Abstract
Fortifying conventional foods with postbiotic powders (PP) is an effective approach for the production of functional products. Preserving both functional properties of PP and the physical characteristics of fortified products is essential during this process. This study aimed to investigate the antioxidant activity and consumer testing of low-fat yoghurt fortified with PP and changes in their physical properties, including rheology, water holding capacity, and microstructure over a 21-day storage period. PP, derived from Bifidobacterium animalis subsp. lactis BB12 grown in cheese whey (CW) and skim milk (SM), was added to the product at 1% individually (B12-CW and B12-SM) and in a mixture (BB12-CW-SM). The results indicated that the antioxidant activity of the samples fortified with PP was 4.6 − 6.3%, almost double the values in samples without PP, despite their similar viability of starters (Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus) (> 8.5 log cfu/g). Adding BB12-CW significantly reduced mouthfeel, flavor, and overall acceptability of the product, while adding BB12-SM and BB12-CW-SM did not alter any consumer acceptability. Similarly, regardless of PP types, their fortification had negligible effects on viscosity, viscoelastic properties, shear-thinning behaviors, water-holding capacity, and microstructure formation, which remained nearly unchanged during storage. Overall, the addition of PP, particularly BB12-SM and BB12-CW-SM, significantly increased antioxidant activity while preserving important physical and consumer acceptance of yoghurt. These findings underscore the potential of postbiotics as functional ingredients, enhancing both the nutritional values and consumer appeal of yoghurt, thereby promoting its health benefits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The widespread popularity and extensive consumption of yoghurt present a compelling opportunity to enhance its nutritional profile by introducing additional elements, such as probiotic bacteria, prebiotics, plant fibers, and various extracts (Fazilah et al., 2018; Ghaderi-Ghahfarokhi et al., 2020). There is also potential for enriching yoghurt with postbiotics derived from beneficial microorganisms, particularly lactic acid bacteria (LAB) and bifidobacteria. These postbiotics can be cultivated in culture media, food, or the intestinal tract. While a universal definition of postbiotics is yet to be established (Aguilar-Toalá et al., 2021; Amiri et al., 2020; Sabahi et al., 2022; Thorakkattu et al., 2022), it is recognized that postbiotics encompass a variety of compounds found both inside and outside bacterial cells. Nevertheless, there is consensus that the removal of bacterial cells is crucial (Moradi et al., 2021). The resulting postbiotic solution consists of safe compositions with distinct chemical structures and an extended shelf life, making it well-suited for integration into various food products (Aguilar-Toalá et al., 2018; Rudke et al., 2023). Hence, the acknowledgment that specific bacteria can contribute to health benefits by producing particular metabolites triggered the emergence of the probiotic concept, along with the development of pharmabiotic metabolites. Postbiotics have garnered increased attention because of their natural stability throughout processing and storage, rendering them particularly well-suited for regions with unreliable cold chain infrastructure. In contrast to probiotics, which often experience a decline in viability during storage, postbiotics maintain their stability over extended periods. Manufacturers of probiotics usually include a surplus of microorganisms to ensure the labeled viable cell counts, which could potentially cause fluctuations in the live-to-dead ratio, affecting their overall effectiveness. In contrast, postbiotics demonstrate impressive stability at room temperature for several years. This feature reduces concerns about viability and facilitates consistent microorganism levels during production, ultimately positioning postbiotics as a promising solution for areas that face challenges related to storage (Moradi et al., 2021; Salminen et al., 2021).
Probiotic microorganisms produce important bioactive elements that are water-soluble. Metabolites collectively referred to as postbiotics encompass bioactive lipids (e.g., conjugated linoleic acid), antimicrobial peptides (e.g., bacteriocins), and exopolysaccharides (EPSs) (Aguilar-Toalá et al., 2018). Notably, these postbiotics offer a diverse range of health benefits, including anti-atherosclerotic, immunomodulatory, anti-diabetic, anti-obesity, antimicrobial, anti-inflammatory, and anti-cancer activities, as evidenced in recent literature (Amiri et al., 2022; Thorakkattu et al., 2022). In recent times, there has been a growing interest in exploring the texture-related and rheological properties of EPSs, which are polysaccharide molecules released by specific bacteria into culture media. These EPSs are now being considered promising candidates for use in the food sector. Ongoing research has shed light on their functional characteristics, emphasizing their potential for immunomodulation, along with their anti-inflammatory, anti-biofilm, and antioxidant properties (Amiri et al., 2021; Kumar et al., 2007). The recognition of potential health benefits associated with functional supplements has spurred the development of various functional food items (Abdel-Hamid et al., 2020), with a significant portion falling within the category of probiotic foods (Aguilar-Toalá et al., 2018). Nonetheless, there is notable concern linked to the utilization of probiotics due to the existence of genes associated with antibiotic resistance within certain probiotic bacteria. These genetic elements possess the capability to be transmitted to pathogenic bacteria through a process known as horizontal gene transfer (Imperial & Ibana, 2016). Another worrisome aspect regarding the formulation of probiotic products, whether in pharmaceutical or commercial food forms, pertains to upholding the viability of bacteria throughout manufacturing and storage. The vulnerability of probiotic organisms in delivery systems is influenced by multiple factors. These include interactions with coexisting microbial species, product acidity, water activity, temperature, nutrient availability, presence of growth stimulants and inhibitors, inoculation levels, fermentation duration, dissolved oxygen, and specific formulation techniques such as freeze-drying, spray drying, or freeze concentration (Sadighbathi et al., 2023). In practical terms, postbiotics have demonstrated greater stability and safety in comparison to the live microorganisms from which they originate, these are applicable in both food and pharmaceutical contexts. This is because their viability is not necessary either during consumption or in the context of large-scale production (Barros et al., 2020).
Bifidobacteria represent a prevalent probiotic genus extensively utilized in functional foods and serve as a fundamental component of the human intestinal microbiota. Notably, postbiotics sourced from BB12 are being harnessed for the creation of functional foods, particularly in the domain of cheese products (Sharafi et al., 2022). Within this group, Bifidobacterium lactis stands out as a Gram-positive, rod-shaped bacterium characterized by its non-gas-producing, non-motile, and catalase-negative nature, thriving specifically in anaerobic conditions (Amiri et al., 2020). In the domain of microbial fermentation, diverse agro-industrial residues — particularly dairy effluents — have emerged as cost-effective alternatives to expensive cultivation media for nurturing microbial cultures. Specifically, whey, the primary byproduct of traditional cheese-making processes and ultra-filtered white cheese production, remains in the forefront. This abundant and economical waste material possesses high lactose concentrations, rendering it a viable carbon source (Amado et al., 2016; Amiri et al., 2022).
Research findings indicate that BB12 has the capacity to produce postbiotics within cheese whey (Amiri et al., 2019, 2020; Meira et al., 2015). Conventionally, in the course of manufacturing probiotics on an industrial scale, postbiotics are produced as secondary products, often ending up as discarded waste. However, the potential of the residual postbiotic solution as a biologically active and cost-efficient resource has been recognized. This solution can serve as an alternative mean for improving the nutritional composition and extending the expiry date of yoghurt in cold storage. Few prior researches have investigated the utilization of postbiotics derived from LAB in food applications, with a focus on preparing postbiotic solutions using the de Man Rogosa and Sharpe (MRS) medium. Nevertheless, there is still the need to identify novel, economically viable, and underutilized agro-industrial residues for the purpose of postbiotic formulation. This research undertook the preparation of postbiotic solutions derived from BB12 within two innovative growth model mediums: cheese whey and skim milk. The resulting postbiotic matrices were later incorporated into yoghurt as powdered nutritional supplements. The fundamental aim of this research was to scrutinize the impact of individual postbiotic powders on the microbial, physicochemical, rheological characteristics, and consumer tests of yoghurt, with a focus on the enhancement achieved through the inclusion of postbiotics.
Material and Methods
Microorganisms and Inoculums
Cultures of BB12, Streptococcus thermophilus, and Lactobacillus delbrueckii subsp. bulgaricus, obtained in freeze-dried form from Chr. Hansen in DK-2970 Hørsholm, Denmark, were acquired and assessed in accordance with the manufacturer’s guidelines. BB12 cultures were grown for 24 h at 37 °C, utilizing MRS medium (Oxoid, Basingstoke, Hampshire, UK) supplemented with 0.1% Tween 80, 0.05% l-cysteine (AppliChem, Darmstadt, Germany), and 0.1% lithium chloride (Sigma-Aldrich, St. Louis, Missouri, USA). S. thermophilus and L. bulgaricus were cultivated for 24 h at 37 °C using M17 medium (Neogen, Michigan, USA) and MRS medium, respectively. After cultivation, the cultures were stored at 4 °C and underwent three successive sub-culturing cycles within the same medium before each experiment.
