Abstract
Fouling, the accumulation of undesirable material on manufacturing equipment surfaces, poses a pervasive challenge in industrial processes. In the food industry, the complex interactions among these compounds can give rise to stubborn deposits that deviate from conventional cleaning protocols. In this work, the forces and removal mechanisms of model fouling agents composed of mixtures of starch, whey protein, and lard deposited on solid surfaces of relevant industrial interest (i.e. stainless steel, aluminium, and PTFE) are investigated using a multi-length scale approach, involving milli-manipulation and a lab-simulated Clean-In-Place (CIP) system. The forces involved in the removal process, the types of failure observed when the deposits are subjected to shear stress (adhesive, mixed, or cohesive), and the performance of the CIP system are systematically analysed as a function of the cleaning treatments applied. For stainless steel surfaces, alkaline treatment seems to facilitate the cleaning of lard and starch deposits, while the whey foulant removal tends to be more effective using hot water under the conditions tested. Hot water is effective for stainless steel and PTFE surfaces, reducing the mechanical shear stress required, while the alkaline treatment demonstrated superior efficacy for aluminium surfaces. These findings emphasise the importance of customising cleaning protocols for CIP optimisation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The accumulation of undesired substances on the surfaces of manufacturing equipment, known as fouling, presents a widespread problem in product manufacturing. This industrial issue arises from a variety of factors, encompassing the inherent characteristics of the processed product, manufacturing conditions, and the presence of impurities or microorganisms. Such deposits give rise to a range of predicaments, including diminished productivity, equipment impairment, higher consumption of energy and resources, and consequential economic and environmental impacts (Mohammad et al., 2012). To enhance the efficiency, safety, and sustainability of manufacturing processes while mitigating the adverse effects of industrial fouling, diverse strategies are being implemented such as the application of anti-fouling coatings (Avila-Sierra et al., 2023; de Vargas et al., 2022; Magens et al., 2017; Şen et al., 2012), optimisation of processing equipment design (EHEDG, 2018), and regular execution of cleaning and maintenance protocols (Wilson et al., 2022). Nevertheless, cleaning processes depend on several factors such as the fouling agent, the nature of the surface, temperature, hydrodynamic forces, detergent formulation, or cleaning time (Basso et al., 2017; Fryer & Asteriadou, 2009; Laukemper et al., 2021; Santos et al., 2013; Wilson et al., 2022).
The issue of fouling in the food industry presents an especially formidable challenge due to the intricate and diverse nature of the food products being processed, as well as the stringent regulations governing hygiene and safety. Among the crucial food constituents that give rise to fouling predicaments, several stand out:
-
(i)
Proteins, possessing substantial molecular complexity and a three-dimensional structure, are susceptible to denaturation in response to environmental factors such as temperature, pH, and ionic strength. This affects their inherent solubility and reactivity, rendering their removal from processing equipment more complex (Avila-Sierra et al., 2021a, 2023; Christian & Fryer, 2006; Felfoul et al., 2015, 2016).
-
(ii)
Carbohydrates, particularly starch, exhibit a complex and branched structure that can lead to the formation of adhesive and viscous deposits on processing equipment. These deposits often prove resistant to conventional cleaning agents and methods (Jurado-Alameda et al., 2015; Jurado et al., 2015).
-
(iii)
Fats, encompassing mixtures of mono-, di-, and triglycerides, as well as other hydrophobic constituents, are insoluble in water, thereby posing challenges for their removal from processing equipment utilising water-based cleaning agents (Ali et al., 2015).
The interaction between multiple food compounds under operational processing conditions gives rise to multicomponent and micro-structured fouling, resulting in the formation of intricate deposits characterised by variations in morphology, topology, and electrostatic conditions across the substrate (Cuckston et al., 2019). For instance, mixtures of starch and proteins may be present in dairy and cereal processing, engendering a complex network of interactions between these compounds (da Silva Pereira et al., 2021) that might give rise to deposits resistant to traditional cleaning agents and methods. Magens et al. (2017) observed that the removal of sponge cake batters, composed of commercial cake mix, egg powder, and vegetable oil, exhibited sensitivity to the oil content. Furthermore, Cuckston et al. (2019) investigated the influence of detergent formulation on the efficiency of cleaning a baked deposit consisting of a complex carbohydrate–fat mixture adhered to stainless steel. Their findings indicated a noticeable increase in removal efficacy with hydration, which was highly dependent on the specific cleaning solution and temperature, resulting in different types of failure during removal. More recently, Herrera-Márquez et al. (2020) explored the effects of systematic variations in fat/starch fractions within binary mixtures on their removal mechanisms. Their study suggested that, under the tested conditions, the effective removal of deposits with high fat content could be achieved using solely a hot (50 °C) neutral pH solution, potentially attributed to the melting point of the fatty phase. Conversely, the use of α-amylase or lipase was not recommended for deposits with high fractions of either starch or fat. However, it is worth noting that overall, there remains a dearth of research examining how systematic alterations in the component fractions of multicomponent fouling can influence the mechanisms of their removal.
In addition to considering the pertinent fouling agents, achieving effective cleanliness within the food industry requires a comprehensive understanding of the surfaces that come into contact with these compounds during food manufacturing, as their characteristics (e.g. surface free energy, roughness) will determine the adhesion strength of the food products during processing (Avila-Sierra et al., 2021b; Laukemper et al., 2021). Among the most used metallic surfaces employed, stainless steel stands out as a desirable option for food-related applications due to its minimal capacity for bacterial retention, ease of cleaning after repeated use, and exceptional resistance to corrosion (Avila-Sierra et al., 2019; Daeschel et al., 2023). Another noteworthy food-grade metal is aluminium, approved not only for food production but also for the safe packaging of food items (Stahl et al., 2017). Additionally, various polymeric surfaces, such as polytetrafluoroethylene (PTFE) coatings, are found in specific sections of processing lines, such as belts and conveyors, commonly used in the mass production of food items (e.g. eggs, bacon, sausage, and hamburgers) (Andreatta et al., 2020; Rondinella et al., 2021). Thus, optimising industrial cleaning procedures necessitates a detailed analysis of the microscopic interactions occurring between the system formed by the surface, the deposit, and the cleaning agent, exploring potential correlations between the composition of the deposits, their mechanical removal, and the efficacy of the cleaning treatment, as well as optimisation according to environmental criteria (Tsai et al., 2021).
