Abstract
Milk fat globule membrane (MFGM) is a lipidic tri-layer structure rich in polar lipids and glycoproteins with promising health and technological benefits, which can be isolated from dairy industry by-products. The present research aims to understand the effect of the addition of an enriched MFGM concentrate on the milk coagulation process and on the structural behavior of ultrafiltered fresh cheeses (UFC). To this end, goat milk was ultrafiltered at 3 protein levels (10, 12, and 14%) and three MFGM contents were evaluated (0, 0.4, and 0.8%). UFC batches were manufactured and characterized using rheology. Coagulation kinetics revealed that ultrafiltration and MFGM addition increased the firmness of the cheese gel. Protein content was the primary factor contributing to gel firmness, and among the mixtures tested, those containing 14% proteins exhibited the highest elastic modulus values. Dynamic and static shear analyses showed that the combination of ultrafiltration and enriched MFGM concentrate supplementation had a synergic effect on cheese viscoelastic behavior. Loss factor (tan(δ)) may be a marker that can be monitored in cheesemaking when adding MFGM concentrate. Overall, MFGM concentrate would improve the rheological characteristics of fresh cheeses, being a promising ingredient for the food industry.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Milk is a complex food that furnishes a broad range of nutritionally relevant compounds, which have physiological, functional, and technological properties (Haug et al., 2007). In recent years, there has been a significant increase in the consumption of goat cheese in Spain, thereby establishing goat’s milk as a prominent raw material for the production of dairy products (MAPA, 2023). In addition, goat’s milk has been recently reported to have health-promoting benefits such as immunomodulatory, anti-inflamatory, or antioxidant effects (ALKaisy et al., 2023). Ultrafiltration (UF) of milk is a widespread procedure that consists of tangential filtration used mainly to concentrate caseins (CN) and standardize protein and fat contents in dairy products (Gavazzi-April et al., 2018). It is commonly used to obtain specific bioactive compounds of dairy origin, depending on the pore size of the membrane (Pouliot, 2008), such as whey proteins (WP) from cheese whey (Baldasso et al., 2011) or milk fat globule membrane (MFGM) from buttermilk (Rezende Costa et al., 2010; Señoráns et al., 2023). There are multiple advantages in using UF for cheesemaking, such as improving yield and efficiency and controlling cheese characteristics, texture, and quality consistency (Banks, 2007). A wide variety of cheeses (mozzarella, cheddar or Burgos-type fresh cheeses, among others) incorporate UF in their manufacture process, leading to a significant increase of their protein content (Covacevich & Kosikowski, 1978; Hueso et al., 2023). This processing technology has a long-lasting effect on the ability of CN to coagulate after rennet, due to induced calcium losses within the internal micelle structure of CN, and produces gels with lower stiffness (Ferrer et al., 2011). Subsequently, cheese characteristics change significantly, mainly the texture and microstructure. The hardness of the cheese increases and the cheese matrix becomes more compact than non-UF cheeses (Deshwal et al., 2020; Ong et al., 2013).
Enhancing the technological and nutritional quality of cheese by the incorporation of bioactive ingredients from dairy by-products has proven to be a promising strategy (Nastaj et al., 2020; Picciotti et al., 2022). Milk proteins, including CN, WP, and milk fat globule membrane proteins (MFGMP), have multiple described health benefits including antioxidant, antihypertensive, immunomodulation, and promoting muscle synthesis (Abd El-Salam & El-Shibiny, 2020; Petrotos et al., 2014). Regarding MFGM, it has been associated to multiple health benefits, especially on preserving cognitive function and the enhancement of immune system and cardiometabolic health (Calvo et al., 2023; Fontecha et al., 2020). Its positive effects would not only be associated with the presence of bioactive lipids or proteins but also with MFGM complex tridimensional structure (Lee et al., 2018). Although previous studies have investigated the addition of WP or buttermilk to different types of cheeses (Danesh et al., 2018; Govindasamy-Lucey et al., 2007), to our knowledge, there is no available information on the effects of adding MFGM to ultrafiltered fresh cheeses (UFC).
Cheese is a hydrocolloidal gel composed of a CN polymeric matrix that collapses with time, releasing whey from its interior in a process called syneresis (Sharma, 2022). The viscoelastic properties of milk are generally characterized by rheology during the cheesemaking process (Karami et al., 2008). The small amplitude oscillatory shear analysis is a useful tool to understand cheese structure and mechanical responses to external forces. These findings can subsequently be correlated with textural and sensory analyses (Drake et al., 1999; Peleg, 2006). On the other hand, creep-recovery assay provides complementary information to evaluate cheese internal structure and its structural variations associated with composition changes (Sharma et al., 2017). Rheology is also useful to study the kinetics of polymerization reactions in food materials (Felfoul et al., 2023). Concerning cheese production, rheology has been previously used to assess the coagulation kinetics when adding new ingredients or replacing milk fat (Arango et al., 2015; Fagan et al., 2006), and even to evaluate the addition of milk subproducts such as buttermilk (Hickey et al., 2018).
The main objective of the present research is to study the combined effect of different protein levels and the addition of an enriched MFGM concentrate on milk coagulation, as well as on the firmness, consistency, and rheological properties of UFC. Milk ultrafiltration was performed to concentrate milk total solids, retaining CN and WP, while coagulation and kinetic studies were carried out to evaluate the impact of incorporating different levels of MFGM concentrate on the cheese matrix. The described procedure can be scaled up and would be of interest to the dairy industry, since it not only allows for the recovery of WPs, but it also incorporates an enriched MFGM fraction obtained from buttermilk by-product with technological, nutritional, and health benefits (Calvo et al., 2020). To the best of our knowledge, this is the first study that evaluates the production of goat fresh cheeses using ultrafiltered milk combined with the incorporation of an enriched MFGM concentrate.
Materials and Methods
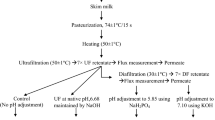
Skimming, Ultrafiltration, and Preparation of Ultrafiltered Milk Blends (UFMB)
Raw goat’s milk (500 L) was heated at 50 °C and skimmed by centrifugation (GEA Westfalia separator, Oelde, Germany). UF of skimmed milks was carried out in an UF pilot plant (Perinox, Villarrobledo, Spain) using 10 kDa ABCOR® 3838 HFK-FYT (Koch separation solutions, Wilmington, MA, USA) spiral membranes at 40 °C and a constant transmembrane pressure of 3 bar. UF cycles were performed until retentate protein content was around 16%, which was determined in situ using a MilkoScan FT+ (FOSS, Hilleroed, Denmark). Cream, UF skimmed milks, and permeates were stored at 4 °C until the mixtures were prepared.