Preparation of Postbiotic Solutions/Powders
Initially, BB12 underwent a 24-h growth period at 37 °C in an MRS medium supplemented as described. After this incubation, a sub-culture was established by transferring 50 µL of the bacterial culture into Falcon tube vials containing 50 mL of media, followed by an overnight incubation at 37 °C. The resultant samples were centrifuged at 4000 × g for 10 min at 20 °C and subjected to two washes with sterilized standard saline solution to collect the biomass of the bacterial culture. These harvested cells, upon resuspension in 10 mL of ultra-high temperature (UHT) milk, were employed as the bacterial culture in the subsequent step. For the postbiotics’ culture media, skim milk (SM) and cheese whey (CW) from Best Way in Haulerwijk, Netherlands, were used. The pH of the media was adjusted to 4.5 with 5N hydrochloric acid (Merck, Darmstadt, Germany) before autoclaving at 121 °C for 15 min. Subsequently, the precipitates were isolated through centrifugation at 2360 × g for 5 min. The sample was then supplemented with 1% peptone, followed by autoclaving the media at 121 °C for 15 min. A variation of the methodology presented in Amiri et al. (2020) and Amiri et al. (2021) was employed optimizing not only the incubation temperature but also the duration for achieving the maximum postbiotic concentration in CW and SM (experimental design not included). Briefly, two different fermentation batches were prepared: BB12-CW (B. lactis BB12 postbiotic-containing cheese whey solution with 1.5% lactose (Fluka, Buchs, Switzerland) and BB12-SM (B. lactis BB12 postbiotic-containing skim milk solution with 0.18% lactose). Following their preparation, the batches were subjected to incubation at two different temperatures: 30 °C for 40.8 h and 30 °C for 48 h, respectively. Throughout the entire duration, the visible growth of bacteria was monitored at 12-h intervals. After the fermentation process, all batches of fermented products underwent freeze-drying using Martin Christ equipment in Osterode am Harz, Germany, at − 60 °C under a pressure of 0.0650 mbar for a duration of 48 h. The resulting powders were subsequently stored in sealed plastic containers at 4 °C for subsequent testing.
Rehydration Properties of Postbiotic Powders
Water absorption capacity (WAC; g water/g sample) was determined following Meena et al. (2022). Briefly, 1 g of each postbiotic powder (PP) sample was weighted (weight of postbiotic powder (WPP)) and dispersed in distilled water (10 mL). The sample underwent homogenization for a duration of 5 min through the use of a vortex mixer, followed by centrifugation at 1200 times the force of gravity (1200 × g) for a period of 30 min. The supernatant was disposed of, and the sediment was gathered, weighed, and designated as the weight of sediment (WS). WAC was calculated using the equation:
The water solubility index (WSI; %) and swelling power (SP; g/g) of the polypropylene were evaluated using the centrifugation method outlined by Meena et al. (2022). In brief, each 5 g sample of PP was measured and dispersed in distilled water (50 mL). The sample underwent centrifugation at 3500 times the force of gravity (3500 × g) for a duration of 20 min at room temperature. The resultant supernatant and sediment were collected and individually weighed, with the sediment represented as the weight of the sediment paste (WSP). Subsequently, the supernatant was subjected to an oven at 105 °C for a duration of 5 h, and measurements were taken upon reaching a steady weight, defined as the dried supernatant weight (DSW). Solubility and swelling power were calculated using the equations:
Preparation of Functional Yoghurts
To produce low-fat yoghurt, a modified procedure outlined by Ghaderi-Ghahfarokhi et al. (2020) was followed, utilizing commercial UHT milk with a composition of 1.5 g/100 g of fat, 12.8 g/100 g of total solids (TS) content, and a pH of 6.67. Four distinct yoghurt formulations, as indicated by sample codes in Table 1, were generated using the process outlined in Fig. 1. The initiation of yoghurt batches involved introducing a starter culture, consisting of Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus, with each component at a concentration of 1% v/v. Following thorough mixing, the resulting yoghurt was placed into sterile 100 mL containers and underwent incubation at 42 °C until reaching a final pH of 4.5. Following that, the samples were cooled to a temperature of 4 °C and maintained in storage over a span of 21 days. The entire yoghurt manufacturing process was conducted in threefold repetition. Throughout the storage period, analysis was done on days 1, 7, 14, and 21 and included the assessment of both physicochemical characteristics and the viability of microorganisms.
Low-fat yoghurt manufacturing flowchart. Yoghurt formulations: Refer to Table 1 for sample codes
Physicochemical Analysis of Yoghurts
The moisture content of the yoghurt samples was evaluated in accordance with the AOAC guidelines (AOAC, 1995). In this procedure, each 10 g portion of yoghurt underwent a drying process at 105 °C for 180 min. The percentage of moisture content was then calculated by dividing the weight after drying by the initial weight of the fresh yoghurt sample.
The AOAC protocol was also followed to measure the ash content of each yoghurt sample at 550 °C (AOAC, 1995), stated as the percentage of inorganic residue remaining after the incineration of the total weight of yoghurt.
The pH levels of the yoghurt were gauged employing a pH meter, specifically the Thermo Orion Model-420A′. Furthermore, the determination of titratable acidity (TTA) in the yoghurt samples followed the official AOAC method, and the results were expressed as a percentage representation of lactic acid (AOAC, 2005).
The evaluation of syneresis levels in the yoghurt samples followed the approach outlined by Li et al. (2022). In brief, 25 g from each yoghurt batch was measured on Whatman paper No. 42 (Whatman) placed on a funnel. The calculation of syneresis involved dividing the whey, which separated from the samples due to gravity during a 2-h drainage period at 4 °C, into a flask with a known weight, relative to the initial mass of the yoghurt.
The assessment of water holding capacity (WHC) in yoghurt samples followed the centrifugation method as detailed by Li et al. (2022). In essence, each 5 g yoghurt sample was placed in a Falcon tube (Mi) and then underwent centrifugation at 3556 × g for 30 min at a temperature of 10 °C. After centrifugation, the supernatant was discarded, and the resultant precipitate was collected and weighed (Mp). The calculation of WHC was carried out utilizing the following formula:
where Mi and Mp were the initial weight of the sample and the final weight of the precipitate, respectively.
The examination of yoghurt gel microstructure involved the use of a scanning electron microscope (SEM, S-4800, Hitachi, Tokyo, Japan) following the methodology outlined by Espírito-Santo et al. (2013). Yoghurt samples, initially stored at 4 °C for both 1 day and 21 days, were subjected to freeze-drying using a freeze-dryer (Martin Christ, Osterode am Harz, Germany) with specific adjustments. The resulting powders were attached to metallic stubs using double-sided carbon tape. Subsequently, the prepared samples underwent a coating process involving the application of a 4 nm-thick gold/palladium layer through two rounds of sputter coating using a high-resolution sputter coater (208HR, Cressington Scientific Instruments, Watford, UK). After the coating procedure, the samples were examined using SEM under specific conditions: an accelerating voltage of 10 kV, emission current set at 10 µA, working distance at 10 mm, and a magnification of 1000 × .
Antioxidant Activity Determination
Yoghurt Sample Extraction
The extraction process for yoghurt samples adhered to the procedures detailed by Demirci et al. (2017). In a concise overview, 5 g of yoghurt was blended with a 25 mL methanol solution (80:20, methanol to distilled water). Following this, the mixture underwent vortexing, centrifugation at 7200 rpm for 10 min at 4 °C, and eventual filtration using Whatman No. 1 filter paper. The resulting liquid fraction was refrigerated at 4 °C for subsequent assessment of antioxidant activity.
DPPH Free Radical Scavenging Activity Assay
The determination of DPPH radical activity followed the protocol elucidated by Demirci et al. (2017). The evaluation of DPPH radical scavenging activity entailed the amalgamation of 100 µL from the extracted sample with 2 mL of 0.2 mM DPPH radicals. This amalgam was allowed to stand undisturbed for 30 min in a darkened room at ambient temperature. Subsequently, 200 µL of the resulting mixture was introduced into the cavity of a 96-well plate, and the absorbance was subsequently gauged (wavelength = 517 nm), employing a blank solution with 80% methanol. The outcomes are expressed as the percentage of DPPH clearance calculated by the microplate reader (Tecan, Hombrechtikon, Switzerland) using a specific formula:
where AC and AA were the absorbance of control and absorbance of extract, respectively.
Assessment of Apparent Viscosity and Gel Structure of Yoghurt
The viscosity of yoghurt samples was evaluated using a HAAKE MARS 40 rheometer (Thermo Scientific, Germany) equipped with a parallel-plate measuring system (35 mm diameter). To determine flow behavior, rotational measurements were conducted, commencing with a gradual increase in shear rate from 0.04 to 100 1/s, followed by a stepwise decrease from 100 to 0.04 1/s. All viscosity assessments were carried out at 4 °C, mirroring the yoghurt’s storage temperature. Before analysis, the sample was allowed to stabilize between the plates for 2 min. Apparent viscosities of the yoghurt were recorded at a shear rate of 50 1/s, corresponding to the initial increase from 0.04 to 100 1/s. The hysteresis loop area, serving as an indicator of structural degradation, was calculated by examining the change in the flow profile area across shear rates ranging from 0.04 to 100 1/s and 100 to 0.04 1/s. This computation was executed using the RheoWin Measuring and Evaluation Software.
