Abstract
This is the first study to produce cucumber pickles using both free and microencapsulated Lactiplantibacillus plantarum HL4 and Pediococcus parvulus HL14, and to investigate the probiotic viability, as well as the physicochemical (pH, total acidity, salt, and color), bioactive (total phenolic content and antioxidant activity) and sensory properties of the pickles during 15 days of fermentation and 9 weeks of storage. L. plantarum HL4 and P. parvulus HL14 were encapsulated with sodium alginate (as a coating agent) and inulin (as a prebiotic source) using an extrusion method. The encapsulation efficiency of L. plantarum HL4 and P. parvulus HL14 was 95.77 ± 6.21% and 94.94 ± 2.94%, respectively. Both free and microencapsulated cells were incorporated into prepared cucumbers at a rate of 1%. Probiotic cucumber pickles kept the highest microencapsulated cell count (> 6 log CFU/g) until the fourth week of storage. This study indicated that the probiotic survivability in samples can be improved by microencapsulation. During fermentation, the pH and total acidity of the samples varied in the range of 3.22–3.97 and 0.19-0.87%, respectively. The antioxidant activity of the samples ranged from 4.54 to 18.70% (DPPH) and from 51.92 to 88.06% (ABTS+). The total phenolic content of the samples varied between 142.83 and 2465.50 mg GAE/L. Moreover, CP-L (samples fermented with L. plantarum HL4) and CP-P (samples fermented with P. parvulus HL14) showed the highest general assessment scores of 6.90 and 6.95 at the end of storage, respectively. This study offers the opportunity for food companies to become competitive in one of the most innovative research areas in the food sector and to meet the requirements and needs of various consumer groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vegetables are considered nutritious, health-promoting and disease-preventing foods due to their nutritional and functional properties. Pickling is one of the most common and low-cost methods of preserving and supplying vegetables in the off-season (Sun et al., 2022). Lactic acid bacteria (LAB) predominate in vegetable fermentation. They are highly valued for their probiotic properties. Numerous LAB species, including the homofermentative Pediococcus spp. and the heterofermentative Lactiplantibacillus plantarum (L. plantarum), are present as a natural microflora on vegetable surfaces and offer the opportunity to be evaluated as probiotic starter cultures for controlled fermentation (Ahmed et al., 2021; Basdogan, 2020; Jang et al., 2021; Page & Pérez-Díaz, 2023).
Probiotics can be taken orally through food formulations. As mentioned above, fermented foods have been extensively studied as potential carriers of probiotics. However, some of them, e.g., pickles, may not have enough viable probiotic cells to be considered a probiotic food, despite their potential probiotic content. It is therefore important to ensure that probiotics maintain their viability during fermentation or storage when added to foods. Another critical challenge in the production of probiotic foods is maintaining cell viability when exposed to gastrointestinal conditions. In this context, probiotics can be microencapsulated to increase their viability under challenging conditions. On the other hand, microencapsulation preserves the sensory qualities of the food. In this process, sodium alginate is widely used as an encapsulation matrix due to its simplicity, non-toxicity, low cost, biocompatibility, and acceptability (Olivares et al., 2019).
Although, there are few studies on the potential use of free probiotic cells in pickles production (Al-Shawi et al., 2019; Çetin, 2011; Fan et al., 2017), there is no published information on the production of probiotic cucumber pickles using microencapsulated cells. The hypothesis of this study is that probiotic cells can be microencapsulated to extend the shelf life of the final product by maintaining cell viability. Therefore, the aim of the present study was to produce probiotic cucumber pickles using microencapsulated L. plantarum HL4 and P. parvulus HL14 by extrusion technique and to assess their viability in cucumber pickles. The effects of the probiotics on the physicochemical, bioactive, and sensory properties of the pickles during fermentation and storage were also studied.
Material and Method
Material
Finding LAB strains that can act as both bioprotectants and autochthonous candidates for cucumber fermentation is important. Therefore, this study focused on the previously characterized potential probiotic strains L. plantarum HL4 and P. parvulus HL14 (Basdogan, 2020) belonging to the culture collection of the Microbiology Research Laboratory of the Ege University (Izmir, Türkiye). The probiotics were activated twice at 37 °C for 18 h in MRS broth (pH 5.6 ± 0.2, Condalab, Spain) with 1% inoculum and subcultured under the same conditions before each assay.
Folin–Ciocalteu phenol reagent, ABTS [2,2’-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid], and DPPH (1,1-Diphenyl-2-picrylhydrazyl) were purchased from Sigma (St. Louis, MO). All other chemicals were of analytical grade.
Fresh cucumbers (Cucumis sativus L.) were purchased from a local market in Izmir, Türkiye. They were sorted, stripped of their flowers, washed, disinfected with a 10% (v/v) vinegar solution for 15 min, and dried under hygienic conditions to avoid any contamination. They were then mixed with brine [rock salt (6%, w/v), vinegar (1%, v/v) and water] and placed in jars (Guney, 2022).
