Abstract
The effect of hyperbaric storage (HS) on polyphenoloxidase activity (PPO) was studied in model solutions and apple juice. Model solutions containing increasing amounts of mushroom (Agaricus bisporus) PPO (up to 26 U) were stored at room temperature at pressure up to 200 MPa. During HS, samples were assessed for residual PPO activity. The enzyme was completely inactivated according to a first-order kinetic model that was used to calculate PPO decimal reduction time (Dp) and pressure sensitivity (zp = 140.8 MPa) in diluted model solutions (2 U PPO). The increase in enzyme concentration (6–26 U) nullified the effect of HS, probably due to protein structure stabilization by self-crowding. The application of HS at 100 and 200 MPa to apple juice promoted a decrease in total bacteria, lactic acid bacteria, yeasts, and molds. These changes occurred in concomitance with the inactivation of PPO (zp = 227.3 MPa). At 200 MPa, PPO inactivation followed a biphasic first-order kinetic, suggesting the presence of PPO isozymes with different pressure sensitivity. The inactivation of PPO was observed to occur more rapidly with increasing storage pressure and led to the maintenance of the original bright juice color. This study proves the capability of HS to control enzyme-related quality decay in fruit juices and, potentially, in many other food matrices suffering enzymatic alteration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperbaric storage (HS) consists in storing perishable foods for long periods of time (up to months) at room temperature under moderate hydrostatic pressure (P < 250 MPa) (Basso et al., 2022b). Although HS shares some technical aspects with high hydrostatic pressure processing (HHP), it substantially differs in the scope and treatment time. In particular, HHP treatments are typically brief (i.e., minutes) and used for non-thermal pasteurization. On the other hand, HS is performed over longer times (i.e., days–months) and has been proposed as an alternative to refrigeration (Fidalgo et al., 2014, 2019; Lemos et al., 2017). Reportedly, the main advantages of HS, as compared to refrigeration, are (i) the very low energetic consumption associated with the maintenance of storage conditions, (ii) the capability to induce substantial non-thermal bacterial inactivation, and (iii) the possibility to steer protein structure and functionality.
Based on its bacteriostatic and bactericidal effect, HS has been mainly applied with the purpose of extending the microbiological safety of perishable matrices, which typically require cold conditions. In raw meat, fish, and seafood, HS not only limited microbial growth, but actively induced up to 5 log reductions of total microbial count, enterobacteria, yeasts, and molds (Fidalgo et al., 2019; Otero & Pérez-Mateos, 2021; Santos et al., 2020). HS was even demonstrated to be a quasi-energetically costless alternative to pasteurization and refrigeration of raw fruit juices, egg white, and milk (Basso et al., 2021; Basso et al., 2022a; Bermejo-Prada et al., 2017; Duarte et al., 2022b; Pinto et al., 2018, 2019). This was proven by the significant inactivation (up to 6 log reductions) induced by HS in pathogenic (e.g., Escherichia coli 8048, Staphylococcus aureus 226, Salmonella enterica), pathogenic-surrogate (e.g., Listeria innocua, Escherichia coli ATCC 25,992), and sporogenic microorganisms (e.g., Bacillus subtilis, Alicyclobacillus acidoterrestris). Similar results were achieved by applying HS (75–100 MPa, 120 days) after inoculation of HHP-pasteurized milk (600 MPa, 90 and 120 s) with Listeria innocua, Escherichia coli, and Salmonella Senftenberg (Lemos et al., 2022). This demonstrated the capability of HS in preventing the effects of post-process contamination.
Besides its antimicrobial activity, HS was also shown to induce modifications in protein structure, leading to the improvement of the technological functionality of protein-rich foods. In particular, HS improved the foam ability of egg white, due to compression and electrostatic stabilization of proteins (Basso et al., 2021). Similar results were achieved in hyperbarically stored raw skim milk. In this case, the better foaming capacity was attributed to the progressive formation of milk proteose peptones under pressure (Basso et al., 2022a). HS also induced the disintegration of myofibrillar proteins in pork meat, enhancing their water-holding capacity and making meat significantly softer and juicer (Santos et al., 2021a, b).