To elucidate the viscoelastic properties of yoghurt during storage, a frequency sweep was performed, ranging from 0.1 to 10 Hz, utilizing oscillatory measurements within the linear viscoelastic range. The storage modulus (G′), loss modulus (G″), and loss tangent (tanδ = G″/G′) were determined at a frequency of 1 Hz. Each time point underwent examination with two biological replicates, and each replicate underwent two measurements for all tests.
Microbiological Determination of Yoghurts
Subsequent to agitation, each yoghurt formulation, weighing 1 g, was mixed with 9 mL of physiological saline solution using a vortex mixer. The resulting diluted samples were then quantified and expressed as log colony-forming units (CFU) per gram of the product (log CFU/g). L. bulgaricus and S. thermophilus enumeration employed MRS agar and M17 agar, respectively. Bacteria underwent incubation under anaerobic conditions at 37 °C for 72 h (L. bulgaricus) and aerobic conditions (S. thermophilus), following the methodology outlined by Batawy and Khalil (2018). Coliform count was ascertained using Violet Red Bile Agar medium (Neogen, Michigan, USA), and the plates were kept at 37 °C for 48 h. Yeasts and molds were quantified on Malt-Extract Agar medium (Neogen, Michigan, USA), and the plates were kept at 25 − 27 °C for 4 days.
Consumer Testing
A group of 20 semi-trained assessors evaluated the consumer acceptance of yoghurt samples such as visual appearance, texture, flavor, and mouth sensation. Evaluators tested the samples on the 10th day of storage. Each sample was served in a transparent cup labeled with three-digit random codes. Participants were instructed to cleanse their mouths before commencing and between sampling each yoghurt. To assess each sample, a hedonic scale consisting of 9 points was utilized, ranging from 1 (indicating strong dislike) to 9 (indicating strong liking).
Statistical Analysis
Triplicate analyses were performed for both physicochemical assessments and microbial counts. The data acquired for evaluating the yoghurt’s physicochemical properties, microbial content, and consumer acceptance underwent analysis of variance (ANOVA) through the general linear model procedure. Mean values, accompanied by their respective standard deviations, are presented in the results. To discern significant differences, mean comparisons were executed using Tukey’s test, with a predetermined level of statistical significance set at p ≤ 0.05. All statistical analyses were carried out using Minitab 21.4 software (Minitab Inc., State College, PA, USA).
Results and Discussion
Rehydration Characterization of Postbiotic Powders
Water absorption capacity significantly influences food products’ texture and development and highlights the potential benefits of probiotic fermentation in modulating food properties (Haque et al., 2016). In our study, the WAC value of BB12-CW was significantly higher than BB12-SM (p < 0.05; Table 2). One plausible reason for this phenomenon could be associated with the water absorption potential of postbiotic compounds, including additional postbiotic, notably EPSs present in BB12-CW powder. EPSs are a function of different factors such as overall charge density, thickness, and hydrophobic nature of the particles (Yang et al., 2022).
The water solubility index was 44.00 ± 0.95% for BB12-CW and 55.95 ± 0.61% for BB12-SM samples (p < 0.05).
These solubility values were lower compared to the WSI reported for freeze-dried yoghurt powder containing free Lactobacillus plantarum and probiotic fruit juice powders (Jouki et al., 2021 and Dias et al., 2018). Notably, the postbiotic powders analyzed in this study displayed predominantly porous surfaces, which have been documented to enhance solubility when food powders are reconstituted (Hardy & Jideani, 2020). Swelling power and WSI are critical parameters for characterizing the rehydration properties of powders. In our study, the swelling power of BB12-CW was significantly higher (4.63 ± 0.10 g/g) than BB12-SM (3.79 ± 0.06 g/g; p < 0.05). This difference can be associated with variations in the structural composition of the powders derived from different materials.
Physico-Chemical Characteristics and Antioxidative Activity of Yoghurts
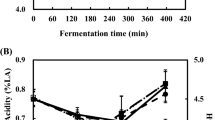
The pH assessments for yoghurt samples were carried out at four distinct time intervals: 1, 7, 14, and 21 days, all during storage at 4 °C. Our observations revealed that both the postbiotic powder type and storage duration exerted a significant influence on the pH values of the resulting yoghurts (p < 0.05). On the initial day of storage, the pH values for all yoghurt samples fell within the range of 4.17 to 4.43 (Fig. 2A). Over the course of storage, this pH index gradually decreased, a trend consistent with findings in previous research studies (Ghaderi-Ghahfarokhi et al., 2021; Karaca et al., 2019). The observed phenomenon of pH decline in yoghurt samples containing BB12-SM powders, compared to BB12-CW and control formulations, can be imputed to the assimilation of organic acids present in the postbiotics. This alteration, likely linked to mass exchange processes, aligns with similar research findings by Sharafi et al. (2022), where significant reductions in pH values were noted for samples containing postbiotics compared to control samples. Consistent with this trend, İncili et al. (2021) observed reduced pH values in breast fillet samples treated with postbiotics compared to control samples.
pH (A) and total titratable acidity (as lactic acid %) (B) in different formulations of yoghurt during storage at 4 °C. Yoghurt formulations: Refer to Table 1 for sample codes. Error bars represent the mean (n = 3) ± standard deviation (SD)
The post-acidification phenomenon persisted throughout the entirety of yoghurt samples, as illustrated in Fig. 2A, primarily driven by continuous fermentation carried out by starter culture bacteria over the entire duration of the shelf life, as noted by Basiri et al. (2018). At the conclusion of the storage period, yoghurts containing BB12-CW, BB12-SM, and BB12-CW-SM showed reductions in pH of approximately 0.24, 0.19, and 0.16 units, respectively, compared to the initial day, while the control experienced a decrease of about 0.21 units. These findings are consistent with the observations of Elsamani and Ahmed (2014), who reported similar pH values in yoghurts produced with or without the incorporation of cheese whey and skim milk.
Lactic acid, identified as the primary acid secreted by probiotic strains according to Ghaderi-Ghahfarokhi et al. (2021), showcased its impact on the yoghurt formulations in Fig. 2B. Throughout the storage duration, all yoghurts demonstrated a raise in TTA coupled with a simultaneous decrease in pH. Our investigation revealed that the type of media employed for generating postbiotic solutions significantly influenced the TTA parameter, resulting in a higher concentration of lactic acid in the BB12-SM formulation compared to BB12-CW (Fig. 2B). Although there were no noteworthy distinctions in TTA content among these samples over the cold storage duration (p > 0.05), certain variations were observed in the TTA index of the yoghurt throughout the storage timeframe, aligning with observations in another study conducted by Gonzaílez-Martí et al. (2002). The varying concentrations of organic acids found in postbiotics produced by different probiotic bacteria may potentially result from the influence of fermentation media on heterofermentative biochemical pathways, particularly those involving lactic acid metabolism as the predominant metabolite product during the growth, which is a distinguishing characteristic of lactic acid bacteria (Chang et al., 2021). Despite the limited understanding of how fermentation media impacts the expression of genes related to organic acid production in each bacterial strain, this research offers foundational insights into how different fermentation media and bacterial strains influence organic acid production in postbiotics.
The physicochemical features and antioxidative activity of the samples were assessed at day 7 of the storage period (Table 3). Statistical analysis indicated significant differences in total solid content between the control and the formulations BB12-SM and BB12-CW-SM (p < 0.05), but not BB12-CW (p > 0.05). It is evident that the postbiotic-yoghurt formulations exhibited higher total solid content compared to the control products, indicating a greater nutrient density in the postbiotic-yoghurts. These findings are consistent with the results of Demirci et al. (2017), who reported a total solid content of 12.66% for yoghurt samples.
In our current investigation, the mean ash content was significantly different in all yoghurt formulations (p < 0.05) (Table 3). These values closely align with those reported by Batawy and Khalil (2018) for yoghurts enriched with maltodextrin (0.78%), although they are lower than the levels found in yoghurts fortified with enzymatically hydrolyzed guar gum (2.56%; Mudgil et al., 2016). Throughout the storage duration, minor fluctuations in ash content were noted in all yoghurt treatments. These differences may be attributed to changes in dry matter content over the storage duration. The moisture content also varied significantly among all yoghurt formulations (p < 0.05) (Table 3). These moisture values were higher than those reported by Sanusi et al. (2022) for yoghurt produced with soursop puree and by Dhillon et al. (2023) for flavored strained yoghurt. Notably, control and BB12-CW samples displayed notably higher moisture content compared to other samples (p < 0.05).