The main objective of this work was to propose a multi-scale methodology to relate physical parameters (shear stress) with detersive efficiency to facilitate the prediction of the removal behaviour of food-based complex deposits (mixtures of starch, whey protein, and lard) in contact with different surfaces (stainless steel, aluminium, and PTFE) using a milli-manipulation and a lab-simulated Clean-In-Place (CIP) system. The forces involved in the removal process, the types of failure observed when the deposits are subjected to shear stress (adhesive, mixed, or cohesive), and the performance of the CIP system are systematically analysed as a function of the chemical treatments applied. These treatments include exposure to hot water (50 °C, 30 min), and exposure to a hot alkaline solution (NaOH, 4 g/L) (50 °C, 30 min). The mechanical shear stress needed for the removal of different deposits in contact with the surfaces studied was evaluated to establish a first approach of this methodology, analysing how cleaning may vary among different materials. Subsequently, the effect of different cleaning solutions on detergency was analysed, and an attempt was made to correlate these results with the shear stress values obtained.
Materials and Methods
Foulants, Surfaces, and Fouling Methods
Foulants
Three different food compounds, corn starch (Maizena®, Spain), whey protein concentrate (Abbot, Spain), and lard (El Pozo®, Spain), were used in this work (Table 1). To prepare the model foulants, the food compounds were blended in accordance with the predetermined proportions outlined in Table 2 for designs 1 and 2. The mixture was then heated up to a temperature of 50 °C, agitated for 5 min using a magnetic stirrer, and homogenised via an Ultra-turrax device (T25 digital, Ika, Spain) for 5 min at a rate of 6800 rpm. The mixture was subsequently autoclaved at 121 ºC for 60 min, then cooled down to 50 ºC, and homogenised once again at 6800 rpm for 5 min. Finally, it was cooled at 30 ºC before being applied upon the clean surfaces.
Surfaces
The surfaces were prepared into square coupons measuring 30 mm by 30 mm. These coupons were from stainless steel (thickness 1.5 mm), aluminium (thickness 2.0 mm), and PTFE (thickness 2.0 mm), as illustrated in Fig. 1a–c. Surface topography and roughness (Ra) were characterised by a Leica TCS SP5 SPECTRAL CONFOCAL microscope with SP detection capable of five channels simultaneously was used. A reflection image of the surface was generated using an Ar/Ar Kr laser (458 nm) with an objective magnification of 10 and numerical aperture set at 0.4 (10 × /0.4), selecting a scan of 512 × 512 pixels. The sample was travelled vertically (z-direction) between the first and last detectable light reflex of each sample, and a z-series of 30 optical sections was generated. The z-series was converted to a greyscale (topographical) image and analysed using ImageJ software and the plugin developed by Chinga et al. (2007). The plugin allows to level and assess surface roughness statistics including root mean square deviation, skewness, and kurtosis. Averaged values of surface roughness (Ra), kurtosis (SKu), Sq/Sa ratio (Sq is the root mean square roughness), and Rp/Rv ratio (Rp is the maximum profile peak height, and Rv the maximum valley depth below the mean line, within a single sampling length) were analysed.
Images of both clean and fouled surfaces. Clean surfaces: stainless steel, aluminium, and PTFE coupons, as depicted in a, b, and c, respectively. Fouled surfaces: stainless steel fouled with Soil C-Design1 (100% lard), aluminium fouled with Soil J-Design2 (17% starch, 66% whey, 17% lard), and PTFE fouled with Soil G-Design2 (50% lard, 50% whey), which are illustrated in d, e, and f. A representative spherical stainless steel wad, fouled with Soil A-Design2 (100% starch), is shown in g
Fouling Method
In the milli-manipulation experiments, coupons were placed within a 13-mm-high mould and subsequently loaded with the foulant of interest at 30 ºC (Table 2), being incubated a subsequent 24-h interval at 20 ºC. The fouled coupons were then de-moulded, as illustrated in Fig. 1d–f.
In the Cleaning-In-Place assays, the surfaces were spherical stainless steel wads measuring 2.0 cm in diameter and weighing between 0.80 and 0.85 g. These coupons were uniformly fouled by rolling them over the foulant being tested (Table 2) at 30 ºC, with incubation at 20 ºC for 24 h (Fig. 1g). Each cleaning test used eight spheres, having a total foulant weight of 2.0 ± 0.2 g.
Statistical Analysis
Design 1 and design 2 were subjected to response surface methodology (RSM) (simplex-centroid design) to ascertain the optimal composition of the samples for the variables examined, i.e. shear stresses and detergency determined from milli-manipulation and cleaning assays, respectively. These two designs facilitate the analysis of the influence of foulants with varying concentrations of solids on the specified variables. Design 1 used more diluted solutions for the formulation of the model foulants (starch (20% w/w)–whey (20% w/w)–lard (100% w/w)), while design 2 used solutions with higher concentrations (starch (30% w/w)–whey (30% w/w)–lard (100% w/w)). Table 2 provides the detailed composition of the model foulants (A–J) required for the simplex-centroid design, and Fig. 2 illustrates them. In both design 1 and design 2, all foulants from A to J underwent testing for the simplex-centroid design, despite differences in solid concentrations. It should be noted that, as indicated in Table 2, model foulant C shares the same composition in both designs, while the remaining model foulants have distinct composition. Response surface equations were generated based on the variables studied and fitted to the special cubic model (Supplementary Information, Sect. 1, Tables SI.1 and SI.2). The statistical analysis was carried out with Statgraphics® software, and the models obtained exhibited a confidence level of over 95%.
Simplex-centroid design. The composition of the samples, which were prepared using starch, whey, and lard, is detailed in Table 2
Milli-manipulation Technique
Liu et al. (2002) expounded on the fundamentals of micro/milli-manipulation technique, which enables the assessment of the force needed to remove a deposit from a solid surface. These devices measure the force required to remove a layer of deposit (cohesion forces) or remove the deposit from the surface (adhesion forces). The force application produces various outcomes. In some instances, the forces can cause the separation of foulant from the surface, resulting in an “adhesive failure”. Conversely, if there is displacement of the top layer of the deposit, a “cohesive failure” is observed. If both phenomena occur in an assay, the behaviour is designated as “mixed failure”.
