and is a significant cause of patient morbidity and mortality.
Several factors are known to influence protein deposition and posterior blood clotting on foreign surfaces. Among these, special emphasis is given to the characteristics of the biomaterial’s surface (namely the composition, the surface energy, the hydrophilicity, and the roughness) to the hemodynamics (the nature of blood flow to which the biomaterial is subjected) and to the biological environment.
Cell adhesion
Once attached to the material, a complex system of communication that is initiated by the proteins begins to take place. This involves the release of signals, the attraction of cells, and the adherence of those cells to the surface. The process of cell attachment to a surface occurs in three consecutive stages: (1) attachment initiation, (2) spreading and growth, and (3) the formation of focal adhesions and stress fibers.13
The adhesion of animal cells is mediated by the adhesion receptor proteins that exist in the cells membranes, the integrins. Structurally, integrins are a large family of transmembrane heterodimers (complexation of nonidentical monomers14) of noncovalently associated α and β subunits. They act as bridges for cell–cell and cell–ECM interactions.15 Despite the existing variety of integrins, their basic shape is similar since they are all composed of subunits with large extracellular domains, a single transmembrane segment, and a relatively short cytoplasmic extremity (Fig. 3).16,17
The first stage of the adhesion process is initiated by contacts between those receptor proteins and the proteins that coat the surface. This phase will result in the activation of the integrins, a phenomenon that includes their clustering and the increase of their affinity toward the protein-coated surface.18,19
Following this process, filaments of actin, the major cytoskeletal protein, are formed by the polymerization of actin and stress fibers are created. This phenomenon allows the cells to spread, enhancing their surface contact area (second stage). Finally, strong points of attachment, termed focal adhesions, are formed. These sites work as linkage spots between the fibers of actin and the extracellular medium, via the transmembrane integrins. At the end of this process, the animal cell is tightly attached to the coated surface. The described process is summarized in Fig. 4.
Stages of cell adhesion to a coated surface: attachment, spreading, and formation of focal adhesions and stress fibers (adapted from Murphy-Ullrich18)
When coated biomaterials are used, cell adhesion may become either an advantage or a shortcoming, depending on the circumstances. While for some applications it is vital that cells become attached, spread, and even divide on the surface (as in the case of the development of materials that are designed for use in tissue engineering), in other cases such processes will result in implant failure and the need for consequential removal and replacement. The most obvious example of undesirable cell adhesion to a material is that which occurs in bacteria attachment.
Bacterial adhesion and biofilm formation
Bacterial attachment and the subsequent spreading on biomaterials results in the formation of a secondary coating, a biofilm, which is frequently associated with the failure of implants, as in the failure seen in joint prostheses, heart valves, vascular catheters, and contact lenses.20 Biofilm formation occurs through several consecutive stages (Fig. 5), starting with the attachment of bacteria to the surface, followed by cell–cell adhesion and proliferation. Finally, cells start to accumulate in multilayer clusters and to produce an ECM (mainly composed of polysaccharides), which works as an enclosing structure. The spreading of the resultant coating biofilm is mainly due to the release of some bacteria from the cluster structures, which then adhere to the coating surface and start a new cycle.21
Studies concerning the mechanisms of bacterial adhesion, leading to an understanding of how to avoid this drawback, are constantly being updated. However, the complexity of such processes and all the factors of relevance (e.g. material properties, bacterial strain type, and patient condition), makes it a task that is/will be difficult to accomplish.
Surface modification
The success or failure of a medical device is often determined by its surface properties and its bulk properties. For a specific medical application, the desired characteristics for the implant must be known prior to its implantation. Thus, implanted devices need to present specific bulk and surface characteristics if they are to be recognized and accepted by the medium into which they are presented.
In most applications, a polymeric material is chosen according to its bulk properties, such as the stiffness, the polymer type, conductivity, the optical properties, the strength, and the freedom from deterioration.22,23 For most biomedical applications, the two properties of greatest importance are the bulk strength (mechanical) and the surface reactivity (chemical). Some synthetic materials have the suitable bulk properties. However, the surfaces of the majority of materials do not have the same composition and features as the bulk. Thus, their surfaces have to be modified, if they are not to be recognized as a foreign material.
Thus, surface modification (coating) plays an important role in biomedical applications since coatings can be used to adapt the surface to the desired characteristics without compromising its bulk properties.
Surface modification techniques are used across various technologies including biomedical devices, textile materials, microelectronic components, and food industry products. Surface modification materials and methods are tried and tested in a variety of fields, giving confidence in the appropriateness of the modification techniques that are available.
Several methods for tailoring surfaces to suit specific applications have been reported. Three major classes of surface modification techniques can be considered. The first concerns surface modifications by physical agents. These include flame treatment, corona discharge treatment, plasma treatment, etching, ultraviolet (UV) radiation exposure, laser ablation treatment, gamma irradiation, and X-ray treatments. The second class includes surface modifications by using chemical agents and functional group incorporation, such as surface oxidation, hydrolysis, chemical grafting, and surface coating.24 Finally, the third class involves the use and immobilization of biological molecules or cells onto the surface of the materials. Thus, several different biomolecules can be immobilized using various techniques.