Postbiotics have been recognized for their diverse functional and bioactive properties, including direct and indirect antioxidant activities (Aguilar-Toalá et al., 2018; Sharma & Shukla, 2016). Among these bioactive compounds, EPSs and peptides stand out for their antioxidant potential. EPSs have shown the capability to alleviate oxidative stress, lipid peroxidation, and inflammation, while peptides have demonstrated anti-aging, anti-inflammatory, antimicrobial, and antioxidant effects. The potential benefits of both peptides and EPSs in promoting health in foods and beverages have been highlighted in studies (Amiri et al., 2019; Chang et al., 2021; Krunić & Rakin, 2022). In our investigation, yoghurt samples fortified with postbiotic supplements exhibited significantly enhanced DPPH scavenging activity, influenced by the formulation on day 7 of storage (p < 0.05). The BB12-SM yoghurt sample displayed the highest radical scavenging activity, showing a 6.30% inhibition on day 7 of storage (Table 3). This result was significantly superior to all other yoghurt formulations (p < 0.05), except for BB12-CW-SM. It is worth mentioning that postbiotics, notably EPS, have bioactivities such as antioxidant activities (Pan & Mei, 2010; Xu et al., 2011). These EPS may be the main reason for the high antioxidant activity observed in postbiotic yoghurt samples (Amiri et al., 2019). These findings are in line with Demirci et al. (2017), who observed a 12.75% increase in DPPH radical scavenging activities when rice bran, known for its antioxidative properties, was added to yoghurt. Some authors have noted that EPS derived from bifidobacteria demonstrates antioxidant properties (Li et al., 2014; Sengül et al., 2011; Xu et al., 2011). However, the mechanism underlying these effects remains not fully understood.
Figure 3 presents the syneresis and WHC of the samples. Monitoring yoghurt coagulum stability is essential for quality assessment during storage (Ghaderi-Ghahfarokhi et al., 2020). The weakening of the gel network could result in spontaneous syneresis, leading to the expulsion of whey from the yoghurt matrix (Ozcan & Kurtuldu, 2014). The degree of syneresis was notably affected by both the yoghurt formulation and the storage duration (p < 0.05).
Syneresis (%) (A) and water holding capacity (%) (B) in different formulations of yoghurt during storage at 4 °C. Yoghurt formulations: Refer to Table 1 for sample codes. Lowercase letters indicate significant differences (p < 0.05) between the storage days of each yoghurt sample. Uppercase letters indicate significant differences (p < 0.05) between different samples at the same storage time. Error bars represent the mean (n = 3) ± standard deviation (SD)
Specifically, syneresis was mitigated and yoghurt texture was enhanced by the addition of CW, SM, and their combination, and the extent of these results depended on specific ratios of the supplements. Initially, whey separation ranged from 20.74 to 25.60% across the different yoghurt samples (p > 0.05). All samples continued to decrease in syneresis throughout the cold storage period. Notably, syneresis significantly declined in the BB12-CW yoghurt samples, dropping from 25.60% on day 1 to 19.86% on day 21 (p < 0.05; Fig. 3A). In contrast, the reduction in the control sample was minor. This observed phenomenon may be explained by the capacity of postbiotic compounds, like EPSs produced by BB12 in BB12-CW powder, as highlighted by Ghaderi-Ghahfarokhi et al. (2020), play a role in retaining water within the gel structure of yoghurt. Instead of relying on food additives, probiotic bacteria capable of producing postbiotic are employed during fermentation to uphold optimal fermentation conditions and enhance the textural qualities of yoghurt. Consequently, this approach can obviate the necessity for supplementary stabilizers (Tiwari et al., 2021). An independent study carried out by Khider et al. (2022) documented a decrease in syneresis in low-fat yoghurt samples that included EPSs, as opposed to the control group. It is plausible that differences in the experimental conditions utilized in these studies may have played a role in the variations observed in the syneresis index, as proposed by Gezginc et al. (2015).
The ability of a gel structure to retain water is a crucial determinant of its capacity to retain serum or whey, making it a fundamental factor in yoghurt production (Kpodo et al., 2014). The incorporation of CW and SM into yoghurt had a noticeable effect on WHC in yoghurt formulations throughout the cold storage period, as depicted in Fig. 3B. Consequently, CW and SM addition were observed to enhance the yoghurt’s propensity to retain water compared to control samples. Notably, the BB12-CW formulation consistently demonstrated the most stable WHC values, ranging from 68.59 to 69.00%, while other formulations experienced negligible decreases in water retention percentage (p > 0.05), except for the control group on day 7 of the storage period (p < 0.05). Yoghurt samples enriched with cheese whey and incorporating the PP of BB12 (BB12-CW and BB12-CW-SM) exhibited the highest WHC values, reaching 70.12% and 70.11%, respectively. In a correlated study, Akalin et al. (2012) investigated the enhancement of yoghurt by fortifying it with skim milk powder, whey protein concentrate (WPC), and sodium calcium caseinate. They found that WPC-fortified samples had a WHC index of 68.78% over a storage duration of 28 days. Delikanli and Ozcan (2014) similarly observed that over a 14-day storage period, WHC was also highest (83.32%) in yoghurt samples enriched with cheese whey. Recent research by Brodziak et al. (2020) affirmed the positive impact of incorporating cheese whey into yoghurt samples, indicating that WHC values significantly rise during storage. Furthermore, as discussed in relation to syneresis, it is noteworthy that EPSs can influence the WHC of yoghurt. Using low-fat yoghurt samples, Khider et al. (2022) showed that an increase in EPS concentration corresponded to elevated water-holding capacity. In another study, the impact of 0.01% EPSs on the texture and microstructure of buffalo yoghurt indicated that the addition of EPS led to denser casein micelles. Furthermore, the incorporation of 0.01% EPS resulted in improved stability in terms of water-holding capacity, viscosity, hardness, and gumminess properties (Yang et al., 2014).
Microscopic imaging was utilized to observe modifications in the microstructure of yoghurt gel and clarify shifts in its physical and structural characteristics resulting from the enrichment with PP and subsequent refrigerated storage. These micrographs unveiled discernible disparities in the gel structures, particularly with regard to the compactness of the three-dimensional network formed by casein micelles and the dimensions of the pores (Fig. 4A–D). The three-dimensional network, which arose from the aggregation of casein micelles, exhibited a pattern of globular shapes interspersed with void zones originating from the original serum. Notably, substantial distinctions were evident between the microstructures of yoghurt samples that were not supplemented with PP and those that were. A similar phenomenon was reported by Espírito-Santo et al. (2013) in yoghurt fortified with passionfruit fiber. However, the yoghurt samples fortified with PP displayed a network with larger pores that is more open (Fig. 4D) in contrast to the control plain yoghurt (Fig. 4A). This divergence may be associated with the thermodynamic incongruity between the polysaccharide’s existent in PP and the milk proteins (Corredig et al., 2011; Grinberg & Tolstoguzov, 1997). These findings align with the observations of Lee and Lucey (2003), who noted increased whey separation in soft yoghurt gels, resulting in relatively larger pores within the gel structure. Tudorica et al. (2004) similarly reported comparable outcomes, linking the presence of larger pore sizes and the formation of a more open network to higher concentrations of β-glucans in milk curds. Furthermore, the existence of larger pores and reduced cross-linking between casein micelles may provide an explanation for the observed decrease in yield stress in yoghurt structures containing postbiotic powder (PP), as outlined in Tables 4, 5, and 6. It is noteworthy that the micrographs of yoghurt samples stored for 21 days exhibited more densely packed casein networks compared to those taken at day 1, suggesting changes in structure occurring throughout the storage period. These rearrangements likely contributed to higher shear stress values and storage modulus at the end of the storage period.
Scanning electron micrographs of yoghurt stored at 4 °C for 21 days. A-1 and A-21: Control samples at days 1 and 21, respectively. B-1 and B-21: BB12-CW samples at days 1 and 21, respectively. C-1 and C-21: BB12-SM samples at days 1 and 21, respectively. D-1 and D-21: BB12-CW-SM at days 1 and 21, respectively. Yoghurt formulations: Refer to Table 1 for sample codes
Rheological Characteristics of Yoghurts
Tables 4, 5, and 6 provide a detailed breakdown of the viscosity, hysteresis loop, and storage and loss moduli for yoghurt samples formulated in various ways. The hysteresis loop refers to the reduction in apparent viscosity over time at a constant temperature, observed when a substance undergoes an elevated shear rate (Mewis & Wagner, 2009). The addition of PP resulted in a noticeable decrease in the apparent viscosity of the yoghurt. Consequently, the viscosity of regular yoghurt was found to be higher than that of all yoghurt variants containing PP, although this difference did not reach statistical significance (p > 0.05). This phenomenon is likely linked to the interaction between caseins and polysaccharides, potentially stabilizing casein aggregates. These results are consistent with the findings of Tseng and Zhao (2013), who observed decreasing apparent viscosity values with the increasing addition of wine grape pomace as a prebiotic in yoghurt. Similarly, El-Said et al. (2014) noted a reduction in viscosity values with the increasing concentration of pomegranate peel extracts in yoghurt, attributing this effect to the impact of pomegranate peel extract on network aggregation within the yoghurt, possibly through electrostatic interactions. In contrast, some researchers reported significantly higher apparent viscosity in yoghurt samples enriched with inulin and peach dietary fiber compared to plain yoghurt (Donkor et al., 2007; Grigelmo-Miguel et al., 1999).