A CAD design of the UGR milli-manipulator is shown in Fig. 3. It comprises a force gauge which is moved by a 2-axis displacement stage at a constant velocity (0.15 cm/s), a scraper, and a sample chamber where the fouled surface is located. Before measuring, the scraper was first positioned 3 mm above the testing surface. Then, the clean surface is replaced by a fouled surface, being located, and immobilised in the sample chamber. The force gauge records the force F (N) required for the scraper to displace the deposit as a function of time t (s). All tests were conducted at least three times, at 20 ºC.
The work needed to move the foulant W (J) was calculated as follows:
where F(t) is the force applied to move the deposit over a distance dx. The distance dx = v·dt, where v is the scraper displacement velocity. Integrating
where t0 is the time at which the scraper contacts the deposit and t1 is the end time of each test (Magens et al., 2017). The shear stress applied to the deposit σ (J/m2) is.
where A (m2) is the contact area between the scraper and the deposit.
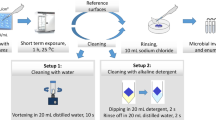
The milli-manipulation tests were performed on three different surfaces (stainless steel, aluminium, PTFE) and ten different foulants for each of the two designs (Table 2). To determine the impact of different environments on the characteristics of the deposits, the fouled surfaces were (i) immersed in water (50 ºC, 30 min) or (ii) in a 4.0 g/L NaOH aqueous solution (50 ºC, 30 min).
Cleaning-In-Place Assays
Cleaning experiments were conducted using a specialised cleaning device, called BSF, previously utilised by Jurado-Alameda et al. (2015), which allows for the adjustment of cleaning time, temperature, surface type, and deposit composition. The device comprises a jacketed tank (1 L) and a thermostated column with a peristaltic pump, which houses eight fouled stainless steel wads (see the “Foulants, Surface and Fouling Methods” section). The device has been employed for cleaning food residues by multiple researchers (Vicaria et al., 2017). The cleaning process entailed two treatments: (method 1) water (50 ºC, 30 min) and (method 2) a 4.0 g/L NaOH aqueous solution (50 ºC, 30 min). During cleaning, the solution (1.2 L) flowed through the tank to the column at a rate of 75 L/h. Upon completion, the surfaces were dried at 60 °C for 24 h, and the quantity of deposit left was determined. The cleaning experiments were conducted at least three times. The initial soil quantity present on the eight coupons was measured before each experiment, and the initial soil moisture content was determined by drying the samples at 60 °C for 24 h. The cleaning efficiency or detergency (De %) was computed using the following formula:
where mi represented the dry weight of foulant present on the stainless steel spheres before the cleaning process and mf was the dry weight of the foulant after cleaning and drying at 60 °C.
Results and Discussion
A comprehensive and systematic analysis of the forces required to remove a series of food complex deposits (Table 2) firmly attached to hard surfaces of industrial relevance, namely, stainless steel, aluminium, and PTFE, was conducted in this section, taking into consideration the composition of the deposit, the nature of the surface, and the removal behaviour of the model foulants without or under chemical treatment. This rigorous examination of the interplay between the surface properties and the characteristics of the deposit was crucial in discerning the underlying mechanisms that govern the cleaning process of these model foulants.
Surface Topographical Characterisation
A topographical analysis of the model surfaces used was conducted according to the method detailed in the “Foulants, Surfaces, and Fouling Methods” section. Average values of surface roughness (Ra), kurtosis (SKu), Sq/Sa, and Rp-Rv ratio are listed in Table 3. The findings indicate that stainless steel and aluminium have similar Ra values, being aluminium slightly rougher. In contrast, PTFE exhibits significantly greater roughness (6.6 ± 1.2 µm). The set of metallic surfaces are characterised by high kurtosis values (SKu > 3), indicating a distribution with sharp peaks, while PTFE has a kurtosis value below 3, suggesting a thin-tailed distribution with infrequent outliers. Additional parameters such as Sq/Sa and Rp-Rv were also used for further surface texture characterisation (Pawlus et al., 2021). The results show similar Sq/Sa values for all surfaces, indicating comparable degrees of surface pattern distribution. However, the peak-valley height of PTFE is double that of both metallic surfaces.
Mechanical Removal of Model Foulants
Within this section, milli-manipulation (see the “Milli-manipulation Technique” section) was employed to determine the force required to remove model deposits present on the surfaces tested (i.e. stainless steel, aluminium, or PTFE). The Supplementary Information (Sect. 2, Tables SI.3-SI.5) exhibits representative removal curves depicting the relationship between force and time for each tested deposit upon stainless steel surfaces. Figure 4 displays response surface plots illustrating the data on shear stress (σ) (see the “Statistical Analysis” section, Table 4), as well as the type of failure observed when the deposits were subjected to stress. This analysis is conducted while considering the percentage of solids for individual fouling agents, design 1 (20% w/w) and design 2 (30% w/w), encompassing either starch or whey protein foulant.
At a lower concentration of solids (design 1), comparable shear stress values and tendencies were observed during the removal of singular fouling agents from the three surfaces studied, particularly in the case of starch-based fouling agent (foulant A, with σ ranging from 14.5 to 21.8 J/m2) and whey protein-based fouling agent (foulant B, with σ ranging from 35.1 to 42.7 J/m2). These findings align with the σ data reported by Herrera-Márquez et al. (2020) and Liu et al. (2006) for potato starch (30% w/w) and whey protein fouling agents (1.49% w/w), respectively. Regardless of the underlying surface type, the mechanical removal of these two deposits predominantly resulted in adhesive failure. For the binary starch–whey mixture at a 1:1 ratio (foulant E), the shear stress required for removal fell within an intermediate range (23.5–27.5 J/m2), exhibiting a mixed failure mechanism on stainless steel and PTFE, while showcasing predominantly adhesive failure on aluminium. Notably, the introduction of lard into the mixture led to clear differences in removal behaviour. Deposits consisting entirely of lard (foulant C) required the highest shear stress for removal (137.0–171.5 J/m2) compared to the single fouling agents. This can be attributed to the increased removal work (indicated by the area under the force curve, as detailed in the Supplementary Information, Sect. 2, Tables SI.3-SI.5) required for their elimination, consistently resulting in adhesive detachment. In fact, the highest shear stress was observed on PTFE, the surface possessing the greatest roughness (see the “Surface Topographical Characterisation” section). When lard was combined with either starch or whey protein fouling agents at a 1:1 ratio, foulants F and G respectively, the shear stress exhibited an increase in comparison to the single starch and whey protein foulants, particularly for deposits adhering to aluminium (for both binary mixtures) and PTFE (in the case of starch–lard deposit). In the latter scenario, the highest shear stress measured in this study (193.5 J/m2) was attained. While the starch–lard deposit consistently exhibited adhesive failure across all surfaces, the removal mechanism for the whey–lard combination was highly dependent on the specific surface under investigation, resulting in cohesive, adhesive, or mixed failures on stainless steel, aluminium, and PTFE, respectively. Introducing the triple combination of starch–lard–whey with an equal fraction of fouling agents (foulant D) yielded the lowest shear stress values (12.4–13.8 J/m2), independently of the investigated surface, and consistently manifesting adhesive failure as removal mechanism. However, significant distinctions emerged when altering the proportion of the ternary fouling agents: an elevated lard content (foulant H) led to an increased shear stress (76.7–98.9 J/m2), while a higher proportion of whey (foulant J, 38.5–50.6 J/m2) or starch (foulant I, 18.3–30.6 J/m2) led to decreased shear stress values, albeit still higher than those observed for the triple mixture at the same proportion of foulants (foulant D). Furthermore, diverse removal mechanisms were observed, as depicted in Fig. 4.