Over the years, several surface modification methods have been developed. Some of these are listed below:
-
The physical deposition of active compounds on the surface by coating.25,26
-
The covalent immobilization of polymer chains onto a surface by chemical reactions, taking place after coating.27
-
The grafting of polymer chains on the surface after and during plasma treatment,28–30 by corona discharge,31–33 and by grafting that has been initiated by γ-radiation treatment.34–36
Surface modification by ultraviolet irradiation
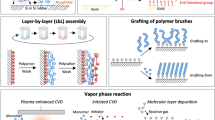
Surface graft polymerization, induced by UV irradiation induced photoactivation (Fig. 6), has been shown to offer advantages over alternative treatments. These include their fast reaction rate, the relatively low cost of processing, the availability of a variety of formulation options and items of equipment, relatively facile development to industrial scale processing, and, probably most importantly, the creation of viable products in which the distribution of the grafted chains is limited to the surface of the material.37
Such grafting can be viewed as a controlled coating option. Surface modification by the photografting of coating formulations provides another basis for the modification of the surface properties without interfering with the bulk material.
Surface polymerization after coating, using UV radiation induction/initiation, is usually performed in the presence of a photoinitiator or a photosensitizer. This photoinitiator (after its photodecomposition), can induce the formation of monomer radicals, leading to subsequent atom abstraction processes, (usually involving H-atoms), resulting in radicals on the backbone of the substrate surface. The introduction of monomers (vapor phase or solution) can bring about graft photopolymerization through the usual routes of propagation and eventual chain termination.38–40 A range of photoactivators is available for use in this context. These allow the initiation of the surface activation process by inducing radical formation in the substrate surface. The radical produced may be symmetrical or asymmetrical, depending on the molecular structure of the initiator. Thus, frequently used photoinitiators, such as the isopropylthioxanthone, xanthone, anthraquinone, 2,2-azo-bis-isobutyronitrile, among others, are used for nonaqueous media.37 Water soluble/compatible derivatives of these initiator types are used when aqueous formulations are required, usually based on quaternary salts and sulphonic acid salts. The curing reaction time and the wavelength of the radiation that is used in the reaction are determined by the chosen photoinitiator and the spectral output of the radiation source. An example is given by 1-[4-(2-hydroxyethoxy)-phenyl]-2-hydroxy-2-methyl-1-propane-1-one (Irgacure®2959), a highly efficient nonyellowing radical photogenerator that is used for the UV curing of systems comprising of unsaturated monomers and pre-polymers, which generates the acetone ketyl radical (Fig. 7).
This photoinitiator has been widely used since Williams et al.41 studied its biocompatibility, along with that of other photoinitiators. In their study, this initiator gave the best results in terms of cytocompatibility.
The main application of UV radiation in the biomaterials field has been targeted toward the modification of surfaces. However, in some cases, such as in the improvement of biocompatibility and haemocompatibility, UV irradiation can be also used to modify the bulk of the material. Moreover, crosslinked materials and cell immobilization systems,42,43 antifouling surfaces,44 and bioscaffolds for tissue engineering,45–47 can also be prepared by UV irradiation-based techniques.
This technique has been used to modify polyurethanes, in order to develop and control cell adhesion on the surface of the material in the development of a new biosensor48–50 and for heart valves.51 The surfaces were activated with Irgacure®2959 and then different vinyl monomers (e.g. acrylic acid, hydroxyethyl methacrylate, sulfobetaine methacrylate) with a desired composition was grafted to the surface, as shown in Fig. 8.
Surface modification by plasma treatment
Nowadays, plasma technologies are being widely used to tailor the surface properties of polymers without affecting their bulk properties. Hydrophilicity, chemical structure, and roughness are some of the surface properties that can be modified by plasma treatment in order to adapt a material for a specific application. Plasma treatment is also being used to free polymer surfaces from organic contamination.52
A plasma is a mixture of highly excited electrons, negatively charged particles and positively charged particles, neutral atoms, and molecules. For this reason the plasma state is sometimes referred to as the fourth state of matter.
Plasma polymerization has been studied intensively since the 1950s and especially in the 1960s. Over the years, plasma polymerization has gained importance as a surface modification technique and, nowadays, is a well-recognized method,53,54 especially in the biomedical field. This is because it is a relatively economical and effective technique for the processing of polymeric materials. Table 2 lists some of the common research areas and applications in which plasma treatment is used for the creation of more effective biomaterials.55
The surface modification of polymers by plasma treatment is widely used with gases such as argon, helium, nitrogen, and oxygen, among others. This treatment leads to the formation of free radicals at the surface of the polymer. These radicals, under appropriate circumstances, are able to react with the excited species in the plasmas. Therefore, treatment with O2 gases leads to oxygen atom containing functionalities such as hydroxyl groups, carbonyl groups and, in some cases, carboxylic groups (Fig. 9). Other chemical groups can be introduced using the appropriate gas (gas mixture). Inert gases can be used if surface roughness features are required.
Schematic representation of the oxygen plasma surface activation mechanism leading to various active centers due to surface oxidation (adapted from Alves et al.49)
In the biomedical field, plasma surface modifications are mostly used to improve adhesion, increase surface wettability, and induce surface roughness. Plasma surface modifications include52:
-
removing the surface contaminants such as air pollutants or fingerprints,
-
etching, leading to an increase of roughness by selective removal of material from surfaces by chemical reaction and/or physical sputtering,
-
substitution of chemical groups on the surface that might allow covalent bonding, by the modification of the surface characteristics with reactive gases.