Although HS has been proven capable to guarantee food safety and to steer protein structure and techno-functionality, limited data are available on the effect of pressurized storage on food proteins with catalytic activity. This is due to the fact that the majority of the published literature studies about HS have focused almost exclusively on the microbiological aspect of the technology (Duarte et al., 2022b; Fidalgo et al., 2014, 2019; Lemos et al., 2017; Otero & Pérez-Mateos, 2021; Pinto et al., 2018, 2019; Santos et al., 2020). To the best of our knowledge, the effect of HS on enzymes has been evaluated in meat, fish, milk, and in some fruit juices. In the case of pork meat and Atlantic salmon, 75 MPa-HS for up to 60 days promoted up to 91% inactivation of acid phosphatase, cathepsins and calpains (Fidalgo et al., 2020; Santos et al., 2021a, b). Significant inactivation (up to 45%) was also demonstrated for alkaline phosphatase and lactoperoxidase in raw milk stored at 75 MPa for up to 31 days (Duarte et al., 2022a, b). In the case of fruit juices, contradicting information is available in the literature. In particular, polyphenoloxidase (PPO) inactivation (−70% residual activity) was achieved by 50-MPa HS for 4 days in watermelon juice, whereas the enzyme was strongly activated (+60% residual activity) in strawberry juice after 7 days at 200 MPa. Such remarkable variability agrees with the body of evidence relevant to the effects of high hydrostatic pressure (HHP; 250–1200 MPa, 5–30 min) on catalytic proteins. The nature (i.e., composition, proteomics) of the enzyme is recognized as a major source of variability (Eisenmenger & Reyes-De-Corcuera, 2009; Mozhaev et al., 1996) and the response of enzymes to pressurized conditions is very often not linearly dependent on the applied pressure and time. For instance, Pinto et al. (2017) showed a non-time-dependent inactivation of PPO upon HS of watermelon juice. Irregular changes in PPO activity were reasonably due to a series of pressure-induced rearrangements of the enzyme structure, which might have promoted less or more active enzyme conformations (Mozhaev et al., 1996). To this regard, it is noteworthy that, when protracted over HS times (i.e., days/months), the effect of minor pressure-induced conformational changes could result in both the complete stabilization of the food matrix or the development of dramatic alterative events. Therefore, the high variability and scarce predictability of HS effects on enzymes are certainly a critical lack in the pressurized storage framework.
Based on these considerations, the aim of the present work was to study the effect of HS on the activity of PPO. The latter was selected based on its critical role for the quality of plant derivatives (Yoruk & Marshall, 2003), which are among the most feasible matrices for HS applications (Otero, 2019). The work was divided in two parts. Initially, a kinetic study of mushroom PPO inactivation by HS at pressure up to 200 MPa was performed in model solutions with different initial enzyme concentration (2–26 U). Following, the results obtained in model systems were validated in fresh apple juice. The latter was specifically selected as a perishable matrix feasible for HS and subjected to the browning activity of PPO (Janovitz-Klapp et al., 1990; Steele et al., 1982). Apple juice was stored at 100 and 200 MPa for up to 6 days. At increasing time during storage, the juice was analyzed not only for PPO activity but also for color and quality-related microbial indexes (i.e., total bacterial count, lactic acid bacteria, and yeasts and molds) to show the industrial relevance of HS in the stabilization of fruit derivatives.
Materials and Methods
Materials
Golden delicious apples were obtained at a local retailer and stored at 4 °C until analysis. Potassium sorbate and sodium benzoate were obtained by Carlo Erba Reagents S.r.l. (Cornaredo, Italy). 3,4-dihydroxy-L-phenylalanine (L-DOPA) and dihydrogen- and monohydrogen-potassium phosphate were obtained by J.T.Baker (Teugseweg, Deventer, Netherlands). Mushroom tyrosinase (5771 U mg−1) was obtained by Sigma-Aldrich (Milano, Italy). Maximum recovery diluent (MRD), plate count agar (PCA), oxytetracycline Glucose Yeast Extract (OGY) agar, and De Man, Rogosa and Sharpe (MRS) agar were obtained from Oxoid (Milan, Italy).
Sample Preparation
Mushroom (Agaricus bisporus) tyrosinase (i.e., PPO) model solutions were prepared by solubilizing increasing amounts of enzyme in pH 7 potassium phosphate buffer with 0.1-M ionic strength. Solutions were frozen and maintained at −30 °C until use to prevent loss of activity (Anese et al., 1994).
Apple juice (dry matter = 11.89 ± 0.05% (w/w), pH 3.7) was obtained from Golden delicious apples as previously described by Manzocco et al. (2013). Briefly, apples were cored and cut into approximately 3 × 3 × 3-cm cubes. Apple cubes were pressed using a domestic juicer (FP800 Kenwood Electronic, Havanthants, UK), collecting the juice in a beaker kept in a water ice bath. The obtained juice was clarified by centrifugation at 5000 rpm at 4 °C for 5 min (Avanti J-25, Beckman Instruments Inc., Palo Alto, CA, USA).