Shifting our focus to an augmentation of the solid concentration (design 2) for singular starch and whey protein fouling agents, remarkable differences in removal behaviour were detected. For the starch-based fouling agent (A), an adhesive failure mechanism was evident, which required higher shear stress values (increasing from approximately 20 J/m2 to 75.8–110.2 J/m2) with shear stress being highly dependent on the underlying surface. Notably, the highest shear stress was observed on stainless steel. In the case of the binary mixture of starch–whey deposit at a 1:1 ratio (foulant E), intermediate shear stress values were required, falling within the ranges reported for the individual fouling agents (36.2–52.3 J/m2). This resulted in adhesive failures on all the surfaces assayed. Once again, with the introduction of a fraction of lard into the mixture, removal characteristics diverged. The binary combination of lard–starch (foulant F) led to increase shear stress on all surfaces inducing adhesive detachments, and lard–whey fouling agents at a 1:1 ratio (foulant G) also required higher shear stress values, except for the aluminium surface which exhibited a slight decrease in shear stress. A mixed failure mechanism was depicted across all surfaces, except for aluminium (adhesive).
Finally, similarly to the observations made for the starch fouling agent, an increase in the solid concentration dramatically elevated the shear stress required for the removal of the triple deposit with the same proportion of foulants (D), escalating from approximately 13 J/m2 to 80.1–140.4 J/m2, particularly pronounced for PTFE. This pattern was similarly observed for the other 3-component mixtures, with the highest shear stress values consistently occurring on PTFE, potentially attributed to its greater surface roughness, followed by stainless steel and aluminium. Generally, a higher proportion of lard in the triple mixture tended to amplify shear stress. Consequently, an elevation in the percentage of solids within the composition of fouling agents suggests an augmentation of cohesive interactions within the model deposits, ultimately leading to a preference for mixed or adhesive failure removal mechanisms under stress conditions.
Mechanical Removal of Food Complex Deposits Under Cleaning Treatments
As in the “Mechanical Removal of Model Foulants” section, milli-manipulation was employed to determine the requisite force for removing model deposits labelled as A to J from the surfaces of interest, i.e. stainless steel, aluminium, and PTFE, under different cleaning treatments: (method1) immersing fouled coupons in water at 50ºC for 30 min and (method 2) immersing fouled coupons in a 4.0 g/L NaOH aqueous solution at 50ºC for 30 min. Shear stress (σ) data are reported in Table 5 as a function of the percentage of solids (design 1, 20% w/w, and design 2, 30% w/w) for starch and whey protein fouling agents, as well as the type of cleaning method employed.
In general, the application of cleaning treatments led to a substantial decrease in shear stress values, with the majority of deposits detaching from the surfaces without the need for mechanical action. For the remaining adhered deposits following cleaning treatment, low shear stress values were measured. Higher proportions of solid content in the single foulants correlated with elevated shear stress, particularly on PTFE surfaces, which possess greater roughness. The most resistant deposits to cleaning procedures were those comprised of starch (A), whey protein (B), starch–lard mixture (F), the triple mixture at the same fraction of foulants (D), and triple mixtures with higher starch (I) or whey protein (J) concentration. According to the results, deposits containing lard or substantial amounts of lard tended to be easily removed using water at 50 ºC, probably because the melting point of lard was below 50 ºC, facilitating the phase change its elimination. In fact, hot water proved more effective than alkaline treatment for eliminating starch–lard deposits with high solid concentrations from stainless steel and PTFE surfaces. Conversely, a higher solid concentration in the triple mixture (D) appeared to facilitate removal by reducing shear stress, irrespective of the cleaning method employed. This observation aligns with the marked decrease in shear stress observed in the “Mechanical Removal of Model Foulants” section. Triple mixtures featuring significant proportions of either starch (I) or whey (J) proved more challenging to remove, especially from stainless steel and PTFE surfaces.
In terms of the cleaning method, hot water demonstrated sufficient efficacy for removing deposits that remained adhered to flat stainless steel and PTFE surfaces, resulting in reduced shear stress values. Conversely, alkaline treatment exhibited greater effectiveness when applied to flat aluminium surfaces. These findings underscore the critical importance of tailoring the cleaning protocol to suit the specific deposit type, its compositional variations during product processing, and the nature of the fouled surface. By optimising the CIP protocol, significant resource savings can be achieved during industrial cleaning endeavours (Dallagi et al., 2023).
Cleaning-In-Place Assays of Food Complex Deposits
Owing to the prevalence of adhesive failures among deposits in the “Mechanical Removal of Food Complex Deposits Under Cleaning Treatments” section after cleaning treatment, this section employs a lab-simulated CIP system (as described in the “Cleaning-In-Place Assays” section) featuring a complex surface structure composed of metallic fibres. The chosen surface is stainless steel, renowned for its relevance in food contact applications. In situ cleaning of the model deposits is conducted here, and the detergency values are documented in Table 6. As in the “Mechanical Removal of Food Complex Deposits Under Cleaning Treatments” section, two cleaning methods are employed: (method 1) which employs water at 50 ºC for 30 min and (method 2) involving the use of an aqueous NaOH solution (4 g/L) at the same temperature and time. Response surface plots illustrating the detergency levels are presented in Fig. 5.