UFMB were prepared using cream and permeate to adjust fat and protein contents. Fat content was set at 5% and the mixtures were prepared at 3 levels of protein: 10, 12, and 14%. Blends (final volume = 30 L) were shaken to properly mix the ingredients and pasteurized at 72 °C for 20 s in a pasteurization pilot plant equipment (Perinox, Spain). Then, sample blends were cooled and stored at 4 °C. The 3 starting mixtures (10, 12, and 14% of protein) were divided into 2 kg batches and heated before coagulation studies to achieve a constant temperature of 40 °C.
The MFGM concentrate powder used in the present investigation was previously obtained by UF of cow buttermilk, which was described by Baliyan et al. (2023). Briefly, buttermilk was initially concentrated by UF, followed by successive cycles of diafiltration to remove WP and lactose from the retentate. The resulting concentrate was then lyophilized and ground into a fine powder. Total dry matter of the enriched MFGM concentrate was 91.17 ± 0.06 g/100 g, and its composition was 67.13 ± 1.12 g of protein, 11.61 ± 0.97 g of fat, and 6.40 ± 0.90 g of lactose per 100 g. Protein and lipid profiles of MFGM concentrate are displayed in Fig. 1 and Online Resource 1. The enriched MFGM concentrate was added to each UFMB (10, 12, and 14% of protein) at ratios 0, 0.4, and 0.8% w/w, and stirred at 40 °C for 5 min. Homogenization of blends after MFGM concentrate addition was done in a two-stage homogenizer (Niro Soavi, Parma, Italy) at 40 °C, with a pressure of 90 and 30 bar at each stage. As a result, 9 UFC batches were obtained: 3 control batches without MFGM (one for each protein content, 10M0, 12M0, and 14M0), 3 batches with 0.4% of MFGM (10M1, 12M1, and 14M1), and 3 batches with 0.8% of MFGM (10M2, 12M2, and 14M2).
Coagulation Kinetics and Cheese Manufacture
Coagulation kinetics and cheese manufacture were carried out simultaneously using 2 L batches of the 9 UFMB, using chymosin (Chymax M 200IMCU/mL, Chr Hansen, Hørsholm, Denmark) at a concentration of 175 IMCU/L. After rennet addition continuously stirring, 18 mL of the mixture was used for coagulation studies and the remaining UFMB with added coagulant were poured into individual serving size plastic containers and placed in a water bath at 40 °C. Approximately 30 cheeses (60 g each) were obtained for each experimental group and cheeses were stored at 4 °C until analysis.
Coagulation kinetics were studied by placing the 18 mL into a cup/cylinder CC27/T200/AL system at a controlled temperature of 40 °C and coagulation tests were carried out within the viscoelastic region in a rheometer (MCR 301, Anton Paar, Graz, Austria). Strain and frequency were applied consistently at 0.5% and 1 Hz, respectively, for the monitoring of storage and loss moduli (G′, G″) during 30 min. The firmness curve (G′ vs. time) and its first derivative were analyzed to determine milk coagulation properties, such as rennet coagulation time (RCT) and curd firming rate (CFR) using Rheoplus 3.62 software (Anton Paar, Graz, Austria). The greatest differences between UFMB were observed at the end of coagulation kinetics, thus G′ values at 24.5 min were analyzed in triplicate to determine significance.
As the coagulation kinetics curve progressed, two cheeses were removed from the water bath at 50, 300, 600, and 900 Pa (G′ values) and stored at 4 °C to stop the reaction. This protocol was carried out to assess whether the coagulation firmness, at which incubation was stopped, was a relevant factor for water-holding capacity (WHC) and firmness (G′) of the cheese gel. The remaining cheeses in the water bath were taken out after 10 min of incubation and stored at 4 °C.
Compositional Analysis of UFMB
General Composition
Total fat, protein, dry matter, lactosem and urea of milk blends were analyzed using a MilkoScan FT+ (FOSS, Hilleroed, Denmark).
Protein Identification by SDS-PAGE
The protein profile of both the MFGM concentrate and the UFMB—without (10M0, 12M0, and 14M0) and with 0.8% of MFGM (10M2, 12M2, and 14M2)—was assessed by means of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using a 12% Bis-Trispolyacrilamide gel (Criterion_XT, Bio-Rad) (Señoráns et al., 2023). Samples were previously standardized according to the protein content (4 mg/mL) and then dissolved in sample buffer (4× XT Sample Buffer, Bio-Rad, Hercules, California, USA) containing 5 mL/100 mL of Tris (2-carboxyethyl) phosphine hydrochloride (TCEP.HCl) until a final concentration of 0.80 mg/mL. Electrophoresis was conducted for 1 h at a constant voltage of 150 V. Subsequently, gels were stained with a dye (Bio-Safe™ Coomassie G-250, Bio-Rad) and scanned with a Versa-Doc image system (Bio-Rad). Finally, the densitometric analysis of the protein bands was carried out using ImageJ software program.
Cheese Characterization
Water-Holding Capacity and Moisture Content
WHC was determined in duplicate at each G′ value (50, 300, 600, and 900 Pa) following (Muñoz et al., 2023). Samples were centrifuged for 10 min at 4000 × g at 5 °C and weighted before and after centrifugation. WHC was determined with the formula proposed by Nassar et al. (2020):
where WDG, weight of drained gel; WS, weight of milk gel sample.
Moisture content was calculated by weight difference following the ISO reference method (2004). Briefly, 5 g of well-homogenized fresh cheese was introduced in a porcelain capsule previously tared. Then, samples were heated in an oven at 103 °C until weighting values were constant using an analytical balance. The weight difference between the sample before and after desiccation represents the moisture content of the cheese sample.
Cheese Texture Analyses
Texture determination of firmness and consistency were carried out in a texturometer (TA-XT2 Aname, Madrid, Spain), following the method previously described in Asensio-Vegas et al. (2018) with a modification for curd. The test was performed by compressing/decompressing of cheese samples (45 mm diameter and 40 mm height) with a speed of 2 m/s and 15 mm of penetration with a cylindrical probe (P/25a, Aname, Quijorna, Spain). Firmness was calculated as the maximum force of penetration and consistency as the area under the force vs. distance curve.