Our analysis revealed that the hysteresis loop, storage modulus (G′), loss modulus (G″), and viscosity of yoghurt samples fortified with postbiotics were generally lower compared to the control samples. However, an exception was noted for the BB12-CW and BB12-CW-SM groups, which exhibited comparable values on days 7, 14, and 21 of storage, and on days 1, 7, and 21 (p > 0.05), respectively. The observation that the storage modulus G′ > loss modulus G″ indicated a gel-like structure in the yoghurt. The strength of this gel relied on the support provided by the milk protein network and the production of exopolysaccharides, as indicated by the loss tangent (tan δ). These findings, along with previous research, provide insight into the mechanisms contributing to the reduction in viscosity due to decreased levels of exopolysaccharides (EPSs). This reduction can be attributed to the hydrolysis of EPSs into their constituent monomers by glycohydrolases (Purwandari et al., 2007). In our study, both the storage (G′) and loss (G″) modulus of BB12-SM were consistently lower than those of the control group throughout the storage period (Table 6). This decrease can be linked to the hydrolysis of EPSs facilitated by glycohydrolases and an increase in acidity, as illustrated in Fig. 2B, compared to the control group. The observed decrease in viscosity in postbiotic-fortified yoghurt samples compared to the control yoghurt may have implications for consumer acceptance. Evaluators favored yoghurt with a slightly thicker consistency, potentially attributed to increased acidity.
The indicators related to viscosity, hysteresis loop, and storage and loss moduli exhibited a remarkable consistency across the diverse formulations of yoghurt (Tables 4, 5 and 6, respectively). This uniformity suggests that the yoghurt products, whether crafted with postbiotic-containing cheese whey powder or skim milk, possessed similar visual and mouthfeel attributes. Notably, these characteristics remained stable over the course of a 21-day storage period. Furthermore, the findings underscored a strong correlation between the viscosity of yoghurt products and their thixotropic behavior, a pivotal aspect of hydrogel rheological properties. Evaluation of the hydrogel’s ability to revert to its original structure, referred to as the hysteresis loop, involved tracking changes in viscosity during the restitution process following shearing, as detailed by Ghica et al. (2016).
Viability of Yoghurt Cultures During Yoghurt Storage
It is evident that the proportions of the cells of S. thermophilus and L. delbrueckii ssp. bulgaricus exhibited a remarkable similarity, with each reaching an approximate concentration of 108 cfu/mL, and they remained consistent throughout the entire cold storage period (Fig. 5). This aligns with the generally accepted standard count for these cultures in yoghurt products, which should ideally be around 107 cfu/mL (Yousefvand et al., 2022). This study investigated the viability of yoghurt cultures throughout a 21-day storage period at 4 °C. The addition of CW, SM, and their combination significantly influenced the growth and survival of these cultures during cold storage. At the beginning of the storage period, the counts of S. thermophilus and L. delbrueckii ssp. bulgaricus in BB12-CW and BB12-CW-SM samples were 8.76 and 8.86 log cfu/g, respectively. These counts were slightly (but not significantly) higher in companion with the other formulation (p > 0.05) (Fig. 5A, B), consistent with earlier reports on yoghurt culture counts (Eskandari et al., 2012). In the initial days of storage, there was a slight decrease in counts for both yoghurt starters; this gradual decline persisted until the conclusion of the storage period.
Viability of S. thermophilus (A) and L. delbrueckii ssp. bulgaricus (B) in different formulations of yoghurt during storage at 4 °C. Yoghurt formulations: Refer to Table 1 for sample codes. Lowercase letters indicate significant differences (p < 0.05) between the storage days of each yoghurt sample. Uppercase letters indicate significant differences (p < 0.05) between different samples at the same storage time. Error bars represent the mean (n = 3) ± standard deviation (SD)
Earlier research conducted by Marafon et al. (2011) and Batawy and Khalil (2018) also observed this trend, as they too noted a decline in the growth of yoghurt cultures during refrigerated storage. Interestingly, the viability of S. thermophilus and L. delbrueckii ssp. bulgaricus remained higher in yoghurt samples fortified with BB12-CW-SM and BB12-SM powders over the duration of storage (p > 0.05), respectively (Fig. 5A, B). This implies that BB12-SM powder might encompass a higher concentration of nutritional compositions, offering enhanced support for yoghurt cultures. On day 21 of storage, the viable counts of starter cultures for S. thermophilus in BB12-CW, BB12-SM, and BB12-CW-SM yoghurt samples were 7.60, 7.26, and 7.51 log cfu/g, respectively; for L. delbrueckii ssp. bulgaricus, they were 6.96, 7.84, and 7.18 log cfu/g, respectively. These results indicate that the yoghurt samples maintained favorable concentrations of starter cultures. It has been shown that supplementing yoghurt with cheese whey and increasing its concentration can uplift the survival of bacteria in yoghurt products throughout the storage period and also facilitate passage through the gastrointestinal tract (Ranok et al., 2021). Glušac et al. (2015) also showed that the addition of cheese whey improved the viability of yoghurt starters, whereas honey did not have a significant impact. Moreover, the inclusion of skim milk in yoghurt has been found to increase the count of S. thermophilus during storage, as demonstrated by Marafon et al. (2011).
Consumer Testing of Yoghurts
Table 7 presents the scores obtained from consumer testing, which encompassed various aspects such as flavor, appearance, body and texture, mouthfeel, and overall acceptability. In this evaluation, it was noted that the BB12-CW yoghurt formulations received the lowest ratings across all categories except appearance and body and texture. Conversely, the control yoghurt samples received the highest ratings in most categories, with the exception of appearance (p > 0.05). This divergence in consumer ratings can be attributed to the distinctive characteristics brought about by the presence of postbiotics. It is likely that yoghurt samples underwent changes in texture and acquired a unique flavor profile due to the postbiotic components. Probiotic supplementation has been documented to notably influence the aroma of yoghurt through elevated synthesis of acetic and various other organic acids, along with acetoin, 2-butanone, and 2-ethyl-1-hexanol (Dimitrellou et al., 2019). Significantly, the satisfactory body and texture noted in the yoghurt samples might be associated with elevated levels of exopolysaccharides found in the postbiotic powders (Amiri et al., 2019; Aziznia et al., 2008; Yousefvand et al., 2022). Our observations are consistent with earlier research (Antunes et al., 2005; Salih & Hamid, 2013), emphasizing the favorable influence of skim milk additions on flavor and viscosity in comparable products. Among the postbiotic-supplemented yoghurt samples, BB12-CW-SM-fortified yoghurt garnered the highest scores across attributes such as flavor, texture, mouthfeel, and overall acceptability (p > 0.05). This suggests that the combination of CW and SM had a particularly favorable consumer acceptance. Nevertheless, Akalin et al. (2012) reported that including or omitting CW did not affect consumer acceptance of experimental yoghurts. In our study, the BB12-CW formulation was slightly less favored by the panelists, whereas the control, BB12-SM, and BB12-CW-SM formulations were notably preferred. This preference for the latter formulations corroborates the finding of Antunes et al. (2005), who reported that incorporating SM supplements positively influenced overall impressions of similar products.
Conclusion
Highlighting the importance of leveraging cost-effective and readily available sources for postbiotic production, whey — often disregarded and discarded as waste in cheese production — holds untapped potential within the food industry to avail as a valuable resource for the production of postbiotic. The examination in this research explored the utilization of cheese whey and skim milk as alternative reservoirs for the synthesis of postbiotics, focusing on cultivating postbiotics from B. animalis subsp. lactis BB12 in both media. The impact of these postbiotic-enriched supplements on yoghurt quality was comprehensively assessed. The postbiotic-enriched yoghurt demonstrated notable antioxidant activity throughout a storage duration of 21 days at 4 °C. Notably, after 10 days of storage, BB12-CW-SM yoghurt received high acceptability ratings in consumer evaluations among the postbiotic-enriched variants, with other formulations also achieving satisfactory consumer acceptance. Our findings concerning syneresis, water retention capacity, and consumer assessments during cold storage suggest that there is great promise for the postbiotic-enriched formulation to become a marketable product. Crucially, the overall characteristics of the yoghurt did not suffer from embedment of postbiotic-fortified powder into yoghurt, highlighting its suitability for integration into the final product. Furthermore, postbiotic solutions derived from probiotics in these dairy side-streams exhibit promises as nutritious beverage options. However, further exploration of postbiotic production using alternative sources, including animal or plant-based waste or byproducts, requires continued investigation. Simultaneously, regulatory standards and accurate labeling guidelines need establishment for food products incorporating postbiotics to ensure their effective integration into the food industry.