As previously mentioned, plasma processing can be used for the cleaning, the activation, and the etching of a surface (metal, ceramics, glass, and plastics) as well as for coating by plasma-induced polymerization. The various effects of the plasma treatment of a polymer surface may be categorized as surface modification, grafting, and film deposition (plasma polymerization).56
Plasma grafting is a two-step, incorporation process whereby functional groups and reactive sites can be created on the polymer surface (Fig. 10). Using inert gas plasmas, free radicals can be formed on the surface. To generate a grafted/coated polymer on this surface, a monomer solution is introduced in the reaction chamber, allowing the monomer to react with the surface free radicals, resulting in a grafted/coated surface treatment.56
Alves et al.48 introduced carboxylic groups onto a thermoplastic polyurethane substrate using the plasma method. In this way, the authors grafted acrylic acid and methacrylic acid onto the surface of the polymeric substrate to improve wettability and to reduce cell adhesion. Poly(dimethyl siloxane) surfaces can be modified by using a low-pressure plasma to improve their characteristics toward biomedical applications. Pluronic®F-68 (polyoxyethylene–polyoxypropylene block copolymer; a relatively nontoxic difunctional surfactant) and poly(ethylene glycol)methyl methacrylate can be grafted to poly(dimethyl siloxane) surfaces to improve their wettability and to reduce bacterial adhesion.57 These surface modification findings show that these kind of materials (polyurethanes and polysiloxanes) can be used in medical devices (e.g. catheters, vascular grafts,51 and voice prostheses.57,58).
Plasma-induced polymerization leads to the formation of a thin coating film that can provide unique chemical properties and physical properties. In order for the polymerization process to occur, radicals can be formed in the presence of the gas in which a monomer is usually mixed. Then, as the molecular weight of the polymer in the chamber increases, it is deposited on the surface of the substrate. The deposition of the polymers is mainly mediated by the geometry of the plasma system, the working gas pressure, the power and frequency of the signal, the temperature of the substrate and the flow rate, and reactivity of the monomer. This technique leads to the production of thin films that are highly crosslinked and are strongly bound to the surface.52,56
Vilani et al.59 studied the influence of the acrylic acid plasma parameters on the treatment of polysiloxane substrates and on polyurethane substrates. They treated both surfaces by using a radio frequency (RF), low-pressure plasma using acrylic acid as the monomer. They concluded that the formed thin film was chemically similar to poly(acrylic acid) films conventionally created.
Biomolecule attachment
As previously mentioned, the adsorption/desorption behavior of blood proteins or the adhesion and proliferation of different types of cells on polymeric materials depends mainly on the surface characteristics of the substrate such as wettability, hydrophilicity/hydrophobicity, bulk chemistry, surface charge type and strength of charge distribution, and surface roughness.60 Routes to dealing with such issues involve the surface modification of biomaterials with proteins to control cell adhesion and the subsequent cellular response to material surfaces as reported.61–64 For this purpose, molecules such as laminin, fibronectin, collagen, and the arginylglycylaspartic acid (RGD, a tripeptide composed of l-arginine, glycine, and l-aspartic acid) can be used. Some studies have shown that by modifying the biomaterial surface with molecules that induce cell adhesion, such as fibronectin, improvement of the cellular response and in tissue repair can be achieved.65,66
Some serum proteins, such as fibronectin and vitronectin, show a preferential adsorption onto moderately aqueous medium wettable surfaces. These proteins are also known as cell-adhesive proteins. If a surface offers suitable hydrophilicity, these proteins can adhere on the surface and, consequently, can improve cell adhesion, spreading, and growth.60 Thus, if a surface is tailored to process the ideal hydrophilicity the cellular response can be controlled.
The RGD peptide sequence is known as a cell recognition site for numerous adhesive proteins that are present in the ECM and in whole blood. Surfaces immobilized with RGD groups are known to enhance cell attachment, while any RGD components that are present in solution can inhibit cell attachment by competing with endogenous ligands for the same recognition site.67
The bovine serum albumin (BSA) protein gives different responses to different interfacial environments. BSA is often used to coat biomedical devices. Albumin is often used for coating vascular grafts in order to passivate surfaces that are in contact with blood and thus minimize surface-induced platelet activation. The albumin reduces both the number of adherent platelets and the extent of platelet activation on the albumin-adsorbed surface.68,69
Poly-d-lysine (PDL) is a synthetic polypeptide which is used as a culture substrate to enhance cell attachment to plastic surfaces and to glass surfaces. It is also used with a wide variety of cell cultures, particularly neurons, glial cells, and transfected cells. In addition, PDL-coated surfaces are often used to reduce cell detachment, which may occur during multiple washing steps that are associated with cell assays.70
Surface coating by polyelectrolyte multilayer (PEMs) thin films
The preparation of polyelectrolyte multilayer (PEMs) thin films via the layer-by-layer assembly (LbL) of oppositely charged polyelectrolytes (PELs) on a charged surface was first introduced by Decher and co-workers in 1992.71 Since then, this concept has generated a new and very active field of research. In the biomedical area, PEMs are being investigated mainly for applications as drug delivery systems and for surface coating and biofunctionalization of biomedical devices. This technique is based on electrostatic attractions, where aqueous solution of two oppositely charged polymers or polyelectrolytes (PELs) are alternatively deposited on a substrate. The deposition of each layer is defined by the surface charge, which is reversed after each dipping cycle. This process allows the formation of a multilayer structure stabilized by electrostatic forces.72 The formation of PEMs by LbL assembly is depicted in Fig. 11.
Each polyelectrolyte layer is formed by the absorption of charged polyelectrolyte species from solution onto an oppositely charged surface. The excess adsorption of the substances, i.e., charge neutralization and resaturation, leads to a reversion of the surface charge, which allows the deposition of an additional layer of a polyelectrolyte with a charge opposite to that of the first one.73 The LbL assembly process is driven by electrostatic interactions that are established between the oppositely charged domains of PELs, although forces of other nature can contribute to the formation and stabilization of the assemblies.74 Also, thermodynamically this process is favored by the gain in entropy due to the release of counterions.