Dynamic Rheology Analysis
Small amplitude oscillatory shear analysis was performed at 8 °C using a TA Instruments Discovery HR 10 hybrid rheometer (Waters TA Instruments, Milford, MA, USA) following Hueso et al. (2023). Rheological analyses were performed using a parallel stainless-steel plate geometry (20 mm diameter, 0.5 cm gap). Cheeses were cut with a copper cylinder (20 mm diameter) and the resulting cheese cylinder was divided with a parallel blade guillotine with a separation of 0.5 cm. The slices were deposited on the lower plate, previously covered with an attached thin sandpaper to avoid sample slip. Then, the upper plate approached the surface of the sample until a minimum contact without significant force was applied (≤ 0.3 N). The linear viscoelastic region was determined via a stress sweep at a fixed frequency of 1 Hz for each sample. Afterwards, a frequency sweep from 0 to 100 rad/s was performed at a constant strain selected within the linear viscoelastic region of all samples, so that the stress and the strain were directly proportional. The oscillation strain was set at 0.5% in the frequency weeps, since the viscoelastic region was linear at that value for all cheese samples analyzed.
Dynamic rheological properties (elastic or storage modulus, G′; viscous or loss modulus, G″; complex modulus, G*; and loss factor, tan(δ)) were determined in quintuplicate for each sample using TRIOS software (TA Instruments, USA) as described in Hueso et al. (2023). A frequency of 1 Hz was selected to determine the mean values of tan(δ). Data were recorded and fitted to the power-law model:
where K was referred to as G′0, G″0, and G*0 at an angular frequency (ω) of 1 rad/s, and n′, n″, and n* denoted the rate of increase of G′, G″, and G* with increasing ω. G′0, G″0, and G*0 were correlated to cheese gel strength, stiffness, and rigidity as described (Peleg, 2006).
Static Rheology Analysis
The creep-recovery test was conducted using the same Discovery HR 10 hybrid rheometer (TA Instruments, New Castle, DE, USA) to acquire information about the cheese deformability and elasticity. The cheese slices were tested at 8 °C for 270 s in total, in which 90 s was for creep (the increase in deformation or strain as a function of time) and 180 s for recovery (the elastic recovery when the load is removed). The ratio of the strain at time t to the constant stress applied in the creep and recovery phases, i.e., compliance (J(t)) and recoverable compliance (JR(t)), respectively, was recorded at 80 s and 100 s, respectively, being the % strain plotted as a function of time, with an initial applied stress of 150 Pa. Stress was selected by preliminary trials to ensure proper compression of samples without signs of breakdown. All static rheological properties were determined in quintuplicate.
Statistical Analyses
Statistical analyses were performed with XLSTAT Premium, version 2018.5.53172 (Addinsoft, Paris, France). Paired comparisons, using a two-way ANOVA, were used to determine differences between the 9 types of cheeses (i.e., composition, texture, rheology, and technological properties). Differences were considered statistically significant at P < 0.05.
An exploratory analysis of the data was performed by principal component analysis (PCA) to identify patterns and search the best separation between groups. A direct visual screen test performed on the eigenvalue distribution showed that two factors were sufficient with 21 selected variables (urea, G′0, G″0, G*0, consistency, firmness, G′15, G″15, CFR, protein, dry matter, WHC, fat, lactose, RCT, t300, t900, J(t), JR(t), fat:protein (F:P) ratio, and tan(δ)). A heatmap was also performed to better understand the relationship between different cheese composition and dependent variables.
Results and Discussion
General Composition of UFMB
Table 1 displays the composition of the 9 UFMB under evaluation. Samples were formulated to differ in protein content, while the fat level was fixed at 5%. As expected, dry matter content primarily increased in correlation with higher protein levels (Table 1), with a secondary but noticeable effect attributed to the addition of MFGM concentrate. These outcomes are consistent with the composition of this ingredient, which is a rich source of proteins (≈ 70%) and polar lipids (≈ 25%) (Baliyan et al., 2023; Señoráns et al., 2023). Moisture content decreased with increasing protein and MFGM concentrate levels, with values ranging from 79.27 g/100 g (10M0) to 74.50 g/100 g (14M2). Lactose content diminished in UFMB with high protein levels, but a slight increase was observed when adding the MFGM concentrate (Table 1).
The SDS-PAGE protein profiles of the MFGM isolate and UFMB without (10M0, 12M0, and 14M0) and with 0.8% of MFGM (10M2, 12M2, and 14M2) are depicted in Fig. 1. Notably, our MFGM concentrate exhibited a protein profile similar to that of previous MFGM obtained by microfiltration (Le et al., 2011) and other valuable sources of MFGM such as by-products of the dairy industry (Señoráns et al., 2023). In fact, it showed a significant presence of high molecular weight membrane proteins (45–200 kDa), alongside with a minimal number of CNs (≈ 25 kDa) and WPs such as α-lactalbumin (14.2 kDa) and β-lactoglobulin (18.3 kDa) (Fig. 1). On the other hand, the UFMB had a similar protein profile with an average concentration of (12.05–14.09) % for MFGM proteins, (64.82–70.68) % for CN, and (16.34–18.64) % for WPs.
Additionally, the densitometric analysis of protein bands illustrated in Fig. 1 revealed variations in MFGM protein concentration among the UFMB. As depicted in Online Resource 2, this analysis highlighted that the addition of the MFGM isolate led to significant variations only in the concentration of xanthine oxidase (XO/XDH) and adipophilin (ADPH), with M2 samples exhibiting a twofold increase compared to the corresponding M0 counterparts. These findings hold significant promise, particularly considering the positive health effects associated with these proteins, such as the antibacterial properties attributed to XO/XDH and the involvement in lipid formation and synthesis processes, demonstrated by ADPH (Mohamed et al., 2022).