Data Availability
Data will be made available on request.
References
Abdel-Hamid, M., Romeih, E., Huang, Z., Enomoto, T., Hung, L., & Li, L. (2020). Bioactive properties of probiotic set-yogurt supplemented with Siraitia grosvenorii fruit extract. Food Chemistry, 303, 125400. https://doi.org/10.1016/j.foodchem.2019.125400
Aguilar-Toalá, J. E., Arioli, S., Behare, P., Belzer, C., Canani, R. B., Chatel, J. M., D’Auria, E., de Freitas, M. Q., Elinav, E., Esmerino, E. A., García, H. S., da Cruz, A. G., González-Córdova, A. F., Guglielmetti, S., de Toledo Guimarães, J., Hernández-Mendoza, A., Langella, P., Liceaga, A. M., Magnani, M., … Zhou, Z. (2021). Postbiotics - When simplification fails to clarify. Nature Reviews Gastroenterology & Hepatology, 18, 825–826. https://doi.org/10.1038/s41575-021-00521-6
Aguilar-Toalá, J. E., Garcia-Varela, R., Garcia, H. S., Mata-Haro, V., González-Córdova, A. F., Vallejo-Cordoba, B., & Hernández-Mendoza, A. (2018). Postbiotics: An evolving term within the functional foods field. Trends in Food Science & Technology, 75, 105–114. https://doi.org/10.1016/j.tifs.2018.03.009
Akalin, A. S., Unal, G., Dinkci, N., & Hayaloglu, A. A. (2012). Microstructural, textural, and sensory characteristics of probiotic yogurts fortified with sodium calcium caseinate or whey protein concentrate. Journal of Dairy Science, 95, 3617–3628. https://doi.org/10.3168/jds.2011-5297
AOAC - Association of Official Analytical Chemists. (1995). Official methods of analysis of the association of official analytical chemists (sixteen). Gaithersburg.
AOAC - Association of Official Analytical Chemists. (2005). Official methods of analysis of the association of official analytical chemists (eighteen). Gaithersburg.
Amado, I. R., & V´azquez, J. A., Pastrana, L., & Teixeira, J. A. (2016). Cheese whey: A cost effective alternative for hyaluronic acid production by Streptococcus zooepidemicus. Food Chemistry, 198, 54–61. https://doi.org/10.1016/j.foodchem.2015.11.062
Amiri, S., Mokarram, R. R., Khiabani, M. S., Bari, M. R., & Alizadeh, M. (2021). Optimization of food-grade medium for co-production of bioactive substances by Lactobacillus acidophilus LA-5 for explaining pharmabiotic mechanisms of probiotic. Journal of Food Science and Technology, 58, 1–12. https://doi.org/10.1007/s13197-020-04894-5
Amiri, S., Mokarram, R. R., Khiabani, M. S., Rari, M. R., & Khaledabad, M. A. (2019). Exopolysaccharides production by Lactobacillus acidophilus LA5 and Bifidobacterium animalis subsp. lactis BB12: Optimization of fermentation variables and characterization of structure and bioactivities. International Journal of Biological Macromolecules, 15, 123, 752–765. https://doi.org/10.1016/j.ijbiomac.2018.11.084
Amiri, S., Mokarram, R. R., Khiabani, M. S., Bari, M. R., & Khaledabad, M. A. (2022). Characterization of antimicrobial peptides produced by Lactobacillus acidophilus LA-5 and Bifidobacterium lactis BB-12 and their inhibitory effect against foodborne pathogens. Journal of Food Science and Technology, 153, 112449. https://doi.org/10.1016/j.lwt.2021.112449
Amiri, S., Mokarram, R. R., Khiabani, M. S., Bari, M. R., & Khaledabad, M. A. (2020). In situ production of conjugated linoleic acid by Bifidobacterium lactis BB12 and Lactobacillus acidophilus LA5 in milk model medium. LWT - Food Science and Technology, 132, 109933. https://doi.org/10.1016/j.lwt.2020.109933
Antunes, A. E. C., Cazetto, T. F., & Bolini, H. M. A. (2005). Viability of probiotic micro-organisms during storage, postacidification and sensory analysis of fat-free yogurts with added whey protein concentrate. International Journal of Dairy Technology, 58, 169–173. https://doi.org/10.1111/j.1471-0307.2005.00203.x
Aziznia, S. A., Khosrowshahi, A., Madadlou, A., & Rahimi, J. (2008). Whey protein concentrate and gum tragacanth as fat replacers in nonfat yogurt: Chemical, physical, and microstructural properties. Journal of Dairy Science, 91, 2545–52. https://doi.org/10.3168/jds.2007-0875
Barros, C. P., Guimarães, J. T., Esmerino, E. A., Duarte, M. C. K. H., Silva, M. C., Silva, R., Ferreira, B. M., & Sant’Ana, A. S., Freitas, M. Q., & Cruz, A. G. (2020). Paraprobiotics and postbiotics: Concepts and potential applications in dairy products. Current Opinion in Food Science, 32, 1–8. https://doi.org/10.1016/j.cofs.2019.12.003
Batawy, O. E., & Khalil, O. S. (2018). Manufacture and properties of low-fat bio yoghurt containing probiotic strains and maltodextrin as prebiotic. Journal of Probiotics and Health, 6(1), 77–90. https://doi.org/10.4172/2329-8901.1000192
Brodziak, A., Król, J., Barłowska, J., Teter, A., & Florek, M. (2020). Changes in the physicochemical parameters of yoghurts with added whey protein in relation to the starter bacteria strains and storage time. Animal, 10, 1350. https://doi.org/10.3390/ani10081350
Basiri, S., Haidary, N., Shekarforoush, S. S., & Niakousari, M. (2018). Flaxseed mucilage: A natural stabilizer in stirred yogurt. Carbohydrate Polymers, 1, 59–65. https://doi.org/10.1016/j.carbp.ol.2018.01.049
Chang, H. M., Foo, H. L., Loh, T. C., Lim, E. T. C., & Abdul Mutalib, N. E. (2021). Comparative studies of inhibitory and antioxidant activities, and organic acids compositions of postbiotics produced by probiotic Lactiplantibacillus plantarum strains isolated from Malaysian foods. Frontiers in Veterinary Science, 7, 602280. https://doi.org/10.3389/fvets.2020.602280
Corredig, M., Sharafbafi, N., & Kristo, E. (2011). Polysaccharide-protein interactions in dairy matrices, control and design of structures. Food Hydrocolloids, 25(8), 1833e1841. https://doi.org/10.1016/j.foodhyd.2011.05.014
Delikanli, B., & Ozcan, T. (2014). Effects of various whey proteins on the physicochemical and textural properties of set type nonfat yoghurt. International Journal of Dairy Technology, 67, 495–503. https://doi.org/10.1111/1471-0307.12142
Demirci, T., Aktaş, K., Sözeri, D., Öztürk, H. İ, & Akın, N. (2017). Rice bran improve probiotic viability in yoghurt and provide added antioxidative benefits. Journal of Functional Foods, 36, 396–403. https://doi.org/10.1016/j.jff.2017.07.019
Dhillon, B., Sodhi, N. S., Kumari, S., Kaur, A., Sharma, S., & Khan, Z. S. (2023). Physico-chemical, antioxidant, sensory and electromyographic analyses of shrikhand (flavored strained yoghurt) formulated for dysphagia patients under IDDSI levels 4 and 5. Journal of Food Measurement and Characterization. https://doi.org/10.1007/s11694-023-02061-w
Dias, C. O., & dos Santos Opuski de Almeida, J., Pinto, S. S., de Oliveira Santana, F. C., Verruck, S., Müller, C. M. O., Prudêncio, L. S., & Dias de Mello Castanho Amboni, R. (2018). Development and physico-chemical characterization of microencapsulated Bifidobacteria in passion fruit juice: A functional non-dairy product for probiotic delivery. Food Bioscience, 24, 26–36. https://doi.org/10.1016/j.fbio.2018.05.006
Dimitrellou, D., Kandylis, P., & Kourkoutas, Y. (2019). Assessment of freeze-dried immobilized Lactobacillus casei as probiotic adjunct culture in yogurts. Foods, 8(9), 374. https://doi.org/10.3390/foods8090374
Donkor, O. N., Nilmini, S. L. I., Stolic, P., Vasiljevic, T., & Shah, N. P. (2007). Survival and activity of selected probiotic organisms in set-type yoghurt during cold storage. International Dairy Journal, 17(6), 657–665. https://doi.org/10.1016/j.idairyj.2006.08.006
Espírito-Santo, A. P., Lagazzo, A., Sousa, A. L. O. P., Perego, P., Converti, A., & Oliveira, M. N. (2013). Rheology, spontaneous whey separation, microstructure and sensorial characteristics of probiotic yoghurts enriched with passion fruit fiber. Food Research International, 50(1), 224–231. https://doi.org/10.1016/j.foodres.2012.09.012
Elsamani, M. O., & Ahmed, I. A. M. (2014). Physicochemical characteristics and organoleptic properties of peanuts milk-based yoghurt fortified with skimmed milk powder. Journal of Research in Applied Sciences, 1, 68–72.