One of the attractive features of the LbL method is its simplicity. In fact, the LbL strategy allows the construction of PEMs films without the need for expensive or sophisticated equipment, using only simple procedures. The dip coating method, illustrated in Fig. 11, is the most simple and common way to LbL built-up. This method consists in the sequential dipping of an object with its charged surface into aqueous solutions of oppositely charged polyelectrolytes. Other techniques that have been developed to produce PEMs by LbL include the spin-coating method75 and spraying LbL.76 The structure and properties of PEMs are determined not only by the intrinsic properties of the polyelectrolytes themselves but also by the experimental conditions, mainly the ionic strength and the pH of the deposition solutions.
Besides its appealing simplicity, the LbL assembly is regarded as an extremely versatile bottom-up nanofabrication technique, since it allows the construction of PEMs films that have a great variety of architectures, by changing the types of polyelectrolyte components, the number of layers, and the layering sequence.73 The LbL process is viewed as being applicable to virtually any pair of synthetic polyelectrolytes or charged biopolymers such as ionic polysaccharides, proteins, enzymes, and nucleic acids. Indeed, all these classes of materials have been already successfully used in the construction of PEMs films.77,78 Already proven to be possible is the incorporation in PEMs films of charged nano-objects such as metal nanoparticles, carbon nanotubes, and clay platelets, among others.79,80 PEMs films can also be assembled on the surface of materials with various shapes and chemistries. Besides being applied to flat supports (glass, metals, and polymers), PEMs films have been used to coat the surface of a variety of device surfaces such as microneedles for transdermal drug delivery,78 stents77 and vascular prostheses,81,82 and even for cryopreserved arteries.83 Polyelectrolyte LbL assembly is also adaptable to the coating of particles of micro- or nano-dimensions.73
PEMs coatings have been extensively used to tailor the biological properties of biomedical devices surfaces. Surfaces that need to possess specific properties, such as antimicrobial surfaces or antithrombogenic surfaces, or those with decreased cell adhesion or enhanced cell adhesion can be constructed by the employing the appropriate combination of polyelectrolytes.84 Often, this meeting of desired characteristics is achieved by the use of natural biopolymers, such as ionic polysaccharides, proteins, and DNA. For example, sulfated polysaccharides, such as heparin and chondroitin sulfate, are frequently used as building blocks for the construction of PEMs coatings that exhibit antithrombogenic and endothelialization properties.85 Also, chitosan, due its inherent antimicrobial properties, is used to produce microbial-resistant LbL coatings.86
The biofunctionalization of surfaces, by PEMs coatings, can also be achieved by the immobilization of bioactive molecules with the structure of the PEMs and their subsequent localized release. In fact, the layer-by layer assembly of PEMs is regarded as being an excellent method for functionalizing surfaces with bioactive molecules without the risk of bioactivity loss. This is because PEMs can be formed in exclusively aqueous environments, at room temperature, and without the need for organic solvents or other aggressive chemicals. The immobilization of growth factors has been regarded with particular interest, since this protein controls the proliferation and differentiation of cells. Basic fibroblast growth factors (bFGFs), vascular endothelial growth factors (VEGFs), and bone morphogenetic proteins (BMPs) are examples of different classes of growth factor that have already been immobilized in LbL made PEMs coatings.87 For example, Hu et al.88 coated titanium substrates with chitosan/gelatin PEMs, with embedded BMP2 (a BMP which is shown to stimulate the production of bone) and fibronectin, demonstrating that this coating was osteoinductive and was able to promote osteogenesis, in vivo.
Conclusions
The surface modification of polymeric materials has been growing in the expansion of the range of biomaterials field over the recent past decades due to its ability to provide materials that have the required bulk properties for use in several biomedical applications, such as their use as catheters, artificial vein, endoprostheses, cardiac valves, and voice prosthesis. For these reasons, this technique is currently a very important issue.
Approaches to creating surface modification techniques that meet essential criteria are converging toward the ability to introduce desired functional groups without leading to irregular etching or the production of significant hazardous waste. However, these functional groups must be presented in as close to a monolayer as possible.
From all the methods available for surface modification, those based on photoactivation (UV and plasma) have been shown to be the simpler, faster, and more effective methods.