Coagulation Kinetic Study
The mean coagulation kinetic curves of triplicates from each sample type are displayed in Fig. 2. Coagulation started between 5 and 10 min after rennet addition at 40 °C. Coagulation kinetics were strongly correlated with the protein content of the mixtures, with higher values of curve slopes for higher protein contents. Significant differences associated to the addition of MFGM concentrate were detected mainly for high protein mixtures (12 and 14%) at the end of the coagulation curves (t = 24.5 min), as increasing values of storage modulus (G′) were observed when concentrate content increased (i.e., 12M0 = 1255 Pa vs. 12M2 = 1598 Pa, P < 0.05; 14M0 = 1858 Pa vs. 14M2 = 2525 Pa, P < 0.001). In contrast, no significant differences were observed between M1 and M2 milk blends at any of the protein concentrations tested.
In relation to the technological properties of UFMB derived from coagulation curves, the rennet coagulation time (RCT) and the curd firming rate (CFR) exhibited opposite behaviors. RCT decreased when protein and MFGM content increased while CFR was higher in blends with more protein and MFGM concentrate (Table 2). Contrarily, Krebs et al. (2024) reported that increasing amounts of buttermilk in UF rennet gels did not significantly impact RCT. These milk coagulation properties determine curd cutting windows in traditional cheesemaking and are important to predict gel firmness (Panthi et al., 2019). In this study, as UFC were directly coagulated in individual containers avoiding cutting and syneresis of curd, both coagulation properties become direct parameters of industrial manufacture process. Therefore, short RCT and high CFR values are preferred to decrease production times while the firmness of the fresh cheeses remain constant. A prior study by Govindasamy-Lucey et al. (2006) revealed that the addition of a concentrate rich in polar lipids from soybeans would affect milk coagulation properties, leading to reduced G′ values in the coagulation curve. In contrast, the present study shows that the enrichment with MFGM concentrate would slightly enhance gel firmness during the coagulation process avoiding phospholipid-related interferences.
Storage and loss moduli at a set time of 15 min (G′15, G″15) also increased depending on protein content (Table 2). Unfortunately, these parameters were not able to discriminate between the two concentrations of MFGM concentrate (i.e., 0.4% M1 vs. 0.8% M2), most likely due to the low amount added. On the other hand, the time at which the gel arrived at a set G′ value was compared, in order to understand which of the samples was quicker at the coagulation process. Both times comparison, at 300 and 900 Pa (t300, t900, Table 2), presented the same tendency, being shorter with increasing protein content and without effect related to the addition of the MFGM concentrate. In this line, Amalfitano et al. (2019) reported that different protein fractions would modify milk coagulation properties, increasing RCT and CFR, when CNs and other dairy proteins are incorporated into milk before enzymatic coagulation. In this line, the addition of our MFGM concentrate that primarily supplies CN and MFGMP, would also improve milk coagulation properties. Gauvin et al. (2018) reported that MFGM fragments isolated from buttermilk may interfere with the formation of curd, resulting in coagulation curves with lower slopes. However, this effect was not observed in M1 and M2 mixtures in any of the different levels of protein (Fig. 2) and further research is encouraged to understand the effect of MFGM addition on rennet coagulation.
Texture and WHC of Manufactured Fresh Cheeses
Figure 3 illustrates the firmness of cheeses manufactured at the established G′ values of 50, 300, 600, and 900 Pa selected from the coagulation curves. In general terms, increasing the protein content in cheesemaking significantly enhanced cheese firmness, whereas the effect of adding MFGM was more limited. Firmness was higher when protein content increased, regardless MFGM concentrate level (Fig. 3a–c), but no significant differences were observed at different G′ values (i.e., 50 Pa vs. 900 Pa). Cheeses with a high protein content (14M0, 14M1, and 14M2) contain more CNs that can create bonds in the protein gel matrix, resulting in a stronger or more cohesive matrix against external forces (Guinee & O’Callaghan, 2013). Samples of any protein content combined with MFGM concentrate exhibited greater firmness and consistency (Table 3). This observation aligns with the findings of Sakkas et al. (2021), that evaluated the incorporation of buttermilk into reduced-fat cheeses and concluded that increasing polar lipids with high emulsion capacity increase cheese hardness. In addition, similar results were obtained in a previous study that evaluated MFGM addition in fermented milks (Elkashef et al., 2022). The effect of the inclusion of MFGM concentrate at different levels was not so clear (Fig. 3) and differences between M1 and M2 cheeses were scarce. These results might be caused by the limited amount of MFGM added to the UFC batches.
Regarding WHC, no significant differences were found when coagulation was stopped between 50 and 900 Pa of gel firmness. However, a significant increase was only observed when the protein concentration of UFMB increased from 10 to 12% (i.e., 10M0 = 92.86 vs. 12M0 = 95.66). The addition of MFGM affected the WHC when protein content was ≤ 12% (i.e., 10M0 = 92.86 vs. 10M2 = 95.17 and 12M0 = 95.66 vs. 12M2 = 98.04), while samples with 14% protein content showed no changes related to the inclusion of MFGM concentrate (Table 3). A previous study reported that adding MFGM from buttermilk to yogurts would increase WHC (Zhao et al., 2020), which may be related to the increase of dry matter in MFMG supplemented samples.
Fresh Cheese Rheology
Small amplitude oscillatory shear analysis revealed a common gel-like behavior between UFC samples with and without MFGM, as G′ values were always higher than G″ (Fig. 4; Table 3). G′ frequency sweeps increased with cheese protein content (Fig. 4a) and, to a lesser extent, with the addition of MFGM concentrate (Fig. 4b). All mechanical spectra fitted well the Power Law model with R2 ≥ 0.98. The lack of significant differences in the viscoelastic Power Law exponents (n′, n″, and n*) for the different UFC samples revealed in essence a similar matrix structural stability. However, mean values of G′0 confirm that gel firmness increases directly rising protein content (999, 1249, and 1817 Pa for 10M0, 12M0, and 14M0, respectively) but only the greatest amount of MFGM significantly increased such parameter (1817, 1980, and 2425 Pa for 14M0, 14M1, and 14M2, respectively). A similar behavior was found for G″0 and G*0, evidencing the reinforcing effect of the increasing protein content and MFGM. In contrast, tan(δ) did not show significant differences for non-enriched cheeses (i.e., 10M0 vs. 12M0 and 14M0), but it tended to decrease after the addition of MFGM (Table 3). These results may be due to changes in the protein composition, since increasing the CN content would modify the CN/WP ratio that is a key factor in the coagulation process and the resulting gel (Kelly & O’Kennedy, 2001). On the other hand, although phospholipids were added in limited quantities, their presence in the MFGM concentrate could also influence the rheological properties. In a study by Zhao et al. (2020), yogurts enriched with 1% or 2% of buttermilk showed greater G′ values tan control yogurts made with skimmed milk powder, which might be indicating the role of phospholipid enrichment. However, G′ decreased when buttermilk content was decreased to 0.5% or increased to 4%.These results might be caused by the selection of the control samples, as adding skimmed milk powder would modify the F:P ratio of the sample, which is the main factor affecting G′ determinations (Hueso et al., 2023). In the present research negative controls to the addition of MFGM concentrate were preferred and the addition of MFGM concentrate only resulted in higher G′ values at 12 and 14% protein levels.