Preparation of Probiotic cell Suspension and Microencapsulation
Briefly, the activated cells were collected by centrifugation (Hettich GmbH & Co. KG, 78.532, Germany) at 6000×g at 4 °C for 10 min. After the removal of the supernatant, the pellets were washed twice with sterile saline solution (0.85% w/v, NaCl) and resuspended in the same solution to obtain free probiotic cells (approximately 9 log CFU/mL). Microencapsulation was performed according to the extrusion method of Krasaekoopt and Watcharapoka (2014), with slight modifications.
The harvested cells were washed twice with sterile saline solution and resuspended in 5 mL of inulin solution (1.5% w/v) (cell suspension, 9.00 ± 0.14 log CFU/mL for L. plantarum HL4 and 9.13 ± 0.07 log CFU/mL for P. parvulus HL14). The sterile 20 mL alginate solution (3% w/v) was added to the cell suspension and gently mixed for 30 min. The homogeneous solution was then loaded into a disposable needle (26G) and dropped from a height of 10 cm into a sterile calcium chloride solution (250 mL, 0.05 M, CaCl2). After 30 min, the solidified microencapsules were collected by filtration (Whatman No. 1). They were then kept in PW (0.1% w/v, pH 7.0 ± 0.2) (Fig. 1). The size range of the microcapsules ranged between 2 and 3 mm.
Encapsulation Efficiency
1 g of microencapsules were dissolved in 9 mL of sodium citrate solution (50 mM, pH 7.5) to release the bacteria from the microcapsules. After the preparation of appropriate dilutions (tenfold serial dilutions in 0.1% PW), 1 mL aliquots were mixed with MRS agar (pH 5.6 ± 0.2) using the pour plate method, and then the plates were incubated at 37 °C for 48–72 h. Encapsulation efficiency (EE) was evaluated using Eq. 1 (Raddatz et al., 2022).
where Nt and N0 represent the count of probiotics after (log CFU/g microencapsule) and before (log CFU/mL) the microencapsulation, respectively.
Cucumber Pickle Production and Sampling
Briefly, both free and microencapsulated cells were incorporated into prepared cucumbers at a rate of 1% as described in Sect. 2.1 (Shoaei et al., 2022) and fermented at room temperature (25 °C) with sampling at 0, 3, 7, 15 days (pickle fermentation) for microbiological, physicochemical, and bioactivity analyses. They were also stored at 4 °C for 9 weeks with weekly sampling for microbiological, physicochemical, bioactivity, and sensory analyses. The negative control (NC) was produced by spontaneous fermentation (Fig. 2).
Methods
Microbiological Analysis
10 g of sample was mixed with PW (90 mL of 0.1%, w/v) and homogenized using a Stomacher Lab-Blender 400 (Seward Medical, London, UK). Appropriate ten-fold dilutions of the homogenate in PW were prepared for further analysis.
Viability Assessment of Free and Microencapsulated Probiotics in Pickles
To determine the free cell count, appropriate ten-fold dilutions of the homogenate (Sect. 2.4.1) were poured into plates and MRS agar containing 4 µg/mL erythromycin was added to enumerate selected probiotics (Pan et al., 2011).
Cell viability in microcapsules was determined as described in Sect. 2.2.
Yeast Count
The yeast count was assessed during fermentation and storage using the pour plate method, which was offered by FDA-BAM (2001). Briefly, PDA (pH 5.6 ± 0.2) containing 10% (w/v) tartaric acid was used, and the plates were incubated at 25ºC for 3–5 days.
Physicochemical, Bioactive and Sensorial Characteristics
Physicochemical Properties
The pH was determined directly using a calibrated portable pH meter (Mettler Toledo, SevenCompact S210, China) (AOAC, 2007). Acidity was determined by titration with 0.1 N sodium hydroxide (NaOH) (Merck, Germany) in the presence of a phenolphthalein indicator until the color change persisted for 30 s (Merck, Germany) and expressed as lactic acid percentage (AOAC, 2007). Salt concentration (NaCl%) was determined based on the Mohr method (Kirk & Sawyer, 1991). Color parameters were determined using a HunterLab Colorflex (CFLX 45–2 Model Colorimeter, HunterLab) set to illuminant D65 (standard daylight) and a 10° observer. The CIE Lab coordinates of L* (lightness), a* (redness or greenness), and b* (yellowness or blueness) were read throughout storage (Kramer & Twigg, 1984). Chroma (C*, color intensity) and hue angle (Hº, properties of the color) were also determined using the following equations (McLellan et al., 1995; Wrolstad & Smith, 2017):
Bioactive Properties
The total phenolic content was determined according to the Folin-Ciocalteu colorimetric method (Cemeroğlu, 2013). The absorbance of the mixture was measured at 760 nm using a spectrophotometer (Agilent Technologies, Carry60 UV–Visible). The results were expressed as mg gallic acid equivalent (GAE)/L.
The antioxidant activity was determined using two different methods. The DPPH radical scavenging method was used, as described by Singh et al. (2002). The absorbance was recorded at 517 nm using a spectrophotometer. The DPPH radical scavenging capacity was calculated using the following Eq. 4:
DPPH radical scavenging activity (%) = [(Ac – As) * 100] / Ac, (Eq. 4)
where Ac is the absorbance of the control and As is the absorbance of the sample.