Eskandari, M. H., Baroutkoub, A., Roushan Zamir, M., Beglarian, R., Ghasemkhani, I., & Shekarforoush, S. S. (2012). Effect of milk supplementation on growth and viability of starter and probiotic bacteria in yogurt during refrigerated storage. Iranian Journal of Veterinary Research, 13(3), 195–202. https://doi.org/10.22099/ijvr.2012.358
El-Said, M. M., Haggag, H. F., Fakhr El-Din, H. M., Gad, A. S., & Farahat, A. M. (2014). Antioxidant activities and physical properties of stirred yoghurt fortified with pomegranate peel extracts. Annals of Agricultural Sciences, 59(2), 207–212. https://doi.org/10.1016/j.aoas.2014.11.007
Fazilah, N. F., Ariff, A. B., Khayat, M. E., Rios-Solis, L., & Halim, M. (2018). Influence of probiotics, prebiotics, synbiotics and bioactive phytochemicals on the formulation of functional yogurt. Journal of Functional Foods, 48, 387–399. https://doi.org/10.1016/j.jff.2018.07.039
Gezginc, Y., Topcal, F., Comertpay, S., & Akyol, I. (2015). Quantitative analysis of the lactic acid and acetaldehyde produced by Streptococcus thermophilus and Lactobacillus bulgaricus strains isolated from traditional Turkish yogurts using HPLC. Journal of Dairy Science, 98, 1426–1434. https://doi.org/10.3168/jds.2014-8447
Ghaderi-Ghahfarokhi, M., Yousefvand, A., Gavlighi, H. A., Zarei, M., & Farhangnia, P. (2020). Developing novel synbiotic low-fat yoghurt with fucoxylogalacturonan from tragacanth gum: Investigation of quality parameters and Lactobacillus casei survival. Food Sciences and Nutrition, 8(8), 4491–4504. https://doi.org/10.1002/fsn3.1752
Ghaderi-Ghahfarokhi, M., Yousefvand, A., Gavlighi, H. A., & Zarei, M. (2021). The effect of hydrolysed tragacanth gum and inulin on the probiotic viability and quality characteristics of low-fat yoghurt. International Journal of Dairy Technology, 74(1), 161–169. https://doi.org/10.1111/1471-0307.12742
Ghica, M. V., Hîrjău, M., Lupuleasa, D., & Dinu-Pîrvu, C. E. (2016). Flow and thixotropic parameters for rheological characterization of hydrogels. Molecules, 21, 786. https://doi.org/10.3390/molecules21060786
Glušac, J., Stijepić, M., Đurđević-Milošević, D., Milanović, S., Kanurić, K., & Vukić, V. (2015). Growth and viability of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus in traditional yoghurt enriched by honey and whey protein concentrate. Iranian Journal of Veterinary Research, 16, 249–254.
Gonzaíez-Martí, C. G., Becerra, M., Chafer, M., Albors, A., Carot, J. M., & Chiralt, A. (2002). Influence of substituting milk powder for whey powder on yoghurt quality. Trends in Food Science & Technology, 3, 816. https://doi.org/10.1016/S0924-2244(02)00160-7
Grigelmo-Miguel, N., Gorinstein, S., & Martı´n-Belloso, O. (1999). Characterisation of peach dietary fibre concentrate as a food ingredient. Food Chemistry, 65(2), 175–181. https://doi.org/10.1016/S0308-8146(98)00190-3
Grinberg, V. Y., & Tolstoguzov, V. B. (1997). Thermodynamic incompatibility of proteins and polysaccharides in solutions. Food Hydrocolloids, 11(2), 145–158. https://doi.org/10.1016/S0268-005X(97)80022-7
Haque, A., Timilsena, Y. P., & Adhikari, B. (2016). Food protein, structure and function food proteins, structure, and function. Reference Module in Food Science. https://doi.org/10.1016/B978-0-08-100596-5.03057-2
Hardy, Z., & Jideani, V. A. (2020). Functional characteristics and microbiological viability of foam-mat dried Bambara groundnut (Vigna subterranea) yogurt from reconstituted Bambara groundnut milk powder. Food Science & Nutrition, 8, 5238–5248. https://doi.org/10.1002/fsn3.951
Imperial, I. C. V. J., & Ibana, J. A. (2016). Addressing the antibiotic resistance problem with probiotics: Reducing the risk of its double-edged sword effect. Frontiers in Microbiology. https://doi.org/10.3389/fmicb.2016.01983
İncili, G. K., Karatepe, P., Akgöl, M., Kaya, B., Kanmaz, H., & Hayaloğlu, A. A. (2021). Characterization of Pediococcus acidilactici postbiotic and impact of postbiotic-fortified chitosan coating on the microbial and chemical quality of chicken breast fillets. International Journal of Biological Macromolecules, 184, 429–437. https://doi.org/10.1016/j.ijbiomac.2021.06.106
Jouki, M., Khazaei, N., Rezaei, F., & Taghavian-Saeid, R. (2021). Production of synbiotic freeze-dried yoghurt powder using microencapsulation and cryopreservation of L. plantarum in alginate-skim milk microcapsules. International Dairy Journal, 122, 105133. https://doi.org/10.1016/j.idairyj.2021.105133
Karaca, O. B., Güzeler, N., Tangüler, H., Yasar, K., & Akın, M. B. (2019). Effects of apricot fibre on the physicochemical characteristics, the sensory properties and bacterial viability of nonfat probiotic yoghurts. Foods, 8, 33. https://doi.org/10.3390/foods8010033
Khider, M., El-Readi, M. Z., Abdalrahim, S., Zohri, A. N., Ibrahim, I. M., & Abulreesh, H. H. (2022). Functional low-fat set yogurt enhanced with microbial exo-polysaccharides-mediated anticancer activity. Journal of Pure and Applied Microbiology, 16(4), 2601–2618. https://doi.org/10.22207/JPAM.16.4.28
Kpodo, F. M. K., Afoakwa, E. O., Amoa, B. B., Budu, A. S., & Saalia, F. K. (2014). Effect of ingredient variation on microbial acidification, susceptibility to syneresis, water holding capacity and viscosity of soy-peanut-cow milk yoghurt. Journal of Nutritional Health & Food Engineering, 1, 74–79. https://doi.org/10.15406/jnhfe.2014.01.00012
Krunić, T., & Rakin, M. B. (2022). Enriching alginate matrix used for probiotic encapsulation with whey protein concentrate or its trypsin-derived hydrolysate: Impact on antioxidant capacity and stability of fermented whey-based beverages. Food Chemistry, 15, 130931. https://doi.org/10.1016/j.foodchem.2021.130931
Kumar, A. S., Mody, K., & Jha, B. (2007). Bacterial exopolysaccharides–A perception. Journal of Basic Microbiology, 47, 103–117. https://doi.org/10.1002/jobm.200610203
Lee, W. J., & Lucey, J. A. (2003). Rheological properties, whey separation, and microstructure in set-style yogurt: Effects of heating temperature and incubation temperature. Journal of Texture Studies, 34(5–6), 515–536. https://doi.org/10.1111/j.1745-4603.2003.tb01079.x
Li, H., Song, W., Liu, T., Xu, S., Zhang, S., Zhang, Y., Liu, D., Li, H., & Yu, J. (2022). Developing novel synbiotic yoghurt with Lacticaseibacillus paracasei and lactitol: Investigation of the microbiology, textural and rheological properties. International Dairy Journal, 135, 105475. https://doi.org/10.1016/j.idairyj.2022.105475
Li, S., Huang, R., Shah, P., & N., Tao, X., Xiong, Y., & Wei, H. (2014). Antioxidant and antibacterial activities of exopolysaccharides from Bifidobacterium bifidum WBIN03 and Lactobacillus plantarum R315. Journal of Dairy Science, 97(12), 7334–7343. https://doi.org/10.3168/jds.2014-7912
Meena, L., Neog, R., Yashini, M., & Sunil, C. K. (2022). Pineapple pomace powder (freeze-dried): Effect on the texture and rheological properties of set-type yogurt. Food Chemistry Advances, 1, 100101. https://doi.org/10.1016/j.focha.2022.100101
Marafon, A. P., Sumi, A., Granato, D., Alcântara, M. R., Tamime, A. Y., & Nogueira de Oliveira, M. (2011). Effects of partially replacing skimmed milk powder with dairy ingredients on rheology, sensory profiling, and microstructure of probiotic stirred-type yoghurt during cold storage. Journal of Dairy Science, 94, 5330–5340. https://doi.org/10.3168/jds.2011-4366
Meira, Q. G. S., Magnani, M., de Medeiros Júnior, F. C., Queiroga, R. C. R. D. E., Madruga, M. S., Gullón, B., Gomes, A. M. P., Pintado, M. M. E., & Souza, E. L. (2015). Effects of added Lactobacillus acidophilus and Bifidobacterium lactis probiotics on the quality characteristics of goat ricotta and their survival under simulated gastrointestinal conditions. Food Research International, 76, 828–838. https://doi.org/10.1016/j.foodres.2015.08.002
Mewis, J., & Wagner, N. J. (2009). Thixotropy. Advances in Colloid and Interface Science, 147–148, 214–227. https://doi.org/10.1016/j.cis.2008.09.005
Moradi, M., Molaei, R., & Guimarães, J. T. (2021). A review on preparation and chemical analysis of postbiotics from lactic acid bacteria. Enzyme and Microbial Technology, 143, 109722. https://doi.org/10.1016/j.enzmictec.2020.109722
Mudgil, D., Barak, S., & Khatkar, B. S. (2016). Development of functional yoghurt via soluble fiber fortification utilizing enzymatically hydrolyzed guar gum. Food Bioscience, 14, 28–33. https://doi.org/10.1016/j.fbio.2016.02.003
Ozcan, T., & Kurtuldu, O. (2014). Influence of dietary fiber addition on the properties of probiotic yogurt. International Journal of Chemical Engineering, 5, 397–401. https://doi.org/10.1016/j.idairyj.2003.08.004
Pan, D., & Mei, X. (2010). Antioxidant activity of an exopolysaccharide purified from Lactococcus lactis subsp. lactis 12. Carbohydrate Polymers, 80, 908–914. https://doi.org/10.1016/j.carbpol.2010.01.005
Purwandari, U., Shah, N. P., & Vasiljevic, T. (2007). Effects of exopolysaccharide-producing strains of Streptococcus thermophilus on technological and rheological properties of set-type yoghurt. International Dairy Journal, 17, 1344–1352. https://doi.org/10.1016/j.idairyj.2007.01.018
Ranok, A., Kupradit, C., Khongla, C., Musika, S., Mangkalanan, S., & Suginta, W. (2021). Effect of whey protein concentrate on probiotic viability and antioxidant properties of yoghurt during storage and simulated gastrointestinal transit. International Food Research Journal, 28, 110–119.