References
Atala, A, “Advances in Tissue and Organ Replacement.” Curr. Stem Cell Res. Ther., 3 (1) 21–31 (2008)
Jiao, Y-P, Cui, F-Z, “Surface Modification of Polyester Biomaterials for Tissue Engineering.” Biomed. Mater., 2 R24–R37 (2007)
Carre, A, Lacarriere, V, “How Substrate Properties Control Cell Adhesion. A Physical-Chemical Approach.” J. Adhes. Sci. Technol., 24 (5) 815–830 (2010)
Singh, M, Berkland, C, Detamore, MS, “Strategies and Applications for Incorporating Physical and Chemical Signal Gradients in Tissue Engineering.” Tissue Eng. B, 14 (4) 341–366 (2008)
Vadgama, P, “Surface Biocompatibility.” Annu. Rep. Prog. Chem. Sect. C, 101 14–52 (2005)
Furno, F, Bayston, R, “Antimicrobial Antibiotic Infection Resistance Materials.” In: Wnek, GE, Bowlin, GL (eds.) Encyclopedia of Biomaterials and Biomedical Engineering, pp. 61–70. Informa Healthcare, New York, 2008
Jung, SY, Lim, SM, Albertorio, F, Kim, G, Gurau, MC, Yang, RD, Holden, MA, Cremer, PS, “The Vroman Effect: A Molecular Level Description of Fibrinogen Displacement.” J. Am. Chem. Soc., 125 (42) 12782–12786 (2003)
Hirsh, SL, McKenzie, DR, Nosworthy, NJ, Denman, JA, Sezerman, OU, Bilek, MM, “The Vroman Effect: Competitive Protein Exchange with Dynamic Multilayer Protein Aggregates.” Colloids Surf. B, 103 395–404 (2013)
Palacio, MLB, Schricker, SR, Bhushan, B, “Bioadhesion of Various Proteins on Random, Diblock and Triblock Copolymer Surfaces and the Effect of ph Conditions.” J. R. Soc. Interface, 8 (58) 630–640 (2011)
Chang, H-I, Wang, Y, “Cell Responses to Surface and Architecture of Tissue Engineering Scaffolds.” In: Eberli, E (ed.) Regenerative Medicine and Tissue Engineering—Cells and Biomaterials, pp. 569–588. InTech, Rijeka, 2011
Andrade, JD, Hlady, V, “Protein Adsorption and Materials Biocompatibility—A Tutorial Review and Suggested Hypotheses.” Adv. Polym. Sci., 79 1–63 (1986)
Qi, P, Maitz, MF, Huang, N, “Surface Modification of Cardiovascular Materials and Implants.” Surf. Coat. Technol., 233 80–90 (2013)
Greenwood, JA, Theibert, AB, Prestwich, GD, Murphy-Ullrich, JE, “Restructuring of Focal Adhesion Plaques by PI 3-kinase: Regulation by Ptdins (3,4,5)-p-3 Binding to Alpha-Actinin.” J. Cell Biol., 150 (3) 627–641 (2000)
Zhanhua, C, Gan, JG-K, Lei, L, Sakharkar, MK, Kangueane, P, “Protein Subunit Interfaces: Heterodimers Versus Homodimers.” Bioinformation, 1 (2) 28–39 (2005)
Brizzi, MF, Tarone, G, Defilippi, P, “Extracellular Matrix, Integrins, and Growth Factors as Tailors of the Stem Cell Niche.” Curr. Opin. Cell Biol., 24 (5) 645–651 (2012)
Calderwood, DA, Shattil, SJ, Ginsberg, MH, “Integrins and Actin Filaments: Reciprocal Regulation of Cell Adhesion and Signaling.” J. Biol. Chem., 275 22607–22610 (2000)
Xiong, JP, Goodman, SL, Arnaout, MA, “Purification, Analysis, and Crystal Structure of Integrins.” Methods Enzymol., 426 307–336 (2007)
Murphy-Ullrich, JE, “The De-adhesive Activity of Matricellular Proteins: Is Intermediate Cell Adhesion an Adaptive State?” J. Clin. Invest., 107 (7) 785–790 (2001)
Ali, O, Guillou, H, Destaing, O, Albigès-Rizo, C, Block, MR, Fourcade, B, “Cooperativity Between Integrin Activation and Mechanical Stress Leads to Integrin Clustering.” Biophys. J., 100 (11) 2595–2604 (2011)
Katsikogianni, M, Missirlis, YF, “Concise Review of Mechanisms of Bacterial Adhesion to Biomaterials and of Techniques Used in Estimating Bacteria–Material Interactions.” Eur. Cells Mater., 8 37–57 (2004)
Lasa, I, “Towards the Identification of the Common Features of Bacterial Biofilm Development.” Int. Microbiol., 9 (1) 21–28 (2006)
Goddard, JM, Hotchkiss, JH, “Polymer Surface Modification for the Attachment of Bioactive Compounds.” Prog. Polym. Sci., 32 (7) 698–725 (2007)
Hu, SW, Ren, XQ, Bachman, M, Sims, CE, Li, GP, Allbritton, NL, “Tailoring the Surface Properties of Poly(dimethylsiloxane) Microfluidic Devices.” Langmuir, 20 (13) 5569–5574 (2004)
McArthur, SL, McLean, KM, “Surface Modification.” In: Wnek, GE, Bowlin, GL (eds.) Encyclopedia of Biomaterials and Biomedical Engineering, pp. 2540–2550. Informa Healthcare, New York, 2008
Ozdemir, M, Yurteri, CU, Sadikoglu, H, “Physical Polymer Surface Modification Methods and Applications in Food Packaging Polymers.” Crit. Rev. Food Sci., 39 (5) 457–477 (1999)