The antioxidant activity of the samples was also determined using the ABTS assay (Re et al., 1999). The absorbance at 734 nm was measured after 6 min using a spectrophotometer. The ABTS+ radical scavenging activity was calculated using the following Eq. 5.
ABTS radical scavenging activity (%) = [(Ac – As) * 100] / Ac, (Eq. 5)
where Ac is the absorbance of the ABTS+ working solution and As is the absorbance of the sample.
Sensory Analysis
Sensory evaluation was performed at the end of the fermentation and during 8 weeks of storage. The sensory properties (color, odor, appearance, taste, hardness, and general assessment) of the probiotic cucumber pickles were evaluated using a 9-point hedonic scale. A three-digit random number code was used for each sample. The samples were then given to the panelists (10 panelists aged between 18 and 30 years) in random order on plastic plates. The mean sensory scores were used in the analysis period (Altug and Elmaci, 2005).
Data Analysis
All the experiments were carried out in triplicate, and the mean ± standard deviation is reported. Data were subjected to a one-way ANOVA and Duncan’s multiple range test to determine significant differences at the 95% level using SPSS 25.
Result and Discussion
Probiotic Count
The free probiotic count in cucumber pickles containing L. plantarum HL4 (CP-L) and cucumber pickles containing P. parvulus HL14 (CP-P) at the beginning of fermentation (day zero) was 7.08 and 7.37 log CFU/g, respectively. During the following days of fermentation, an increase in the free probiotic count was observed, and this count was maintained at a certain level (> 7 log units) throughout the fermentation (P > 0.05) (Table 1).
Microcapsules containing probiotic cells were formed by applying the extrusion process and using alginate as a coating agent and inulin as a prebiotic source. The encapsulation efficiency of L. plantarum HL4 and P. parvulus HL14 was 95.77 ± 6.21% and 94.94 ± 2.94%, respectively (Fig. 3). This is consistent with the results published in the studies of Gul and Dervisoglu (2017) by 95.92–99.75%, Holkem et al. (2016) by 89.91% and Mahmoud et al. (2020) by 96.27% using sodium alginate as a coating material. Considering the results, it can be seen that the probiotics were successfully entrapped and a higher count of probiotic cells (> 1 log unit) were added to the cucumber pickles by microencapsulation, as indicated in Table 1. As the fermentation progressed, the microencapsulated probiotic counts in CP-L and CP-P decreased (P < 0.05), and CP-P had the highest probiotic count (7.52 log CFU/g microencapsule) at the end of fermentation (P > 0.05). This may be due to the inability of the microencapsulated cells to benefit from the nutrients in the environment, a lack of nutrients, or to the intrusion of unfavorable environmental conditions (such as excessive salt concentrations, high acidity) into the microencapsules. Consequently, the pickles met the “>6 log CFU/g live probiotic cell criteria” required for probiotic products, as the research showed that potential probiotic cultures could maintain their viability > 7 log CFU/g until the end of the fermentation. On the other hand, it is not known whether the LAB found in NC is probiotic or not, where the count was 6.60 log CFU/g at the end of the fermentation (P < 0.05).
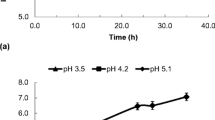
Storage time, storage temperature, pH and salt concentration have a significant effect on the changes in probiotic cell counts. In the first week of storage, the free probiotic count in CP-L decreased from the initial count of > 7 log units to 5 log units (Table 1). This situation may be related to the temperature shock experienced by the microorganism. However, there was a greater decrease in the count of viable cells, ranged from 5.83 to 2.18 log CFU/g for CP-L (P < 0.05) (Table 1). Similar findings were reported by Mousanejadi et al. (2023), Reddy et al. (2015), and Sun et al. (2022), who observed a decrease in viable probiotic cells in various foods during storage. However, CP-P had the highest probiotic counts at the end of the storage (5.01 log CFU/g in free form and 5.99 log CFU/g in microencapsulated form; P > 0.05). A food must contain at least 6 log CFU/mL-g of viable probiotics to be considered as a probiotic food. In this context, CP-P could be considered as a probiotic product for up to four weeks of storage, according to the microencapsulated probiotic counts. Compared to P. parvulus HL14, L. plantarum HL4 has a shorter metabolic cycle, is more susceptible to temperature fluctuations, and is less tolerant to the changes in pH and salt concentration. Figure 4 shows that the pH of the samples fluctuated between 3.15 and 3.58 throughout storage. Considering these values, P. parvulus grows better than L. plantarum at low pH (Pérez-Díaz et al., 2019), which explains why the cell count of samples containing P. parvulus was higher than that of samples containing L. plantarum (Table 1). As can be seen, P. parvulus is more resistant to harsh environmental conditions than L. plantarum, although the coating material can be affected by unfavorable conditions. Therefore, P. parvulus benefits more from the microencapsulation than L. plantarum. On the other hand, the metabolites produced by the free L. plantarum cells, which is a heterofermentative microorganism, in the samples can be linked to the quick decline of the viable cell counts in the microcapsules containing L. plantarum. These metabolites can be absorbed by the sodium alginate and cause damage to both the microcapsule and the cells (Olivares et al., 2019). In this study, P. parvulus was the probiotic with the highest viability in both free and microencapsulated forms. Olivares et al. (2019) investigated the growth of free and microencapsulated forms of Lac. casei in various fruit juices (orange, pineapple, and raspberry). Depending on the type of fruit juice, a low pH adversely affected the viability of Lac. casei throughout storage (28 days). Some microencapsules added to pineapple juice were also damaged, but it was noted that the microencapsules were recovered at the end of the storage (2.3 × 107 CFU/g capsule). Similarly, in this study, low pH, high salt content, and nutritional deficiencies affected the probiotic cell counts, especially L. plantarum counts, in cucumber pickles during storage.