Rudke, C. R. M., Camelo-Silva, C., Rudke, A. R., Prudencio, E. S., & de Andrade, C. J. (2023). Trends in dairy products: New ingredients and ultrasound-based porocessing. Food and Bioprocess Technology. https://doi.org/10.1007/s11947-023-03153-7
Sabahi, S., Homayouni Rad, A., Aghebati-Maleki, L., Sangtarash, N., Ozma, M. A., Karimi, A., Hosseini, H., Hosseini, H., & Abbasi., A. (2022). Postbiotics as the new frontier in food and pharmaceutical research. Critical Reviews in Food Science and Nutrition, 29, 1–28. https://doi.org/10.1080/10408398.2022.2056727
Sadighbathi, S., Saris, P. E. J., Amiri, S., & Yousefvand, A. (2023). Development and properties of functional yoghurt enriched with postbiotic produced by yoghurt cultures using cheese whey and skim milk. Frontiers in Microbiology, 14, 1276268. https://doi.org/10.3389/fmicb.2023.1276268
Salih, M. M., & Hamid, O. I. A. (2013). Effect of fortifying camel’s milk with skim milk powder on the physicochemical, microbiological and sensory characteristics of set yoghurt. Advance Journal of Food Science and Technology, 5, 765–770. https://doi.org/10.19026/ajfst.5.3161
Salminen, S., Collado, M. C., Endo, A., Hill, C., Lebeer, S., & Quigley, E. M. M. (2021). The international scientific association of probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nature Reviews Gastroenterology & Hepatology, 18, 649–667. https://doi.org/10.1038/s41575-021-00440-6
Sanusi, M. S., Raji, A. O., & Ayilaran, E. O. (2022). Kinetic acidification and quality composition of yoghurt produced with soursop puree. Journal of Food Measurement and Characterization, 16, 2229–2239. https://doi.org/10.1007/s11694-022-01339-9
Şengül, N., Işık, S., Aslım, B., & Uc¸ar, G., & Demirbag˘, A. E. (2011). The effect of exopolysaccharide-producing probiotic strains on gut oxidative damage in experimental colitis. Digestive Diseases and Sciences, 56, 707–714. https://doi.org/10.1007/s10620-010-1362-7
Sharafi, H., Moradi, M., & Amiri, S. (2022). Application of cheese whey containing postbiotics of Lactobacillus acidophilus LA5 and Bifidobacterium animalis BB12 as a preserving liquid in high-moisture Mozzarella. Foods, 11, 3387. https://doi.org/10.3390/foods11213387
Sharma, M., & Shukla, G. (2016). Metabiotics: One step ahead of probiotics; an insight into mechanisms involved in anticancerous effect in colorectal cancer. Frontiers in Microbiology, 7, 1940–1940. https://doi.org/10.3389/fmicb.2016.01940
Thorakkattu, P., Khanashyam, A. C., Shah, K., Babu, K. S., Mundanat, A. S., Deliephan, A., Deokar, G. S., Santivarangkna, C., & Nirmal, N. P. (2022). Postbiotics: Current trends in food and pharmaceutical industry. Foods, 11, 3094. https://doi.org/10.3390/foods11193094
Tiwari, S., Kavitake, D., Devi, P. B., & Halad, P. S. (2021). Bacterial exopolysaccharides for improvement of technological, functional and rheological properties of yoghurt. International Journal of Biological Macromolecules, 183, 1585–1595. https://doi.org/10.1016/j.ijbiomac.2021.05.140
Tseng, A., & Zhao, Y. (2013). Wine grape pomace as antioxidant dietary fibre for enhancing nutritional value and improving storability of yogurt and salad dressing. Food Chemistry, 138(1), 356–365. https://doi.org/10.1016/j.foodchem.2012.09.148
Tudorica, C. M., Jones, T. E. R., Kuri, V., & Brennan, C. S. (2004). The effects of refined barley b-glucan on the physico-structural properties of low-fat dairy products: Curd yield, microstructure, texture and rheology. Journal of the Science of Food and Agriculture, 84(10), 1159–1169. https://doi.org/10.1002/jsfa.1789
Xu, R., Shang, N., & Li, P. (2011). In vitro and in vivo antioxidant activity of exopolysaccharide fractions from Bifidobacterium animalis RH. Anaerobe, 17(5), 226–231.
Yang, F., Li, H., Wang, S., Zhao, F., Fang, F., Guo, J., & Shen, Y. (2022). Differences in exopolysaccharides of three microbial aggregates. Environmental Technology, 43(19), 2909–2921. https://doi.org/10.1080/09593330.2021.1909658
Yang, T., Wu, K., Wang, F., Liang, X., Liu, Q., Li, G., & Li, Q. (2014). Effect of exopolysaccharides from lactic acid bacteria on the texture and microstructure of buffalo yoghurt. International Dairy Journal, 34(2), 252–256. https://doi.org/10.1016/j.idairyj.2013.08.007
Yousefvand, A., Huang, X., Zarei, M., & Saris, P. E. J. (2022). Lacticaseibacillus rhamnosus GG survival and quality parameters in kefir produced from kefir grains and natural kefir starter culture. Food, 11, 523. https://doi.org/10.3390/foods11040523
Funding
Open Access funding provided by University of Helsinki (including Helsinki University Central Hospital). Financial support for this study was provided by the Finnish Food Research Foundation (Grant no. 3032022), proof-of-concept funding from the Faculty of Agriculture and Forestry at the University of Helsinki (Grant no. 11142022), the Finnish Society of Sciences and Letters (Grant no. 220041), and the Oskar Öflund Foundation (Grant no. 230121). Furthermore, Thao M. Ho expresses gratitude for the support from the Academy of Finland (Grant no. 346839). Open access funding is covered by the Helsinki University Library.
Author information
Authors and Affiliations
Contributions
Amin Yousefvand: conceptualization, methodology, formal analysis, funding acquisition, project administration, data curation, supervision, writing — original draft, writing — review and editing. Quang-Hieu Pham: investigation, methodology, data curation, writing — review and editing. Thao Minh Ho: investigation, methodology, data curation, writing — review and editing. Saber Amiri: conceptualization, writing — review and editing. Noora Mäkelä-Salmi: conceptualization, writing — review and editing. Per Erik Joakim Saris: conceptualization, funding acquisition, writing — review and editing.
Corresponding author
Ethics declarations
Ethics Approval
Each individual provided informed consent before participating in the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yousefvand, A., Pham, QH., Ho, T.M. et al. Bifidobacterium animalis subsp. lactis BB12-Derived Postbiotic Powders Enhance Antioxidant and Physicochemical Properties of Low-Fat Yoghurt. Food Bioprocess Technol (2024). https://doi.org/10.1007/s11947-024-03405-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11947-024-03405-0