Small amplitude oscillatory shear analysis of manufactured goat ultrafiltered fresh cheeses. a Storage modulus (G′) frequency sweep curves of cheeses without MFGM at 3 protein contents (10M0, 12M0, and 14M0). b G′ frequency sweep curves of cheeses with 14% protein at 3 MFGM levels (14M0, 14M1, and 14M2)
The creep-recovery provides insight into the deformability of the cheese matrix against a constant applied stress over a selected period of time, and how the deformed material regains its original shape when the stress is removed, as an indicator of elasticity. This assay revealed a higher % strain, both in the creep and recovery parts of the test, in UFC with the lowest protein contents, which would indicate a lower cohesiveness of the cheese matrix. Figure 5a illustrates % strain curves of control samples (10M0, 12M0, and 14M0) with different protein contents while Fig. 5b compares increasing amounts of MFGM concentrate (10M0, 10M1, and 10M2). The mean values of J(t) and JR(t) were significantly higher for UFC containing 10% protein, while no differences were detected between cheeses with 12 and 14% protein content (Table 3). Therefore, protein content is a key factor for cheese cohesiveness and deformability. On the other hand, the addition of MFGM concentrate tends to diminish the percentage of strain, but it was only significant for UFC with 10% protein and the two levels of addition showed similar behaviors (Fig. 5b; Table 3). It has been previously reported that low-fat cheeses improved texture parameters when lecithin was incorporated as an ingredient (Drake et al., 1996), which suggest that the presence of phospholipids is a key factor for cohesiveness. In the present research, as fat content was fixed at 5%, it was observed hat protein content was the main factor modifying cheese cohesiveness and creep-recovery assay. In this line, it has been suggested that the CN/WP ratio is a key factor that affects the coagulation of milk in rennet-induced gels (Kelly & O’Kennedy, 2001). The addition of MFGM concentrate to UFC (M1 and M2) would increase the CN/WP ratio and, therefore, would improve cheese cohesiveness. The reduction of J(t) and JR(t) values was especially observed in cheeses with the lowest protein content (Fig. 5b; Table 3).
Exploratory Data Modeling and Clustering
In order to discriminate the effects of protein content and the addition of MFGM concentrate on the coagulation process as well as on the structure of UFC, a PCA was performed. In the PCA map, the first two principal components accounted for 83.7% and 6.8% of the variance in the first and second dimension, respectively (Fig. 6a). The score plot showed that the strong effect of protein content is observed along PC1, while PC2 was more related to the addition of MFGM. Cheese samples with 14% protein segregated to the right in PC1, while 10% samples showed a negative response and 12% were close to the center. Along PC2, the centroids of M0 and M1 UFC were positive at all protein levels and M2 centroids presented negative responses (Fig. 6b). Tan(δ), which is the main contributor to PC2, is a rheological parameter that has been used to describe meltability in different types of cheeses (Fu & Yano, 2022), as well as to compare viscoelastic behaviors in gels (Lai et al., 2008). It is important to highlight that the grouping of samples at different levels of MFGM concentrate (Fig. 6b) was correlated to the values of tan(δ) (Table 3). In contrast, no significant differences were observed for tan(δ) in commercial UFC, even with different composition, dynamic moduli, and creep-recovery response (Hueso et al., 2023). The addition of MFGM concentrate would modify the rheological characteristics of UFC and especially the tan(δ), possibly due to the emulsifying capacity of the phospholipids present in MFGM (Calvo et al., 2020). Additional research is needed to understand the underlying mechanism.
Loading plot (a) and scores plot (b) for principal component analysis (PCA) carried out on composition, rheological, textural, and coagulation properties of ultrafiltered milk blends and goat ultrafiltered fresh cheese batches at 3 protein contents (10, 12, and 14%) and 3 levels of MFGM concentrate (0, 0.4, and 0.8%)
Figure 7 illustrates the heat map performed using the same variables as the PCA. Correlations between dependent variables and samples were represented from red (least correlated, − 1) to green (most correlated, + 1). Groups of samples at different protein levels were clearly observed and specific characteristics were associated to each group of UFC. Certain coagulation properties (RCT, t300, t900), J(t), and JR(t) were notably linked to the F:P ratio, distinguishing cheeses with 10% protein content. In contrast, samples with 14% protein showed strong associations with dynamic rheological properties (G′0, G″0, G*0), firmness, consistency, dry matter, and coagulation properties (G′15, G″15, CFR). 12M0, 12M1, and 12M2 samples displayed intermediate correlations across the dependent variables. The dynamic rheological variables were closely related to texture (consistency and firmness) and composition (dry matter and urea) parameters, showing high values in samples with 14% protein. In contrast, lactose content was inversely associated with texture and dynamic rheological parameters. A similar approach was also successful to establish relationships between general composition of commercial UFC and their rheological parameters, showing that the F:P ratio was a key discrimination factor (Hueso et al., 2023). Overall, our results show that coagulation properties, cheese texture, and rheological properties were mainly affected by protein content. The MFGM levels tested did not negatively affect the coagulation process of UFMB and the changes observed for the different variables were limited. Future studies will be directed towards the addition of higher MFGM concentrate contents to determine the effect of this bioactive ingredient in milk coagulation and dairy products production.