The viability of probiotic bacteria was enhanced by microencapsulation. Research has shown that the addition of prebiotic ingredients to the solution prepared for use in microencapsulation significantly increases the viability of probiotics (Bustamante et al., 2020; Donthidi et al., 2010; Homayouni et al., 2008). This is because probiotics can exert their positive health effects at > 106 log CFU/g-mL (FAO/WHO, 2002). On the other hand, calcium alginate (sodium alginate and calcium chloride interaction) capsules, which have a porous structure, can be negatively affected by ions and chelating molecules and high or low pH (Zanjani et al., 2014). In addition, probiotic cells can leak from capsules during storage. Capsule size is another factor affecting probiotic viability. In this study, the size range of microcapsules ranged from 2 to 3 mm. The sensory qualities of the product are badly impacted by large capsules, but the probiotics cannot be sufficiently protected by small capsules (Motalebi Moghanjougi et al., 2021).
Consequently, these findings demonstrate that the use of P. parvulus HL14 as a starter culture and microencapsulation technology enables the production of cucumber pickles with a high count of viable probiotic cells among the endogenous cultures found in pickles. Using this technology, the samples could be considered probiotic for the microencapsulated cells when stored for four weeks, which means the microencapsulation improved the survivability of the cells. On the other hand, the leakage of cells from the microcapsules may be the reason why the count of free cells in CP-P remained relatively unchanged during the 6 weeks of storage. Double coating technology can reduce the damaging effects of high acidity and saltiness, such as damage to the capsule structure, and increase viability.
Yeast Count
Yeast count is one of the most important factors for the shelf life of pickles (Çetin, 2011). Some yeasts are able to grow at a relatively low pH and in the presence of low concentrations of lactic acid. In this study, the probiotic added to the medium negatively affects yeast growth by creating a competitive environment, which is why the yeast count at the end of fermentation is lower in CP-L and CP-P than in NC (Table 2).
At the end of the storage, the highest yeast count was found in NC (4.13 log CFU/g) (P > 0.05). It is generally accepted that the critical yeast count required to cause a technological failure is greater than 106 CFU/g (Aljahani, 2020). Therefore, the yeast counts observed in the current study are acceptable, especially for samples containing probiotics, as they might not affect the quality of the pickles. Consequently, the use of probiotic starter cultures in the fermentation of cucumber pickles has been shown to inhibit yeast growth, which is consistent with the findings of our study (Al-Shawi et al., 2019; Alan & Yildiz, 2021).
Physicochemical Properties
While pH is crucial in determining whether microorganisms can grow in food, total acidity is a more reliable indicator of how organic acids in food affect flavour. A low pH can prevent the growth of spoilage and pathogen microorganisms to maintain the product quality and safety, as well as have a significant influence on the sensory quality of foods. The pH of all samples decreased towards the end of the fermentation while the total acidity increased (Fig. 4). This can be attributed to the accumulation of organic acids produced by microorganisms (Aljahani, 2020). A similar result was obtained by Ahmed et al. (2021), who performed controlled fermentation of cucumber. When these findings were compared with those of our previous study (Guney, 2022), it was clear that the addition of microencapsulated cells had no effect on the pH or total acidity of the samples. Raddatz et al. (2022) found a similar result. While the pH for strawberry pulp produced with free cells was 3.61, it was ranged from 3.48 to 3.70 for the strawberry pulp produced with microencapsulated cells. They stated that the microencapsulated cells had no significant effect on pH. During storage, the pH and total acidity of the samples containing probiotics fluctuated (P > 0.05), while the pH of NC increased slightly. This may be related to the growth of undesirable microorganisms in the environment due to the lack of starter culture. Consequently, the appropriate salt concentration, fermentation type, and starter culture used affect the pH and total acidity of the samples.
The use of salt in the production of pickles not only adds flavor to the product, but also prevents tissue softening and prolongs shelf life by inhibiting microbial growth. The salt concentration in the samples varied between 5.44% and 6.32% during fermentation and remained at approximately 5.5% during storage (Fig. 5).