At a lower concentration of solids (design 1), the starch-containing foulant (A) exhibits the lowest detergency values (31.4%) in water, followed by lard (C, 42.9%) and whey (B, 50.5%). The impact of alkaline cleaning on the removal of these three model deposits is observed to differ: it diminishes the removal of whey foulant (from 50.5 to 40.9%) and enhances the removal of lard by approximately 6%, while exerting no significantly discernible effect on the cleaning process for starch foulant under the conditions investigated. Consistent with previous research, where the effectiveness of alkaline solutions in the removal of fat-based fouling agents through hydrolysis has been established (Herrera-Márquez et al., 2020; Otto et al., 2016).
In the case of binary mixtures, the combination of starch and whey (E) yields detergency levels comparable to those exhibited by starch deposits (~ 27%) in water. Furthermore, when starch and whey foulants are mixed with lard, their removal becomes easier, leading to detergency levels of 55.2% and 65.9%, respectively. Alkaline treatment significantly facilitates the removal of these binary mixtures containing starch (E and F), resulting in detergency levels surpassing 75%. However, alkaline treatment diminishes the removal of the whey–lard combination by approximately 13%. The detergency values obtained align with the findings reported by Serrano-Haro et al. (2019) and Herrera-Márquez et al. (2020) concerning the cleaning efficacy of combinations involving potato starch and Iberian pork lard. Specifically, these studies observed detergency values of approximately 60% when using neutral pH solutions, whereas alkaline cleaning solutions yielded detergency values of around 90%.
On the other hand, the triple mixture composed of equal fractions of starch, lard, and whey (D) achieves detergency levels of approximately 50% regardless of the cleaning method employed. Nevertheless, varying the proportions of foulants within the triple mixtures yields distinct removal levels: while the triple mixture with a high lard content (H) remains largely unaltered under both cleaning treatments, the alkaline solution augments the removal of the mixture with a higher proportion of starch (I) by approximately 40%, while simultaneously decreasing its removal by roughly 16% for the triple mixture in which whey (J) predominates.
A higher percentage of solids (design 2) appears to diminish the removal percentage of starch (A) and whey (B) single foulants when subjected to both water and alkaline cleaning conditions. While alkaline cleaning slightly increases the removal efficiency of the starch-based foulant by approximately 5%, it decreases the effectiveness of cleaning for the whey-based foulant by about 9%. The efficacy of alkaline solutions in targeting amylaceous deposits has been demonstrated, attributing its efficacy to the transformative influence exerted by these solutions on the starch structure, resulting in a decrease in polymerisation degree, while promoting swelling, solubility, and facilitating the subsequent removal process (Han & Lim, 2004; Lai et al., 2004; Nor Nadiha et al., 2010; Wang & Copeland, 2012; Vicaria et al., 2017; Herrera-Márquez et al., 2019; Avila-Sierra et al., 2021c). On the other hand, the cleaning efficiency of whey protein deposits is significantly affected by pH due to the consequential alteration of protein structure: at neutral pH, proteins may retain their natural state, rendering them soluble and easily removable from the substrate, while an alkaline pH may facilitate protein denaturation, resulting in a gel-like appearance and partial insolubility that can condition the removal process.
Similar trends are observed for their binary mixtures, with removal decreasing as the percentage of solids increases. Alkaline treatment proves advantageous for the removal of all investigated binary mixtures, although minimal differences of approximately 2% are found for the starch–whey combination. Conversely, a higher percentage of solids in the triple mixture composed of equal fractions of starch, lard, and whey (D) appears to marginally decrease removal in water by approximately 4%, but no significant differences are observed under alkaline treatment, where removal is seemingly favoured. In water, a higher percentage of solids in the triple mixtures formed by different fractions of foulants (H, I, J) reduces removal, except for those with a high proportion of lard, which remain largely unaffected. Under alkaline conditions, a higher percentage of solids reduces removal for all triple mixtures, particularly those with higher starch (I) and lard (H) content, resulting in a decline in detergency of approximately 60 and 16%, respectively.
Cleaning Mechanisms and Efficiency of Model Foulants on Stainless Steel Surfaces
Expanding upon the preceding sections, here we present a comprehensive examination of the cleaning mechanisms followed for the model fouling agents employed in this study on stainless steel surfaces. In terms of low solid concentrations, starch exhibited a lower shear stress requirement compared to whey-based deposits (see the Mechanical Removal of Model Foulants” section), a trend that persisted during mechanical removal and in situ cleaning with both water and NaOH (see the “Mechanical Removal of Food Complex Deposits Under Cleaning Treatments” and “Cleaning-In-Place Assays of Food Complex Deposits” sections). Although the lard-based fouling agent necessitated the highest shear stress among the three single agents investigated (see the “Mechanical Removal of Model Foulants” section), the cleaning temperature of 50 °C, surpassing the melting point of the fatty phase, significantly facilitated its removal (see the “Mechanical Removal of Food Complex Deposits Under Cleaning Treatments” and “Cleaning-In-Place Assays of Food Complex Deposits” sections).
Concerning binary mixtures, those containing lard demanded higher shear stresses in the absence of cleaning treatment (see the “Mechanical Removal of Model Foulants” section). However, the application of cleaning solutions resulted in complete adhesive failure for these mixtures in most cases, irrespective of any mechanical action (see the “Mechanical Removal of Food Complex Deposits Under Cleaning Treatments” section).
Under alkaline treatment, binary mixtures containing starch demonstrated a substantial increase in removal efficacy, while the whey–lard mixture experienced a decline in performance compared to hot water treatment (see the “Cleaning-In-Place Assays of Food Complex Deposits” section). In fact, for in situ cleaning, the starch–whey binary mixture exhibited the lowest removal percentage when using hot water (see the “Cleaning-In-Place Assays of Food Complex Deposits” section).
For three-component mixtures with identical compound fractions, removal percentages generally hovered around 50% (see the “Cleaning-In-Place Assays of Food Complex Deposits” section). Alkaline treatment proved slightly more effective as solids concentration increased (see the “Cleaning-In-Place Assays of Food Complex Deposits” section), especially for those necessitating higher shear stresses (see the “Mechanical Removal of Model Foulants” section). However, no significant disparities were reported in terms of cleaning mechanisms (see the “Mechanical Removal of Food Complex Deposits Under Cleaning Treatments” and “Cleaning-In-Place Assays of Food Complex Deposits” sections). Notably, hot water was preferred for triple mixtures with a high lard content, particularly at higher solids concentrations (see the “Cleaning-In-Place Assays of Food Complex Deposits” section). Although in situ alkaline cleaning enhanced detergency for the triple mixture with high a fraction of starch and low solid concentration, removal levels were similar to those achieved with hot water at higher concentrations (see the “Cleaning-In-Place Assays of Food Complex Deposits” section), aligning with the findings of the “Mechanical Removal of Food Complex Deposits Under Cleaning Treatments” section. Similarly, for three-component mixtures with a high whey content, hot water was the preferred method at lower solids concentrations (see the “Mechanical Removal of Food Complex Deposits Under Cleaning Treatments” and “Cleaning-In-Place Assays of Food Complex Deposits” sections).