Bosco, R, Beucken, JVD, Leeuwenburgh, S, Jansen, J, “Surface Engineering for Bone Implants: A Trend from Passive to Active Surfaces.” Coatings, 2 95–119 (2012)
Jia, Z, Du, S, Tian, G, “Surface Modification of Acrylic Fiber by Grafting of Casein.” J. Macromol. Sci. A, 44 (3) 299–304 (2007)
Song, YQ, Sheng, J, Wei, M, Yuan, XB, “Surface Modification of Polysulfone Membranes by Low-Temperature Plasma-Graft Poly(ethylene glycol) onto Polysulfone Membranes.” J. Appl. Polym. Sci., 78 (5) 979–985 (2000)
Suzuki, M, Kishida, A, Iwata, H, Ikada, Y, “Graft-Copolymerization of Acrylamide onto a Polyethylene Surface Pretreated with a Glow-Discharge.” Macromolecules, 19 (7) 1804–1808 (1986)
Friedrich, J, The Plasma Chemistry of Polymer Surfaces: Advanced Techniques for Surface Design, pp. 294–298. Wiley-VCH, Germany, 2012
Lei, JX, Liao, X, “Surface Graft Copolymerization of Acrylic Acid onto LDPE Film Through Corona Discharge.” Eur. Polym. J., 37 (4) 771–779 (2001)
Lei, JX, Shi, MW, Zhang, JC, “Surface Graft Copolymerization of Hydrogen Silicone Fluid onto Fabric through Corona Discharge and Water Repellency of Grafted Fabric.” Eur. Polym. J., 36 (6) 1277–1281 (2000)
Kim, MS, Khang, G, Lee, HB, “Gradient Polymer Surfaces for Biomedical Applications.” Prog. Polym. Sci., 33 138–164 (2008)
Bucio, E, Arenas, E, Burillo, G, “Radiation Grafting of n-Isopropylacrylamide onto Polypropylene Films by Preirradiation Method.” Mol. Cryst. Liquid Cryst., 447 521–531 (2006)
Mok, S, Worsfold, DJ, Fouda, A, Matsuura, T, “Surface Modification of Polyethersulfone Hollow-Fiber Membranes by Gamma-Ray Irradiation.” J. Appl. Polym. Sci., 51 (1) 193–199 (1994)
Alvarez-Lorenzo, C, Bucio, E, Burillo, G, Concheiro, A, “Medical Devices Modified at the Surface by Gamma-Ray Grafting for Drug Loading and Delivery.” Expert Opin. Drug Deliv., 7 (2) 173–185 (2010)
Deng, J, Wang, L, Liu, L, Yang, W, “Developments and New Applications of UV-Induced Surface Graft Polymerizations.” Prog. Polym. Sci., 34 (2) 156–193 (2009)
Ebara, M, Hoffman, JM, Stayton, PS, Hoffman, AS, “Surface Modification of Microfluidic Channels by UV-Mediated Graft Polymerization of Non-Fouling and ‘Smart’ Polymers.” Radiat. Phys. Chem., 76 (8–9) 1409–1413 (2007)
Guan, JJ, Gao, CY, Feng, LX, Shen, JC, “Surface Modification of Polyurethane for Promotion of Cell Adhesion and Growth 1: Surface Photo-Grafting with N,N-Dimethylaminoethyl Methacrylate and Cytocompatibility of the Modified Surface.” J. Mater. Sci., 128 (5) 447–452 (2001)
Masson, F, Decker, C, Andre, S, Andrieu, X, “UV-Curable Formulations for UV-Transparent Optical Fiber Coatings I. Acrylic Resins.” Progr. Org. Coat., 49 (1) 1–12 (2004)
Williams, CG, Malik, AN, Kim, TK, Manson, PN, Elisseeff, JH, “Variable Cytocompatibility of Six Cell Lines with Photoinitiators Used for Polymerizing Hydrogels and Cell Encapsulation.” Biomaterials, 26 (11) 1211–1218 (2005)
Hipler, UC, Elsner, P, Fluhr, JW, “Antifungal and Antibacterial Properties of a Silver-Loaded Cellulosic Fiber.” J. Biomed. Mater. Res. B, 77B 156–163 (2006)
Jayakumar, R, Nwe, N, Tokura, S, Tamura, H, “Sulfated Chitin and Chitosan as Novel Biomaterials.” Int. J. Biol. Macromol., 40 (3) 175–181 (2007)
Pinto, S, Alves, P, Santos, AC, Matos, CM, Oliveiros, B, Goncalves, S, Gudina, E, Rodrigues, LR, Teixeira, JÁ, Gil, MH, “Poly(dimethyl siloxane) Surface Modification with Biosurfactants Isolated from Probiotic Strains.” J. Biomed. Mater. Res. A, 98A (4) 535–543 (2011)
Thom, VH, Altankov, G, Groth, T, Jankova, K, Jonsson, G, Ulbricht, M, “Optimizing Cell-Surface Interactions by Photografting of Poly(ethylene glycol).” Langmuir, 16 (6) 2756–2765 (2000)
Janorkar, AV, Fritz, EW, Jr, Burg, KJL, Metters, AT, Hirt, DE, “Grafting Amine-Terminated Branched Architectures from Poly(l-lactide) Film Surfaces for Improved Cell Attachment.” J. Biomed. Mater. Res. B, 81B (1) 142–152 (2007)
Cheung, HK, Han, TT, Marecak, DM, Watkins, JF, Amsden, BG, Flynn, LE, “Composite Hydrogel Scaffolds Incorporating Decellularized Adipose Tissue for Soft Tissue Engineering with Adipose-Derived Stem Cells.” Biomaterials, 35 (6) 1914–1923 (2014)