One of the characteristics that customers pay attention to when evaluating the quality of a product is the color. During fermentation, the L* values, representing brightness, ranged from 24.56 to 32.93 (Table 3). The L* values of the samples prepared with the starter cultures fluctuated during the fermentation, but they eventually reached the value recorded at the beginning of the fermentation. The control sample had become whiter as the value measured at the end of the fermentation was higher than the value obtained at the beginning. This demonstrates that controlled fermentation can prevent product discolouration. However, the effect of probiotics and storage time on the L* values of the samples was not significant. Table 3 shows that the green color was dominant in the cucumber pickles on day zero (P > 0.05), a* value increased and red color was dominant on the other days of the fermentation. Additionally, the highest b* value among the samples was measured on the third day in CP-L with 27.13 (Table 3) (P < 0.05). It was determined that C and H° values were in the range of 10.86–27.31 and 1.28-188.46, respectively. Hue angle is expressed as red for 0°/360°, yellow for 90°, green for 180° and blue for 270°. The H° value indicates the perceived color of the sample and the C value indicates the vividness and opaqueness properties of the products. In this context, the dark and bright green color of the cucumber samples, which was dominant at the beginning of the fermentation, turned into light and bright red during fermentation, and this result showed a positive correlation with a* values. CP-L and NC had a more vivid color, CP-P was found to have an opaque color (Table 3). During storage, the increase in the C value of the samples resulted in an increase in their brightness. The H° values of the samples fluctuated during storage, and the values were close to 0, which, when evaluated together with the C values, indicated that the bright red-yellow color was dominant.
Food processing destroys the chlorophyll pigments that give vegetables and fruit their distinctive green color, changing the color of the food. It is claimed that the synthesis of pheophorbide and pheophytin pigments is responsible for the color changes in cucumbers exposed to acidic and salty conditions. Pickled cucumbers lose their bright green color and develop an olive green or olive yellow hue due to the breakdown of chlorophyll into pheophorbide and pheophytin (McMurtrie & Johanningsmeier, 2018). At the end of the fermentation, cucumbers lose their yellowish-green color. Kazancı (2008) examined the effect of different acids (acetic, lactic and citric acid) and different concentrations of these acids (0.2%, 0.4%, 0.6%, 0.8% and 1%) on the color stability and hardness of cucumber pickles. Although the results obtained by Kazancı (2008) were similar to the L* and b* values obtained in this study, the samples in our study had a red color inside the yellow color (Table 3). The use of starter culture, fermentation conditions, as well as the metabolites produced in the environment, can all be linked to this variation.
Bioactive Properties
Fermentation could boost the antioxidant activity of several vegetables (Ng et al., 2020). At the end of the fermentation, CP-P had the highest DPPH% value (14.72%, P > 0.05) (Table 4). Studies in the literature demonstrate that antioxidant activity increases during fermentation but subsequently falls over time. This indicates that a number of variables, including temperature, pH, and fermentation conditions, have an impact on antioxidant activity (Parada et al., 2023; Zubaidah et al., 2020). At the end of storage, the samples containing probiotics had higher DPPH% values than NC (P < 0.05), indicating the advantage of using an endogenous starter culture.
The antioxidant activity of the samples was also examined using the ABTS+ method. ABTS+ values of the samples were between 51.92% and 88.06% and increased during fermentation (Table 4). During the storage, ABTS+ values of the samples ranged between 59.96% and 94.50% and decreased over time (Table 4). At the end of the storage, the lowest ABTS+ value belonged to NC with 75.95 (P > 0.05). Kumar and Kumar (2016) aimed to produce probiotic yogurt using Lac. rhamnosus in free (3%, v/v) and microencapsulated forms (3%, w/v). In the study, various fruits [apricot (Prunus armeniaca), raspberry (Rubus ellipticus), plum (Prunus domestica)] were also used to increase the antioxidant activity of yogurt and the DPPH% values of the samples were examined at 4 °C for 15 days. At the end of storage, the DPPH% values (60%) of yogurt samples containing free cells were higher than those of yogurt samples containing microencapsulated cells (50–55%). These values are lower than those found in our study. The differences in the results can be attributed to the different starter cultures and raw materials used.
Polyphenols are essential secondary metabolites found in plant foods and are typically used as dietary antioxidants to protect human health. At the end of the fermentation, the highest phenolic content was determined in CP-P with 2465.50 mg GAE/L (P < 0.05), while the lowest phenolic content was determined in NC with 1500.50 mg GAE/L (P < 0.05) (Table 4). At the end of the fermentation, the highest DPPH% value was found in CP-P with 14.72% and ABTS+ value was found in CP-L with 88.06%, and the lowest DPPH% (14.32%) and ABTS+ (80.70%) values were also found in NC, which showed that the phenolic content and antioxidant values of the samples, especially DPPH% values, were in parallel. As a result, this study showed that at the beginning and end of the fermentation, the phenolic content and antioxidant values of the probiotic-added samples were higher than those of NC (P < 0.05). The fluctuations were observed in the phenolic content of the samples during storage (Table 4). Consequently, the total phenolic content of the samples containing probiotic starter cultures had the highest antioxidant activity values.