Overall, an increase in solid concentration generally increased the shear stresses required for deposit removal from stainless steel surfaces without the involvement of cleaning agents (see the “Mechanical Removal of Model Foulants” section). An increased concentration in solids also seemed to enhance the cohesiveness of those single fouling agents, favouring their adhesive detachment under cleaning treatment, but without necessitating mechanical action (see the “Mechanical Removal of Food Complex Deposits Under Cleaning Treatments” section). However, for persistently adhered deposits, higher shear stresses were typically required for removal compared to deposits at lower solids concentrations, regardless of the cleaning treatment employed. This observation also corresponds to the data reported in the “Cleaning-In-Place Assays of Food Complex Deposits” section for in situ cleaning, where removal efficacy diminished as solids concentration increased in most cases.
Conclusions
In this work, using a multi-length scale approach involving milli-manipulation and a lab-simulated CIP system, we investigated the forces required and mechanisms for removing model fouling agents comprising mixtures of starch, whey, and lard from industrially relevant solid surfaces (i.e. stainless steel, aluminium, and PTFE). The forces involved in the removal process, the types of failure observed when the deposits are subjected to shear stress (adhesive, mixed, or cohesive), and the performance of the CIP system are systematically analysed as a function of the cleaning treatments applied. These treatments include chemical treatment, exposure to hot water (50 °C, 30 min), and exposure to a hot alkaline solution (NaOH, 4 g/L) (50 °C, 30 min).
Foulants have a complex composition and structure that will differ depending on the concentration of solids. Based on the results of cleaning on stainless steel surfaces, irrespective of the percentage of solids, alkaline treatment seems to enhance the cleaning of lard and starch deposits, while whey foulant removal is more effective using hot water. For binary mixtures, the alkaline solution demonstrates better efficacy in removing starch–lard and starch–whey combinations, especially when the percentage of solids is lower for the latter. For the binary mixture whey–lard, and triple mixtures contain a high proportion of lard or whey protein, the use of hot water allows somewhat higher detergency values to be obtained for lower solids concentrations. In these cases where the starch concentration is zero (binary mixture) or very low, the positive effect of the basic medium on cleaning is not as remarkable. For the removal of these foulants, cleaning at 50 °C seems to have a greater effect, probably due to the melting of the lard fraction, limiting the chemical modification of the whey proteins.
In the case of the triple mixture, alkaline conditions yield superior results at higher percentages of solids. When the triple mixture contains a high proportion of lard or whey protein, hot water is recommended under the experimental conditions tested. However, the percentage of solids significantly impacts the removal of the triple mixture with a high fraction of starch, as it determines the most efficient cleaning approach. Alkaline cleaning proves more favourable at lower percentages of solids, while no differences are found between alkaline and water treatments as the solid fraction increases in the mixture. In general, foulants easily removed with water or NaOH solutions (shear stress 0 or close to 0, stainless steel) exhibited higher detergency values. This phenomenon was predominantly noticeable in instances involving foulants containing substantial lard content, implying that the temperature played a role in diminishing shear stress values of the foulants. Nevertheless, a similar reduction in shear stress values was also evident in binary mixtures of whey and starch. The nature of the surface to which the fouling agent adheres is also a crucial factor to consider. Notably, hot water proved to be sufficiently effective in removing deposits from flat stainless steel and flat PTFE surfaces, leading to reduced shear stress requirements. In contrast, alkaline treatment demonstrated superior efficacy when applied to flat aluminium surfaces. These findings emphasise the paramount significance of customising cleaning protocols for CIP optimisation, by carefully considering the deposit nature, its compositional fluctuations throughout product processing, and the characteristics of the surface it comes into contact with.
These results may also serve as a reliable dataset for the development and validation of new computational cleaning models, an emerging field that offers numerous benefits such as cost savings, process optimisation, product and equipment development, risk assessment, regulatory compliance, and predictive maintenance. However, in our view, further work is still needed to correlate physical parameters (e.g. shear stress) more precisely with detergency. Factors that should be further studied include the influence of cleaning temperature, time, and the composition of cleaning solutions. Extension of the results obtained to CIP systems would require validation through a pilot plant to assess the cleaning performance under real industrial conditions.
Data Availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information file. Should any raw data files be needed in another format, they are available from the corresponding author upon reasonable request.