Heat map and agglomerative hierarchical clustering dendrogram carried out on composition, rheological, textural, and coagulation properties of ultrafiltered milk blends and goat ultrafiltered fresh cheese batches at 3 protein contents (10, 12, and 14%) and 3 levels of MFGM concentrate (0, 0.4, and 0.8%)
Conclusions
The present study shows the combined effect of milk with different protein contents obtained by UF and the addition of MFGM concentrate from cow buttermilk in the production of UFC. From a technological point of view, the simultaneous use of UF and MFGM improves the coagulation properties of milk since the desired firmness is obtained in shorter times. Ultrafiltered milks with a high protein content produce harder cheeses with a more cohesive structure. The addition of MFGM concentrate at low levels (˂ 1%) also contributed to the firmness of UFC, which would be closely related to the amount added and may be monitored by tan(δ). Additional research is encouraged to evaluate the addition of other MFGM concentrates, of different origin and composition, to fully understand the effects of this bioactive ingredient on the technological properties of milk and the nutritional quality of dairy products. Finally, it is important to note that protein enrichment and the addition of MFGM concentrates in UFC are considered a novel development that has not been previously described and that, in addition to improving their rheological structure, could also be a product with recognized health properties, and well accepted by both the dairy market and consumers.
Data Availability
No datasets were generated or analyzed during the current study.
References
Abd El-Salam, M. H., & El-Shibiny, S. (2020). Milk fat globule membrane: An overview with particular emphasis on its nutritional and health benefits. International Journal of Dairy Technology, 73(4), 639–655. https://doi.org/10.1111/1471-0307.12730
ALKaisy, Q. H., Al-Saadi, J. S., AL-Rikabi, A. K. J., Altemimi, A. B., Hesarinejad, M. A., & Abedelmaksoud, T. G. (2023). Exploring the health benefits and functional properties of goat milk proteins. Food Sci Nutr, 11, 5641–5656. https://doi.org/10.1002/FSN3.3531
Amalfitano, N., Cipolat-Gotet, C., Cecchinato, A., Malacarne, M., Summer, A., & Bittante, G. (2019). Milk protein fractions strongly affect the patterns of coagulation, curd firming, and syneresis. Journal of Dairy Science, 102(4), 2903–2917. https://doi.org/10.3168/JDS.2018-15524
Arango, O., Trujillo, A. J., & Castillo, M. (2015). Predicting coagulation and syneresis parameters of milk gels when inulin is added as fat substitute using infrared light backscatter. Journal of Food Engineering, 157, 63–69. https://doi.org/10.1016/J.JFOODENG.2015.02.021
Asensio-Vegas, C., Tiwari, B., Gredilla, A. E., Bueno, F., Delgado, D., & Martín-Diana, A. B. (2018). Development of yoghurt from ovine milk with enhanced texture and flavour properties. International Journal of Dairy Technology, 71(1), 112–121. https://doi.org/10.1111/1471-0307.12341
Baldasso, C., Barros, T. C., & Tessaro, I. C. (2011). Concentration and purification of whey proteins by ultrafiltration. Desalination, 278(1–3), 381–386. https://doi.org/10.1016/J.DESAL.2011.05.055
Baliyan, S., Calvo, M. V., Piquera, D., Montero, O., Visioli, F., Venero, C., & Fontecha, J. (2023). Milk fat globule membrane concentrate as a nutritional supplement prevents age-related cognitive decline in old rats: A lipidomic study of synaptosomes. Food Research International, 163, 112163. https://doi.org/10.1016/j.foodres.2022.112163
Banks, J. M. (2007). Ultrafiltration of cheesemilk. In P. L. H. McSweeney (Ed.), Cheese problems solved (1st ed., pp. 30–33). Cambridge: Woodhead Publishing. https://doi.org/10.1533/9781845693534.30
Calvo, M. V., Martín-Hernández, M. C., García-Serrano, A., Castro-Gómez, M. P., Alonso-Miravalles, L., García-Martín, R., Megino-Tello, J., Alonso, L., & Fontecha, J. (2020). Comprehensive characterization of neutral and polar lipids of buttermilk from different sources and its milk fat globule membrane isolates. Journal of Food Composition and Analysis, 86, 103386. https://doi.org/10.1016/J.JFCA.2019.103386
Calvo, M. V., Kohen, L., Díaz-Mardomingo, V., García-Herranz, C., Baliyan, S., Tomé-Carneiro, S., Colmenarejo, J., Visioli, G., Venero, F., C., & Fontecha, J. (2023). Milk fat globule membrane-enriched milk improves episodic memory: A randomized, parallel, double-blind, placebo-controlled trial in older adults. Journal of Functional Foods, 111, 105849. https://doi.org/10.1016/J.JFF.2023.105849
Covacevich, H. R., & Kosikowski, F. V. (1978). Mozzarella and cheddar cheese manufacture by Ultrafiltration principles. Journal of Dairy Science, 61(6), 701–709. https://doi.org/10.3168/JDS.S0022-0302(78)83636-4
Danesh, E., Goudarzi, M., & Jooyandeh, H. (2018). Transglutaminase-mediated incorporation of whey protein as fat replacer into the formulation of reduced-fat Iranian white cheese: Physicochemical, rheological and microstructural characterization. Journal of Food Measurement and Characterization, 12(4), 2416–2425. https://doi.org/10.1007/S11694-018-9858-5/FIGURES/4
Deshwal, G. K., Ameta, R., Sharma, H., Singh, A. K., Panjagari, N. R., & Baria, B. (2020). Effect of ultrafiltration and fat content on chemical, functional, textural and sensory characteristics of goat milk-based Halloumi type cheese. LWT, 126, 109341. https://doi.org/10.1016/J.LWT.2020.109341
Drake, M. A., Herrett, W., Boylston, T. D., & Swanson, B. G. (1996). Lecithin improves texture of reduced Fat cheeses. Journal of Food Science, 61(3), 639–642. https://doi.org/10.1111/J.1365-2621.1996.TB13176.X
Drake, M. A., Gerard, P. D., Truong, V. D., & Daubert, C. R. (1999). Relationship between instrumental and sensory measurements of cheese texture. Journal of Texture Studies, 30(4), 451–476. https://doi.org/10.1111/j.1745-4603.1999.tb00230.x