Sensory Analysis Results
The changes in sensory properties of the samples during storage are indicated in Fig. 6 using radar charts. The color score of all samples decreased significantly as a function of storage time (P < 0.05), this effect was more intense in NC. At the end of the storage, CP-P had the highest color score (6.85) compared to the other samples (P < 0.05); which is supported by the colorimetric results. While the odor scores of NC decreased with storage time, the odor scores of the samples containing probiotics fluctuated and remained relatively stable. At the end of the storage, CP-L had the highest appearance score (6.80), while NC had the lowest appearance score (4.80) (P < 0.05). Most of the microcapsules remained in the brine, with many adhering to the surface of the cucumber, but were not noticed by the panelists. At the end of storage, the most favored sample in terms of taste was CP-P with 6.80 scores. The hardness scores of CP-L and CP-P ranged between 6.80 and 8.10, while it was in the range of 5.20 and 6.95 in NC. At the beginning (6.95, P > 0.05) and end of the storage (6.95, P < 0.05), CP-P had the highest general assessment score. However, NC was the least liked sample in terms of general assessment (5.45, P < 0.05). Similarly, Kowsalya et al. (2023) and Mousanejadi et al. (2023) indicated that all panellists chose probiotic enriched samples. It is crucial that consumers accept products with probiotic supplements, as they avoid functional products with unpleasant flavours, regardless of the health benefits. In general, all panelists preferred the samples containing probiotics and the addition of microencapsulated probiotics had no negative effect on the sensory properties of cucumber pickles.
Conclusion
Pickles are a potential matrix for carrying probiotic strains; however unfavorable conditions such as low pH and high salt content have an adverse effect on the viability of these microorganisms. On the other hand, once microencapsulated, the probiotic cells could be protected from harsh conditions during fermentation and storage. In the study, microencapsulation using sodium alginate and inulin increased the viability of probiotic cells and provided stability during a storage period of 4 weeks for the sample containing P. parvulus. Therefore, given the required level for probiotic foods, the formulated pickles can be qualified as probiotic products up to week 4 when stored at 4 °C. Hence, an acceptable, safe and nutritious probiotic pickle can be developed from cucumber using P. parvulus HL14. On the other hand, further research is needed to increase the viability of probiotics using double coating, novel coating agents, or various encapsulation techniques.
Data Availability
Data supporting the findings of this study are available upon request from the corresponding author.
References
Ahmed, S., Ashraf, F., Tariq, M., & Zaidi, A. (2021). Aggrandizement of fermented cucumber through the action of autochthonous probiotic cum starter strains of lactiplantibacillus plantarum and Pediococcus pentosaceus. Annals of Microbiology, 71(1), 1–23. https://doi.org/10.1186/s13213-021-01645-5.
Al-Shawi, S. G., Swadi, W. A., & Hussein, A. A. (2019). Production of probiotic (Turshi) pickled vegetables. Journal of Pure and Applied Microbiology, 13(4), 2287–2293. https://doi.org/10.22207/JPAM.13.4.43.
Alan, Y., & Yildiz, N. (2021). Effects of Lactobacillus used as the starter culture on naturally fermented pickled cabbage. Food Science and Technology, 42, e45020. https://doi.org/10.1590/fst.45020.
Aljahani, A. H. (2020). Microbiological and physicochemical quality of vegetable pickles. Journal of the Saudi Society of Agricultural Sciences, 19(6), 415–421. https://doi.org/10.1016/j.jssas.2020.07.001.
Altug, T., & Elmaci, Y. (2005). Sensory evaluation in foods. Meta.
AOAC (2007). Official Methods of Analysis of the Association of Official Analytical Chemists (18th edition). USA: Association of Offical Analytical Chemistry.
Basdogan, M. G. (2020). Determination of probiotic potential of lactic acid bacteria isolated from pickles produced traditionally, Master thesis, 111 p, Ege University, Türkiye.
Bustamante, M., Laurie-Martínez, L., Vergara, D., Campos-Vega, R., Rubilar, M., & Shene, C. (2020). Effect of three polysaccharides (inulin, and mucilage from Chia and flax seeds) on the survival of probiotic bacteria encapsulated by spray drying. Applied Sciences, 10(13), 4623. https://doi.org/10.3390/app10134623.
Cemeroğlu, B. (2013). Some special analysis methods applied to foods. In B. Cemeroglu (Ed.), Food Analysis (pp. 101–108). Bizim Grup Basımevi.
Çetin, B. (2011). Production of probiotic mixed pickles (Tursu) and microbiological properties. African Journal of Biotechnology, 10(66), 14926–14931. https://doi.org/10.5897/AJB11.2621.
Donthidi, A. R., Tester, R. F., & Aidoo, K. E. (2010). Effect of lecithin and starch on alginate-encapsulated probiotic bacteria. Journal of Microencapsulation, 27(1), 67–77. https://doi.org/10.3109/02652040902982183.
FAO/WHO. Food and Agriculture Organization/World Health Organization. (2002). Guidelines for the evaluation of probiotics in food, Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food, London, Ontario, Canada (2002). Retrieved January 1, 2024 from https://ftp.fao.org/es/esn/food/wgreport2.pdf
Fan, S., Breidt, F., Price, R., & Pérez-Díaz, I. (2017). Survival and growth of probiotic lactic acid bacteria in refrigerated pickle products. Journal of Food Science, 82(1), 167–173. https://doi.org/10.1111/1750-3841.13579.
FDA-BAM (Food and Drug Administration-Bacteriological Analytical Manual) (2001). Yeasts, molds and mycotoxins, Retrieved February, 28, from https://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm071435.htm.
Gul, O., & Dervisoglu, M. (2017). Application of multicriteria decision technique to determine optimum sodium alginate concentration for microencapsulation of Lactobacillus casei Shirota by extrusion and emulsification. Journal of Food Process Engineering, 40(3), e12481. https://doi.org/10.1111/jfpe.12481.