Alves, P, Pinto, S, Kaiser, J-P, Bruinink, A, de Sousa, HC, Gil, MH, “Surface Grafting of a Thermoplastic Polyurethane with Methacrylic Acid by Previous Plasma Surface Activation and by Ultraviolet Irradiation to Reduce Cell Adhesion.” Colloid. Surf. B, 82 (2) 371–377 (2011)
Alves, P, Pinto, S, Ferreira, P, Kaiser, J-P, Bruinink, A, Sousa, HC, Gil, MH, “Improving Cell Adhesion: Development of a Biosensor for Cell Behavior Monitoring by Surface Grafting of Sulfonic Groups onto a Thermoplastic Polyurethane.” J. Mater. Sci., 25 (8) 2017–2026 (2014). doi:10.1007/s10856-014-5233-1
Alves, P, Ferreira, P, Kaiser, J-P, Salk, N, Bruinink, A, Sousa, HC, Gil, MH, “Surface Grafting of Carboxylic Groups onto Thermoplastic Polyurethanes to Reduce Cell Adhesion.” Appl. Surf. Sci., 283 744–750 (2013)
Alves, P, Cardoso, R, Correia, TR, Antunes, BP, Correia, IJ, Ferreira, P, “Surface Modification of Polyurethane Films by Plasma and Ultraviolet Light to Improve Haemocompatibility for Artificial Heart Valves.” Colloid. Surf. B, 113 25–32 (2014)
Gomathi, N, Sureshkumar, A, Neogi, S, “RF Plasma-Treated Polymers for Biomedical Applications.” Curr. Sci. India, 94 (11) 1478–1486 (2008)
Biederman, H, Slavinska, D, “Plasma Polymer Films and Their Future Prospects.” Surf. Coat. Technol., 125 (1–3) 371–376 (2000)
Petersen, J, Michel, M, Toniazzo, V, Ruch, D, Schmerber, G, Ihiawakrim, D, Muller, D, Dinia, A, Ball, V, “Atmospheric Plasma Polymer Films as Templates for Inorganic Synthesis to Yield Functional Hybrid Coatings.” RSC Adv., 2 9860–9866 (2012)
Chu, PK, Chen, JY, Wang, LP, Huang, N, “Plasma-Surface Modification of Biomaterials.” Mater. Sci. Eng. R, 36 (5–6) 143–206 (2002)
Inagaki, N, Plasma Surface Modification and Plasma Polymerization. Technomic Publishing, Lancaster, 1996
Pinto, S, Alves, P, Matos, CM, Santos, AC, Rodrigues, LR, Teixeira, JA, Gil, MH, “Poly(dimethyl siloxane) Surface Modification by Low Pressure Plasma to Improve Its Characteristics Towards Biomedical Applications.” Colloid. Surf. B, 81 (1) 20–26 (2010)
Ferreira, P, Carvalho, A, Correia, TR, Antunes, BP, Correia, IJ, Alves, P, “Functionalization of Polydimethylsiloxane Membranes to be Used in the Production of Voice Prosthesis.” Sci. Technol. Adv. Mater., 14 1–8 (2013)
Vilani, C, Weibel, DE, Zamora, RRM, Habert, AC, Achete, CA, “Study of the Influence of the Acrylic Acid Plasma Parameters on Silicon and Polyurethane Substrates Using XPS and AFM.” Appl. Surf. Sci., 254 (1) 131–134 (2007)
Lee, HB, Khang, G, Lee, JH, “Polymeric Biomaterials.” In: Park, JB, Bronzino, JD (eds.) Biomaterials: Principles and Applications, pp. 55–78. CRC Press, Boca Raton, 2003
Ohya, Y, Matsunami, H, Yamabe, E, Ouchi, T, “Cell Attachment and Growth on Films Prepared from Poly(depsipeptide-co-lactide) Having Various Functional Groups.” J. Biomed. Mater. Res. A, 65A (1) 79–88 (2003)
Wan, YQ, Yang, J, Yang, JL, Bei, JZ, Wang, SG, “Cell Adhesion on Gaseous Plasma Modified Poly-(l-lactide) Surface Under Shear Stress Field.” Biomaterials, 24 (21) 3757–3764 (2003)
Garcia, AJ, Vega, MD, Boettiger, D, “Fibronectin Coating of Oxygenator Membranes Enhances Endothelial Cell Attachment.” BioMed. Eng. OnLine, 12 1–8 (2013)
Lee, I-C, Young, T-H, “Preparation of PLLA Membranes with Different Morphologies for Culture of Ligament Cells.” Biomed. Eng., 18 (4) 185–189 (2006)
Franck, D, Gil, ES, Adam, RM, Kaplan, DL, Chung, YG, Estrada, CR, Mauney, JR, “Evaluation of Silk Biomaterials in Combination with Extracellular Matrix Coatings for Bladder Tissue Engineering with Primary and Pluripotent Cells.” PLoS ONE, 8 (2) e56237 (2013)
Shin, H, Jo, S, Mikos, AG, “Biomimetic Materials for Tissue Engineering.” Biomaterials, 24 (24) 4353–4364 (2003)
Armstrong, J, Salacinski, HJ, Mu, QS, Seifalian, AM, Peel, L, Freeman, N, Holt, CM, Lu, JR, “Interfacial Adsorption of Fibrinogen and Its Inhibition by RGD Peptide: A Combined Physical Study.” J. Phys. Condens. Matter., 16 (26) S2483–S2491 (2004)
Maalej, N, Albrecht, R, Loscalzo, J, Folts, JD, “The Potent Platelet Inhibitory Effects of S-Nitrosated Albumin Coating of Artificial Surfaces.” J. Am. Coll. Cardiol., 33 (5) 1408–1414 (1999)
Sivaraman, B, Latour, RA, “The Adherence of Platelets to Adsorbed Albumin by Receptor-Mediated Recognition of Binding Sites Exposed by Adsorption-Induced Unfolding.” Biomaterials, 31 (6) 1036–1044 (2010)