References
Ali, A., & de’Ath, D., Gibson, D., Parkin, J., Alam, Z., Ward, G., Wilson, D.I. (2015). Development of a ‘millimanipulation’ device to study the removal of soft solid fouling layers from solid substrates and its application to cooked lard deposits. Food and Bioproducts Processing, 93, 256–268. https://doi.org/10.1016/j.fbp.2014.09.001
Andreatta, F., Lanzutti, A., Aneggi, E., Gagliardi, A., Rondinella, A., Simonato, M., & Fedrizzi, L. (2020). Degradation of PTFE non-stick coatings for application in the food service industry. Engineering Failure Analysis, 115, 104652. https://doi.org/10.1016/j.engfailanal.2020.104652
Avila-Sierra, A., Huellemeier, H. A., Zhang, Z. J., Heldman, D. R., & Fryer, P. J. (2021a). Molecular understanding of fouling induction and removal: Effect of the interface temperature on milk deposits. ACS Applied Materials & Interfaces, 13(30), 35506–35517. https://doi.org/10.1021/acsami.1c09553
Avila-Sierra, A., Moreno, J. A., Goode, K., Zhu, T., Fryer, P. J., Taylor, A., & Zhang, Z. J. (2023). Effects of structural and chemical properties of surface coatings on the adsorption characteristics of proteins. Surface and Coatings Technology, 452, 129054. https://doi.org/10.1016/j.surfcoat.2022.129054
Avila-Sierra, A., Vicaria, J. M., Jurado-Alameda, E., & Martínez-Gallegos, J. F. (2020). Removal of food soil by ozone-based oxidation processes: Cleaning and wastewater degradation in a single step. Journal of Food Engineering, 272, 109803. https://doi.org/10.1016/j.jfoodeng.2019.109803
Avila-Sierra, A., Vicaria, J. M., Lechuga, M., Martínez-Gallegos, J. F., Olivares-Arias, V., Medina-Rodríguez, A. C., Jiménez-Robles, R., & Jurado-Alameda, E. (2021b). Insights into the optimisation of the Clean-In-Place technique: Cleaning, disinfection, and reduced environmental impact using ozone-based formulations. Food and Bioproducts Processing, 129, 124–133. https://doi.org/10.1016/j.fbp.2021.08.003
Avila-Sierra, A., Zhang, Z. J., & Fryer, P. J. (2019). Effect of surface characteristics on cleaning performance for CIP system in food processing. Energy Procedia, 161, 115–122. https://doi.org/10.1016/j.egypro.2019.02.067
Ávila-Sierra, A., Zhang, Z. J., & Fryer, P. J. (2021c). Effect of surface roughness and temperature on stainless steel - Whey protein interfacial interactions under pasteurisation conditions. Journal of Food Engineering, 301, 110542. https://doi.org/10.1016/j.jfoodeng.2021.110542
Basso, M., Simonato, M., Furlanetto, R., & De Nardo, L. (2017). Study of chemical environments for washing and descaling of food processing appliances: An insight in commercial cleaning products. Journal of Industrial and Engineering Chemistry, 53, 23–36. https://doi.org/10.1016/J.JIEC.2017.03.041
Chinga, G., Johnssen, P. O., Dougherty, R., Lunden-Berli, E., & Walter, J. (2007). Quantification of the 3-D microstructure of SC surfaces. Journal of Microscopy, 227(3), 254–265. https://doi.org/10.1111/j.1365-2818.2007.01809.x
Christian, G. K., & Fryer, P. J. (2006). The effect of pulsing cleaning chemicals on the cleaning of whey protein deposits. Food and Bioproducts Processing, 84, 320–328. https://doi.org/10.1205/FBP06039
Cuckston, G. L., Alam, Z., Goodwin, J., Ward, G., & Wilson, D. I. (2019). Quantifying the effect of solution formulation on the removal of soft solid food deposits from stainless steel substrates. Journal of Food Engineering, 243, 22–32. https://doi.org/10.1016/j.jfoodeng.2018.08.018
da Silva Pereira, G. V., da Silva Pereira, G. V., Xavier Neves, E. M. P., Albuquerque, G. A., Rêgo, J. A. R., Cardoso, D. N. P., Brasil, D. S. B., & Joele, M. R. S. P. (2021). Effect of the mixture of polymers on the rheological and technological properties of composite films of acoupa weakfish (Cynoscion acoupa) and cassava starch (Manihot esculenta C.). Food Bioprocess Technology, 14, 1199–1215. https://doi.org/10.1007/s11947-021-02622-1
Daeschel, D., Rana, Y. S., Chen, L., Cai, S., Dando, R., & Snyder, A. B. (2023). Visual inspection of surface sanitation: Defining the conditions that enhance the human threshold for detection of food residues. Food Control, 149, 109691. https://doi.org/10.1016/j.foodcont.2023.109691
Dallagi, H., Jha, P. K., Faille, C., Le-Bail, A., Rawson, A., & Benezech, T. (2023). Removal of biocontamination in the food industry using physical methods; an overview. Food Control, 148, 109645. https://doi.org/10.1016/j.foodcont.2023.109645
de Vargas, V. H., Marczak, L. D. F., Flores, S. H., et al. (2022). Advanced technologies applied to enhance properties and structure of films and coatings: A review. Food and Bioprocess Technology, 15, 1224–1247. https://doi.org/10.1007/s11947-022-02768-6
EHEDG. (2018). Doc. 8. Hygienic design principles. Third Edition. https://www.ehedg.org/guidelines-working-groups/guidelines/guidelines/detail/hygienic-design-principles.
Felfoul, I., Beaucher, E., Cauty, C., Attia, H., Gaucheron, F., & Ayadi, M. A. (2016). Deposit generation during camel and cow milk heating: Microstructure and chemical composition. Food and Bioprocess Technology, 9, 1268–1275. https://doi.org/10.1007/s11947-016-1714-1
Felfoul, I., Lopez, C., Gaucheron, F., Attia, H., & Ayadi, M. A. (2015). Fouling behavior of camel and cow milks under different heat treatments. Food and Bioprocess Technology, 8, 1771–1778. https://doi.org/10.1007/s11947-015-1529-5
Fryer, P. J., & Asteriadou, K. (2009). A prototype cleaning map: A classification of industrial cleaning processes. Trends in Food Science & Technology, 20, 255–262. https://doi.org/10.1016/j.tifs.2009.03.005
Han, J. A., & Lim, S. T. (2004). Structural changes in corn starches during alkaline dissolution by vortexing. Carbohydrate Polymers, 55(2), 193–199. https://doi.org/10.1016/j.carbpol.2003.09.006
Herrera-Márquez, O., Serrano-Haro, M., Vicaria, J. M., Jurado, E., Fraatz-Leál, A. R., Zhang, Z. J., Fryer, P. J., & Avila-Sierra, A. (2020). Cleaning maps: A multi length-scale strategy to approach the cleaning of complex food deposits. Journal of Cleaner Production, 261, 121254. https://doi.org/10.1016/j.jclepro.2020.121254
Herrera-Márquez, O., Vicaria, J. M., & Jurado-Alameda, E. (2019). Experimental design for optimizing the cleaning of starch adhering to stainless-steel surfaces using nonionic surfactants and silica microparticles. Journal of Surfactants and Detergents, 22(3), 559–569. https://doi.org/10.1002/jsde.12251