Elkashef, H., Mobdy, A. A., & Hassan, A. (2022). Texture, microstructure, and antioxidant characteristics of bio-fermented milk fortified with buttermilk nano-powder. International Dairy Journal, 126, 105248. https://doi.org/10.1016/J.IDAIRYJ.2021.105248
Fagan, C. C., O’Donnell, C. P., Cullen, P. J., & Brennan, C. S. (2006). The effect of dietary fibre inclusion on milk coagulation kinetics. Journal of Food Engineering, 77(2), 261–268. https://doi.org/10.1016/J.JFOODENG.2005.06.030
Felfoul, I., Bouazizi, A., Burgain, J., Perroud, C., Gaiani, C., Scher, J., Attia, H., & Petit, J. (2023). Enzymatic coagulation of raw and reconstituted skim dromedary and cows’ milk powders: Kinetics, rheological and morphological properties. International Dairy Journal, 137, 105509. https://doi.org/10.1016/J.IDAIRYJ.2022.105509
Ferrer, M. A., Alexander, M., & Corredig, M. (2011). Does ultrafiltration have a lasting effect on the physico-chemical properties of the casein micelles? Dairy Science and Technology, 91(2), 151–170. https://doi.org/10.1007/S13594-011-0002-0/FIGURES/4
Fontecha, J., Brink, L., Wu, S., Pouliot, Y., Visioli, F., & Jiménez-Flores, R. (2020). Sources, production, and clinical treatments of milk fat globule membrane for infant nutrition and well-being. Nutrients 2020, 12(6), 1607. https://doi.org/10.3390/NU12061607
Fu, W., & Yano, H. (2022). Exploring melting behaviors of different cheese products by structural characteristics and rheological properties. International Journal of Dairy Technology, 75(4), 874–881. https://doi.org/10.1111/1471-0307.12895
Gauvin, M. P., Pouliot, Y., & Britten, M. (2018). Rennet coagulation properties of milk in the presence of MFGM fragments isolated from raw – and pasteurised-cream buttermilk. International Dairy Journal, 85, 153–158. https://doi.org/10.1016/J.IDAIRYJ.2018.06.006
Gavazzi-April, C., Benoit, S., Doyen, A., Britten, M., & Pouliot, Y. (2018). Preparation of milk protein concentrates by ultrafiltration and continuous diafiltration: Effect of process design on overall efficiency. Journal of Dairy Science, 101(11), 9670–9679. https://doi.org/10.3168/JDS.2018-14430
Govindasamy-Lucey, S., Lin, T., Jaeggi, J. J., Johnson, M. E., & Lucey, J. A. (2006). Influence of condensed sweet cream buttermilk on the manufacture, yield, and functionality of pizza cheese. Journal of Dairy Science, 89(2), 454–467. https://doi.org/10.3168/JDS.S0022-0302(06)72109-9
Govindasamy-Lucey, S., Lin, T., Jaeggi, J. J., Martinelli, C. J., Johnson, M. E., & Lucey, J. A. (2007). Effect of type of concentrated Sweet cream buttermilk on the manufacture, yield, and functionality of pizza cheese. Journal of Dairy Science, 90(6), 2675–2688. https://doi.org/10.3168/JDS.2006-681
Guinee, T. P., & O’Callaghan, D. J. (2013). Effect of increasing the protein-to-fat ratio and reducing fat content on the chemical and physical properties of processed cheese product. Journal of Dairy Science, 96(11), 6830–6839. https://doi.org/10.3168/JDS.2013-6685
Haug, A., Christophersen, O. A., Høstmark, A. T., & Harstad, O. M. (2007). Milk and health. Tidsskrift for Den Norske Laegeforening, 127(19), 2542–2545. https://doi.org/10.1056/NEJMRA1903547/SUPPL_FILE/NEJMRA1903547_DISCLOSURES.PDF
Hickey, C. D., O’Sullivan, M. G., Davis, J., Scholz, D., Kilcawley, K. N., Wilkinson, M. G., & Sheehan, J. J. (2018). The effect of buttermilk or buttermilk powder addition on functionality, textural, sensory and volatile characteristics of Cheddar-style cheese. Food Research International, 103, 468–477. https://doi.org/10.1016/J.FOODRES.2017.09.081
Hueso, D., Gómez-Guillén, M. C., Fontecha, J., & Gómez-Cortés, P. (2023). Rheological characterization of commercial Burgos-type ultrafiltered fresh cheeses. LWT-Food Science and Technology, 190(115525). https://doi.org/10.1016/j.lwt.2023.115525
International Organization for Standardization. (2004). Whey cheese — Determination of dry matter (Reference method) (ISO Standard No. 2920:2004). https://dgn.isolutions.iso.org/obp/ui#iso:std:iso:2920:ed-2:v1:en
Karami, M., Ehsani, M. R., Mousavi, S. M., Rezaei, K., & Safari, M. (2008). Changes in the rheological properties of Iranian UF-Feta cheese during ripening. Food Chemistry. https://doi.org/10.1016/j.foodchem.2008.06.003
Kelly, P. M., & O’Kennedy, B. T. (2001). The effect of casein/whey protein ratio and minerals on the rheology of fresh cheese gels using a model system. International Dairy Journal, 11(4–7), 525–532. https://doi.org/10.1016/S0958-6946(01)00076-0
Krebs, L., Larouche, J., Remondetto, G. E., Chamberland, J., Venema, K., & Brisson, G. (2024). The effect of concentrated buttermilk on cheese milk rennet-induced coagulation and rheological properties at various buttermilk to skim milk ratios. Food Hydrocolloids, 150, 109664. https://doi.org/10.1016/J.FOODHYD.2023.109664
Lai, G., Li, Y., & Li, G. (2008). Effect of concentration and temperature on the rheological behavior of collagen solution. International Journal of Biological Macromolecules, 42(3), 285–291. https://doi.org/10.1016/J.IJBIOMAC.2007.12.010
Le, T. T., van Camp, J., Pascual, P. A. L., Meesen, G., Thienpont, N., Messens, K., & Dewettinck, K. (2011). Physical properties and microstructure of yoghurt enriched with milk fat globule membrane material. International Dairy Journal, 21(10), 798–805. https://doi.org/10.1016/J.IDAIRYJ.2011.04.015
Lee, H., Padhi, E., Hasegawa, Y., Larke, J., Parenti, M., Wang, A., Hernell, O., Lönnerdal, B., & Slupsky, C. (2018). Compositional dynamics of the milk fat globule and its role in infant development. Frontiers in Pediatrics, 6, 313. https://doi.org/10.3389/FPED.2018.00313/BIBTEX
Mohamed, H. J. J., Lee, E. K. H., Woo, K. C. K., Sarvananthan, R., Lee, Y. Y., & Mohd Hussin, Z. A. (2022). Brain-immune-gut benefits with early life supplementation of milk fat globule membrane. JGH Open: An Open Access Journal of Gastroenterology and Hepatology, 6(7), 454–461. https://doi.org/10.1002/JGH3.12775