Harnett, EM, Alderman, J, Wood, T, “The Surface Energy of Various Biomaterials Coated with Adhesion Molecules Used in Cell Culture.” Colloid. Surf. B, 55 (1) 90–97 (2007)
Decher, G, Hong, JD, Schmitt, J, “Buildup of Ultrathin Multilayer Films by a Self-Assembly Process. 3. Consecutively Alternating Adsorption of Anionic and Cationic Polyelectrolytes on Charged Surfaces.” Thin Solid Films, 210 (1–2) 831–835 (1992)
Xiang, Y, Lua, S, Jiang, SP, “Layer-by-Layer Self-Assembly in the Development of Electrochemical Energy Conversion and Storage Devices from Fuel Cells to Supercapacitors.” Chem. Soc. Rev., 41 7291–7321 (2012)
Ariga, K, Hill, JP, Ji, Q, “Layer-by-Layer Assembly as a Versatile Bottom-up Nanofabrication Technique for Exploratory Research and Realistic Application.” Phys. Chem. Chem. Phys., 9 (19) 2319–2340 (2007)
Klitzing, R, “Internal Structure of Polyelectrolyte Multilayer Assemblies.” Phys. Chem. Chem. Phys., 8 (43) 5012–5033 (2006)
Chiarelli, PA, Johal, MS, Holmes, DJ, Casson, JL, Robinson, JM, Wang, H-L, “Polyelectrolyte Spin-Assembly.” Langmuir, 18 (1) 168–173 (2001)
Krogman, KC, Zacharia, NS, Schroeder, S, Hammond, PT, “Automated Process for Improved Uniformity and Versatility of Layer-by-Layer Deposition.” Langmuir, 23 (6) 3137–3141 (2007)
Jewell, CM, Zhang, J, Fredin, NJ, Wolff, MR, Hacker, TA, Lynn, DM, “Release of Plasmid DNA from Intravascular Stents Coated with Ultrathin Multilayered Polyelectrolyte Films.” Biomacromolecules, 7 (9) 2483–2491 (2006)
Saurer, EM, Flessner, RM, Sullivan, SP, Prausnitz, MR, Lynn, DM, “Layer-by-Layer Assembly of DNA- and Protein-Containing Films on Microneedles for Drug Delivery to the Skin.” Biomacromolecules, 11 (11) 3136–3143 (2010)
Podsiadlo, P, Shim, BS, Kotov, NA, “Polymer/Clay and Polymer/Carbon Nanotube Hybrid Organic–Inorganic Multilayered Composites Made by Sequential Layering of Nanometer Scale Films.” Coord. Chem. Rev., 253 (23–24) 2835–2851 (2009)
Li, W, Xu, D, Hu, Y, Cai, K, Lin, Y, “Surface Modification of Titanium Substrates with Silver Nanoparticles Embedded Sulfhydrylated Chitosan/Gelatin Polyelectrolyte Multilayer Films for Antibacterial Application.” J. Mater. Sci. Mater. Med., 25 (6) 1435–1448 (2006)
Brynda, E, Houska, M, Jiroušková, M, Dyr, JE, “Albumin and Heparin Multilayer Coatings for Blood-Contacting Medical Devices.” J. Biomed. Mater. Res., 51 (2) 249–257 (2000)
Paternotte, E, Kerdjoudj, H, Kokten, T, Stoltz, JF, Kearney-Schwartz, A, Voegel, JC, Menu, P, “Endothelialized and Preconditioned Natural Umbilical Arteries with Long Term Patency Open the Route for Future Human Uses.” Clin. Hemorheol. Microcirc., 54 (3) 223–234 (2013)
Kerdjoudj, H, Boura, C, Moby, V, Montagne, K, Schaaf, P, Voegel, JC, Stoltz, JF, Menu, P, “Re-endothelialization of Human Umbilical Arteries Treated with Polyelectrolyte Multilayers: A Tool for Damaged Vessel Replacement.” Adv. Funct. Mater., 17 (15) 2667–2673 (2007)
Boudou, T, Crouzier, T, Ren, K, Blin, G, Picart, C, “Multiple Functionalities of Polyelectrolyte Multilayer Films: New Biomedical Applications.” Adv. Mater., 22 (4) 441–467 (2010)
Meng, S, Liu, ZJ, Shen, L, Guo, Z, Chou, LSL, Zhong, W, Du, QG, Ge, J, “The Effect of a Layer-by-Layer Chitosan–Heparin Coating on the Endothelialization and Coagulation Properties of a Coronary Stent System.” Biomaterials, 30 (12) 2276–2283 (2009)
Zhou, B, Hu, Y, Jing, L, Bin, L, “Chitosan/Phosvitin Antibacterial Films Fabricated Via Layer-by-Layer Deposition.” Int. J. Biol. Macromol., 64 402–408 (2014)
Gribova, V, Auzely-Velty, R, Picart, C, “Polyelectrolyte Multilayers Assemblies in Materials Surfaces: From Cell Adhesion to Tissue Engineering.” Chem. Mater., 24 (5) 854–869 (2012)
Hu, Y, Cai, K, Luo, Z, Zhang, Y, Li, L, Lai, M, Hou, Y, Huang, Y, Li, J, Ding, X, et al., “Regulation of the Differentiation of Mesenchymal Stem Cells In Vitro and Osteogenesis In Vivo by Microenvironmental Modification of Titanium Alloy Surfaces.” Biomaterials, 33 (13) 3515–3528 (2012)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferreira, P., Alves, P., Coimbra, P. et al. Improving polymeric surfaces for biomedical applications: a review. J Coat Technol Res 12, 463–475 (2015). https://doi.org/10.1007/s11998-015-9658-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-015-9658-3