Guney, D. (2022). Production of pickles enriched with probiotics. Master thesis, Ege University, Türkiye.
Holkem, A. T., Raddatz, G. C., Nunes, G. L., Cichoski, A. J., Jacob-Lopes, E., Grosso, C. R., & de Menezes, C. R. (2016). Development and characterization of alginate microcapsules containing Bifidobacterium BB-12 produced by emulsification/internal gelation followed by freeze drying. LWT-Food Science and Technology, 71, 302–308. https://doi.org/10.1016/j.lwt.2016.04.012.
Homayouni, A., Azizi, A., Ehsani, M. R., Yarmand, M. S., & Razavi, S. H. (2008). Effect of microencapsulation and resistant starch on the probiotic survival and sensory properties of synbiotic ice cream. Food Chemistry, 111(1), 50–55. https://doi.org/10.1016/j.foodchem.2008.03.036.
Jang, H. J., Son, S., Kim, J. A., Jung, M. Y., Choi, Y. J., Kim, D. H., Lee, H. K., Shin, D., & Kim, Y. (2021). Characterization and functional test of canine probiotics. Frontiers in Microbiology, 12, 625562. https://doi.org/10.3389/fmicb.2021.625562.
Kazancı, Y. T. (2008). Effect of different acid and ph on color stability in cucumber pickle production PhD Thesis Bursa Uludag University, Türkiye.
Kirk, S., & Sawyer, R. (1991). Pearson’s composition and analysis of foods. Longman Group Ltd.
Kowsalya, M., Sudha, K. G., Ali, S., Velmurugan, T., & Rajeshkumar, M. P. (2023). Sustainability and controlled release behavior of microencapsulated Lactobacillus plantarum PRK7 and its application in probiotic yogurt production. Food Bioscience, 52, 102430. https://doi.org/10.1016/j.fbio.2023.102430.
Kramer, A., & Twigg, B. A. (1984). Quality Control for the Food Industry. The Avi Publishing Company.
Krasaekoopt, W., & Watcharapoka, S. (2014). Effect of addition of inulin and galactooligosaccharide on the survival of microencapsulated probiotics in alginate beads coated with chitosan in simulated digestive system, yogurt and fruit juice. LWT-Food Science and Technology, 57(2), 761–766. https://doi.org/10.1016/j.lwt.2014.01.037.
Kumar, A., & Kumar, D. (2016). Development of antioxidant rich fruit supplemented probiotic yogurts using free and microencapsulated Lactobacillus rhamnosus culture. Journal of Food Science and Technology, 53, 667–675. https://doi.org/10.1007/s13197-015-1997-7.
Mahmoud, M., Abdallah, N. A., El-Shafei, K., Tawfik, N. F., & El-Sayed, H. S. (2020). Survivability of alginate-microencapsulated Lactobacillus plantarum during storage, simulated food processing and gastrointestinal conditions. Heliyon, 6(2020), e03541. https://doi.org/10.1016/j.heliyon.2020.e03541.
McLellan, M. R., Lind, L. R., & Kime, R. W. (1995). Hue angle determinations and statistical analysis for multiquadrant Hunter L, a, b data. Journal of Food Quality, 18(3). https://doi.org/10.1111/j.1745-4557.1995.tb00377.x. 235 – 40.
McMurtrie, E. K., & Johanningsmeier, S. D. (2018). Quality of cucumbers commercially fermented in calcium chloride brine without sodium salts. Journal of Food Quality, 2018, 1–3. https://doi.org/10.1155/2018/8051435.
Motalebi, Moghanjougi, Z., Rezazadeh, Bari, M., Alizadeh Khaledabad, M., Amiri, S., & Almasi, H. (2021). Microencapsulation of Lactobacillus acidophilus LA-5 and Bifidobacterium animalis BB‐12 in pectin and sodium alginate: A comparative study on viability, stability, and structure. Food Science & Nutrition, 9(9), 5103–5111. https://doi.org/10.1002/fsn3.2470.
Mousanejadi, N., Barzegar, H., Behbahani, A., B., & Jooyandeh, H. (2023). Production and evaluation of a functional fruit beverage consisting of mango juice and probiotic bacteria. Journal of Food Measurement and Characterization, 3, 1–4. https://doi.org/10.1007/s11694-023-01862-3.
Ng, T. K., Yu, A. Q., Ling, H., Juwono, N. K., Choi, W. J., Leong, S. S., & Chang, M. W. (2020). Engineering Yarrowia Lipolytica towards food waste bioremediation: Production of fatty acid ethyl esters from vegetable cooking oil. Journal of Bioscience and Bioengineering, 129(1), 31–40. https://doi.org/10.1016/j.jbiosc.2019.06.009.
Olivares, A., Soto, C., Caballero, E., & Altamirano, C. (2019). Survival of microencapsulated Lactobacillus casei (prepared by vibration technology) in fruit juice during cold storage. Electronic Journal of Biotechnology, 42, 42–48. https://doi.org/10.1016/j.ejbt.2019.10.002.