Muñoz, M., Fernández, D., Fresno, J., & Delgado, D. (2023). Effect of high hydrostatic pressure processing on the rennet coagulation kinetics and physicochemical properties of sheep milk rennet-induced gels. Journal of Dairy Science, 106(4), 2314–2325. https://doi.org/10.3168/jds.2022-21879
Nassar, K. S., Lu, J., Pang, X., Ragab, E. S., Yue, Y., Zhang, S., & Lv, J. (2020). Rheological and microstructural properties of rennet gel made from caprine milk treated by HP. Journal of Food Engineering, 267, 109710. https://doi.org/10.1016/J.JFOODENG.2019.109710
Nastaj, M., Terpiłowski, K., & Sołowiej, B. G. (2020). The effect of native and polymerised whey protein isolate addition on surface and microstructural properties of processed cheeses and their meltability determined by Turbiscan. International Journal of Food Science & Technology, 55(5), 2179–2187. https://doi.org/10.1111/IJFS.14471
Ong, L., Dagastine, R. R., Kentish, S. E., & Gras, S. L. (2013). Microstructure and composition of full fat cheddar cheese made with ultrafiltered milk retentate. Foods MDPI, 2, 310–331. https://doi.org/10.3390/FOODS2030310
Panthi, R. R., Kelly, A. L., Sheehan, J. J., Bulbul, K., Vollmer, A. H., & McMahon, D. J. (2019). Influence of protein concentration and coagulation temperature on rennet-induced gelation characteristics and curd microstructure. Journal of Dairy Science, 102(1), 177–189. https://doi.org/10.3168/JDS.2018-15039
Peleg, M. (2006). On fundamental issues in texture evaluation and texturization—A view. Food Hydrocolloids, 20(4), 405–414. https://doi.org/10.1016/J.FOODHYD.2005.10.008
Petrotos, K., Tsakali, E., Goulas, P., & D’Alessandro, A. G. (2014). Casein and whey proteins in human health. Milk and Dairy Products as Functional Foods, 4, 94–146. https://doi.org/10.1002/9781118635056.CH4
Picciotti, U., Massaro, A., Galiano, A., & Garganese, F. (2022). Cheese fortification: Review and possible improvements. Food Reviews International, 38(S1), 474–500. https://doi.org/10.1080/87559129.2021.1874411
Pouliot, Y. (2008). Membrane processes in dairy technology - From a simple idea to worldwide panacea. International Dairy Journal, 18(7), 735–740. https://doi.org/10.1016/J.IDAIRYJ.2008.03.005
Rezende Costa, M., Elias-Argote, E., Jiménez-Flores, R., & Lúcia Gigante, M. (2010). Use of ultrafiltration and supercritical fluid extraction to obtain a whey buttermilk powder enriched in milk fat globule membrane phospholipids. International Dairy Journal, 20(9), 1–5. https://doi.org/10.1016/j.idairyj.2010.03.006
Sakkas, L., Alatini, E., & Moatsou, G. (2021). Use of sweet sheep buttermilk in the manufacture of reduced-fat sheep milk cheese. International Dairy Journal, 120, 105079. https://doi.org/10.1016/J.IDAIRYJ.2021.105079
Señoráns, M., Gallo, V., Calvo, M. V., & Fontecha, J. (2023). Lipidomic and proteomic profiling of the milk fat globule membrane from different industrial by-products of the butter and butter oil manufacturing process. Foods MDPI, 12(4), 750. https://doi.org/10.3390/foods12040750
Sharma, P. (2022). ADSA Foundation Scholar Award: Materials science approach to the study of mechanical and diffusion properties in cheese. Journal of Dairy Science, 105(6), 4711–4721. https://doi.org/10.3168/JDS.2021-21093
Sharma, P., Munro, P. A., Gillies, G., Wiles, P. G., & Dessev, T. T. (2017). Changes in creep behavior and microstructure of model mozzarella cheese during working. LWT - Food Science and Technology, 83, 184–192. https://doi.org/10.1016/J.LWT.2017.05.003
Spanish Ministry of Agriculture, Fisheries and Food. (2023). Caracterización del sector ovino y caprino de leche en España. Accessed March 15, 2024. https://www.mapa.gob.es/es/ganaderia/temas/produccion-y-mercados-ganaderos/caracterizacionovinoycaprinolechedatos2022_tcm30-562416.pdf
Zhao, L., Feng, R., & Mao, X. (2020). Addition of buttermilk powder improved the rheological and storage properties of low-fat yogurt. Food Science & Nutrition, 8(7), 3061–3069. https://doi.org/10.1002/FSN3.1373
Acknowledgements
The authors wish to thank the Interprofessional Dairy Laboratory of Castilla y León (LILCYL, S.A.) for the gross composition analysis, and the Analytical, Instrumental and Microbiological Techniques Service Unit (ICTAN, CSIC) for the moisture content analysis. Diego Hueso gratefully acknowledges the Spanish National Research Council for his predoctoral contract.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research was funded by the Spanish Ministry of Science and Innovation (Projects PDI2020-114821RB-I00 MCIN/AEI//FEDER and PDC2021-121528-I00) and the Spanish National Research Council (Projects 20217AT002 and 202270I067).
Author information
Authors and Affiliations
Contributions
DD, MCG-G, JF, and PG-C contributed to the conception and design of the study. DH and DD prepared the ultrafiltered milk blends, performed coagulation kinetics, cheesemaking, and determined the composition of all samples. VG performed the protein identification by SDS-PAGE and MCG-G carried out cheese rheological measurements. DH performed the data analysis and wrote the first draft of the manuscript. DD, VG, MCG-G, JF, and PGC supervised the research, reviewed, and edited the manuscript. JF and PGC carried out the project administration and funding acquisition. All authors have read and approved the final submitted version.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hueso, D., Delgado, D., Gallo, V. et al. The Effect of Milk Fat Globule Membrane (MFGM) Concentrate Addition on Ultrafiltered Goat Milk Coagulation and Fresh Cheese Rheology. Food Bioprocess Technol (2024). https://doi.org/10.1007/s11947-024-03441-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11947-024-03441-w