Jurado-Alameda, E., Herrera-Márquez, O., Martínez-Gallegos, J. F., & Vicaria, J. M. (2015). Starch-soiled stainless steel cleaning using surfactants and α-amylase. Journal of Food Engineering, 160, 56–64. https://doi.org/10.1016/j.jfoodeng.2015.03.024
Jurado, E., Herrera-Marquez, O., Plaza-Quevedo, A., & Vicaria, J. M. (2015). Interaction between non-ionic surfactants and silica micro/nanoparticles. Influence on the cleaning of dried starch on steel surfaces. Journal of Industrial and Engineering Chemistry, 21, 1383–1388. https://doi.org/10.1016/j.jiec.2014.06.011
Lai, L. N., Karim, A. A., Norziah, M. H., & Seow, C. C. (2004). Effects of Na2CO3 and NaOH on pasting properties of selected native cereal starches. Journal of Food Science, 69, 249–256. https://doi.org/10.1111/j.1365-2621.2004.tb06324.x
Laukemper, R., Becker, T., & Jekle, M. (2021). Surface energy of food contact materials and its relation to wheat dough adhesion. Food and Bioprocess Technology, 14, 1142–1154. https://doi.org/10.1007/s11947-021-02625-y
Liu, W., Christian, G. K., Zhang, Z., & Fryer, P. J. (2002). Development and use of a micromanipulation technique for measuring the force required to disrupt and remove fouling deposits. Food and Bioproducts Processing, 80, 286–291. https://doi.org/10.1205/096030802321154790
Liu, W., Fryer, P. J., Zhang, Z., Zhao, Q., & Liu, Y. (2006). Identification of cohesive and adhesive effects in the cleaning of food fouling deposits. Innovative Food Science & Emerging Technologies, 7(4), 263–269. https://doi.org/10.1016/j.ifset.2006.02.006
Magens, O. M., Liu, Y., Hofmans, J. F. A., Nelissen, J. A., & Wilson, D. I. (2017). Adhesion and cleaning of foods with complex structure: Effect of oil content and fluoropolymer coating characteristics on the detachment of cake from baking surfaces. Journal of Food Engineering, 197, 48–59. https://doi.org/10.1016/j.jfoodeng.2016.11.004
Mohammad, A. W., Ng, C. Y., Lim, Y. P., & Ng, G. H. (2012). Ultrafiltration in food processing industry: Review on application, membrane fouling, and fouling control. Food and Bioprocess Technology, 5, 1143–1156. https://doi.org/10.1007/s11947-012-0806-9
Nor Nadiha, M. Z., Fazilah, A., Bhat, R., & Karim, A. A. (2010). Comparative susceptibilities of sago, potato and corn starches to alkali treatment. Food Chemistry, 121(4), 1053–1059. https://doi.org/10.1016/j.foodchem.2010.01.048
Otto, C., Zahn, S., Hauschild, M., Babick, F., & Rohm, H. (2016). Comparative cleaning tests with modified protein and starch residues. Journal of Food Engineering, 178, 145–150. https://doi.org/10.1016/j.jfoodeng.2016.01.015
Pawlus, P., Reizer, R., & Wieczorowski, M. (2021). Functional importance of surface texture parameters. Materials, 14, 5326. https://doi.org/10.3390/ma14185326
Rondinella, A., Andreatta, F., Turrin, D., & Fedrizzi, L. (2021). Degradation mechanisms occurring in PTFE-based coatings employed in food-processing applications. Coatings, 11, 1419. https://doi.org/10.3390/coatings11111419
Santos, A. L., Pires, A. C. S., Behaine, J. J. S., Araújo, E. E., de Andrade, N. J., & de CVarvalho, A. F. (2013). Effect of cleaning treatment on adhesion of Streptococcus agalactiae to milking machine surfaces. Food and Bioprocess Technology, 6, 1868–1872. https://doi.org/10.1007/s11947-011-0665-9
Şen, Y., Bağcı, U., Güleç, H. A., & Mutlu, M. (2012). Modification of food-contacting surfaces by plasma polymerization technique: Reducing the biofouling of microorganisms on stainless steel surface. Food and Bioprocess Technology, 5, 166–175. https://doi.org/10.1007/s11947-009-0248-1
Serrano-Haro, M., Tirado-Delgado, M., Herrera-Márquez, O., Vicaria, J. M., & Jurado-Alameda, E. (2019). Characterization of mixed fatty-starchy soils for cleaning studies in food industry. Chemical Engineering Transactions, 75, 217–222. https://doi.org/10.3303/CET1975037
Stahl, T., Falk, S., Rohrbeck, A., Georgii, S., Herzog, C., Wiegand, A., Hotz, S., Boschek, B., Zorn, H., & Brunn, H. (2017). Migration of aluminum from food contact materials to food—A health risk for consumers? Part I of III: Exposure to aluminum, release of aluminum, tolerable weekly intake (TWI), toxicological effects of aluminum, study design, and methods. Environmental Sciences Europe, 29(19), 1–8. https://doi.org/10.1186/s12302-017-0116-y
Tsai, J. H., Huang, J. Y., & Wilson, D. I. (2021). Life cycle assessment of cleaning-in-place operations in egg yolk powder production. Journal of Cleaner Production, 278, 123936. https://doi.org/10.1016/j.jclepro.2020.123936
Vicaria, J. M., Jurado-Alameda, E., Herrera-Márquez, O., Olivares-Arias, V., & Ávila-Sierra, A. (2017). Analysis of different protocols for the cleaning of corn starch adhering to stainless steel. Journal of Cleaner Production, 168, 87–96. https://doi.org/10.1016/j.jclepro.2017.08.232
Wang, S., & Copeland, L. (2012). Effect of alkali treatment on structure and function of pea starch granules. Food Chemistry, 135(3), 1635–1642. https://doi.org/10.1016/j.foodchem.2012.06.003
Wilson, D. I., Christie, G., Fryer, P. J., Hall, I. M., Landel, J. R., & Whitehead, K. A. (2022). Lessons to learn from roadmapping in cleaning and decontamination. Food and Bioproducts Processing, 135, 156–164. https://doi.org/10.1016/j.fbp.2022.07.011
Funding
Funding for open access publishing: Universidad de Granada/CBUA. Funding for open access charge: Universidad de Granada / CBUA. This work was supported by the Regional Government of Andalusia-FEDER ANDALUCÍA 2014‐2020 (grant number A-TEP-030-UGR18).
Author information
Authors and Affiliations
Contributions
José María Vicaria, Germán Luzón, and Alejandro Ávila-Sierra defined the experimental methodology. María José Sáenz-Espinar, Marina Arroyo-Camarena, and José María Vicaria planned and made the experimental assays. All authors wrote and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sáenz-Espinar, M.J., Arroyo-Camarena, M., Vicaria, J.M. et al. Multi-length Scale Approach to Investigate Cleaning of Food-Derived Deposits Adhered to Hard Surfaces: Mixtures of Starch, Whey Protein, and Lard. Food Bioprocess Technol (2024). https://doi.org/10.1007/s11947-024-03330-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11947-024-03330-2