Page, C. A., & Pérez-Díaz, I. M. (2023). Whole-genome sequencing and annotation of Pediococcus ethanolidurans and Pediococcus pentosaceus isolates from commercial cucumber. Fermentation Microbiology Resource Announcements, 12(5), e00050–e00023. https://doi.org/10.1128/mra.00050-23.
Pan, D. D., Zeng, X. Q., & Yan, Y. T. (2011). Characterisation of Lactobacillus fermentum SM-7 isolated from koumiss, a potential probiotic bacterium with cholesterol‐lowering effects. Journal of the Science of Food and Agriculture, (3), 512–518. https://doi.org/10.1002/jsfa.4214.
Parada, R. B., Marguet, E., Campos, C., & Vallejo (2023). Improved antioxidant capacity of three Brassica vegetables by two-step controlled fermentation using isolated autochthone strains of the genus Leuconostoc spp. and Lactiplantibacillus spp. Food Chemistry: Molecular Sciences, 6, 100163. https://doi.org/10.1016/j.fochms.2023.100163.
Pérez-Díaz, I. M., Hayes, J. S., Medina, E., Webber, A. M., Butz, N., Dickey, A. N., & Azcarate-Peril, M. A. (2019). Assessment of the non-lactic acid bacteria microbiota in fresh cucumbers and commercially fermented cucumber pickles brined with 6% NaCl. Food Microbiology, 77, 10–20. https://doi.org/10.1016/j.fm.2018.08.003.
Raddatz, G. C., Pinto, V. S., Zepka, L. Q., Barin, J. S., Cichoski, A. J., da Silva, C. D., Lozano-Sánchez, J., da Cruz, A. G., & de Menezes, C. R. (2022). Use of red onion (Allium cepa L.) residue extract in the co-microencapsulation of probiotics added to a vegan product. Food Research International, 161, 111854. https://doi.org/10.1016/j.foodres.2022.111854.
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolarization assay. Free Radical Biology and Medicine, 26, 1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3.
Reddy, L. V., Min, J. H., & Wee, Y. J. (2015). Production of probiotic mango juice by fermentation of lactic acid bacteria. Microbiology and Biotechnology Letters, 43(2), 120–125. https://doi.org/10.4014/mbl.1504.04007.
Shoaei, F., Heshmati, A., Mahjub, R., Garmakhanyi, A. D., & Taheri, M. (2022). The assessment of microencapsulated Lactobacillus plantarum survivability in rose petal jam and the changes in physicochemical, textural and sensorial characteristics of the product during storage. Scientific Reports, 12(1), 6200. https://doi.org/10.1038/s41598-022-10224-w.
Singh, R. P., & Chidambara Murthy, K. N., Jayaprakasha,G.K (2002). Studies on antioxidant activity of pomegranate (Punica granatum) peel extract using in vivo models. Journal of Agricultural and Food Chemistry, 50(17), 4791–4795. https://doi.org/10.1021/jf010865b.
Sun, W., Nguyen, Q. D., Sipiczki, G., Ziane, S. R., Hristovski, K., Friedrich, L., & Bujna, E. (2022). Microencapsulation of Lactobacillus plantarum 299v strain with whey proteins by lyophilization and its application in production of probiotic apple juices. Applied Sciences, 13(1), 318. https://doi.org/10.3390/app13010318.
Wrolstad, R. E., & Smith, D. E. (2017). Color analysis. Food Analysis, 545, 55.
Zanjani, M. A., Tarzi, B. G., Sharifan, A., & Mohammadi, N. (2014). Microencapsulation of probiotics by calcium alginate-gelatinized starch with chitosan coating and evaluation of survival in simulated human gastro-intestinal condition. Iranian Journal of Pharmaceutical Research, 13(3), 843.
Zubaidah, E., Arum, M. S., Widyaningsih, T. D., & Rahayu, A. P. (2020). Sauerkraut with the addition of Lactobacillus casei: Effects of salt and sugar concentrations on fermentation and antioxidant activity. Current Nutrition & Food Science, 16(8), 1265–1269. https://doi.org/10.2174/1573401316666200217112642.
Funding
This study was supported by Ege University Scientific Research Project Commission for the project entitled “Production of pickles enriched with endogenous probiotic lactic acid bacteria” (Project No: FGA-2022-23495).
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
Aysegul Kirmizigul Peker: Conceptualization, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Damla Guney: Conceptualization, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Ilkin Sengun: Conceptualization, Validation, Formal analysis, Investigation, Supervision, Writing – original draft, Writing – review & editing.
Corresponding author
Ethics declarations
Ethical Approval
The sensory evaluation of the cucumber pickles was approved by the Ethics Committee for Scientific Research and Publication of Ege University (Ethics ID: E.806332). Informed consent was obtained from human panelists for the sensory evaluation. All methods were performed according to the relevant guidelines and regulations.
Competing interest
The authors declare that they have no known competing financial interests or personal relationships.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kirmizigul Peker, A., Guney, D. & Sengun, I. The Use of both Free and Microencapsulated Lactiplantibacillus plantarum and Pediococcus parvulus in Cucumber Pickles. Food Bioprocess Technol (2024). https://doi.org/10.1007/s11947-024-03400-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11947-024-03400-5