Appropriate aliquots of PPO solutions (1 mL) or apple juice (10 mL) were packaged inside PP/EVOH/PE pouches (5 × 3 cm; 80-μm thickness, water vapor permeability < 1 g m−2 24 h−1; Niederwieser Group S.p.A., Campogalliano, Italy) and heat-sealed (Orved VM-16, Musile di Piave, Italy) with minimal headspace.
Hyperbaric Storage
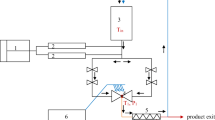
Samples were stored for up to 10 days at 100 and 200 MPa in a hyperbaric storage pilot plant assembled by Comer S.r.l. (Bologna, Italy) and kept in a thermostated room at 20 ± 1 °C. The working unit consisted of a water-tight steel vessel (Hystat, Slaithwaite, Huddersfield, UK) with a maximum working pressure of 200 MPa, pressurized by a Haskel International high-pressure pump (Burbank, CA, USA). An aqueous solution containing 0.2% (w/w) potassium sorbate and 0.2% (w/w) sodium benzoate was used as pressure-mediating fluid to prevent mold growth in the fluid reservoir. Control samples were stored at room pressure and temperature conditions (0.1 MPa, 20 ± 1 °C).
Polyphenoloxidase Activity
Activity of mushroom PPO solutions and apple juice samples was determined during storage according to Manzocco et al., (2009, 2013). Briefly, 20 µL of PPO solutions or apple juice was added to 1980 µL of 1.5 mM L-DOPA in 0.10-M potassium phosphate buffer pH 7. Following the addition of the enzyme to L-DOPA, absorbance at 420 nm was determined at increasing time for up to 10 min using a UV-2501 PC spectrophotometer (Shimadzu Kyoto, Japan). Absorbance increase rate (Abs min−1) was calculated by applying a zero-order kinetic model to the absorbance curves within the first 3 min of assay. Fitting of the kinetic model was deemed acceptable with values of the adjusted determination coefficient (R2adj) > 0.9. The enzymatic unit (U) was defined as the amount of enzyme capable to induce a 0.001 Abs min−1 increase in absorbance at 420 nm in the described testing conditions. Sample residual activity was calculated during storage using Eq. 1.
where \(RA\) is the residual PPO activity, \({A}_{t}\) (Abs min−1) is the PPO activity of the samples stored for a time \(t\) (h), and \({A}_{0}\) (Abs min−1) is the activity of the samples before storage.
Kinetic Modeling
Zero-, first-, second-, and nth-order models were used to fit the data. The two-fraction model (Eq. 2) was used to fit PPO inactivation curves, which showed a biphasic behavior during storage, and estimates the relative abundance of the two PPO fractions (Weemaes et al., 1998):
where \({RA}_{t}\) (%) is the estimated residual PPO activity at storage time t (h), \({RA}_{0}^{f}\) and \({RA}_{0}^{s}\) (%) are the estimated initial PPO activity of the two PPO fractions, and \({k}^{f}\) and \({k}^{s}\) (h−1) are the relevant inactivation kinetic rates.
First-order kinetic rates (k, h−1) were used to estimate the decimal reduction time under pressure (Dp, h) and pressure sensitivity (zp, MPa) of PPO according to Manzocco et al. (2016). In particular, Dp was computed using Eq. 3.
The decimal logarithm of Dp was then linearly regressed versus storage pressure (P) according to Eq. 4.
where P is the storage pressure (MPa). zp was derived as the negative reciprocal of the regression line slope.
Microbiological Analyses
Appropriate serial dilutions of apple juice samples with MRD were plated on PCA for total bacteria count (TBC), OGY for yeasts and molds (YM), and MRS agar for lactic acid bacteria (LAB). Plated samples were incubated at 30 °C, for 24–48 h for TBC and LAB, and for 48–72 h for YM. Analyses were carried out in sterile conditions and microbial counts were expressed as log CFU mL−1.
Color
Apple juice color was determined using a tristimulus colorimeter (Chromameter-2 Reflectance, Minolta, Osaka, Japan) equipped with a CR-300 measuring head and calibrated against a standard tile before use. Four-milliliter aliquots of apple juice samples were poured into a Petri dish (5-cm diameter, 1-cm height) positioned over the instrument calibration tile. Color measurements were taken placing the instrument lens onto the juice surface.
Image Acquisition
Images (300-dpi vertical and horizontal resolution) were acquired using an image acquisition cabinet (Immagini & Computer, Bareggio, Italy) equipped with a digital camera (EOS 550D, Canon, Milano, Italy) placed on an adjustable stand positioned 45 cm above a black cardboard base. Uniform lighting was guaranteed by 4100-W frosted photographic floodlights.
Data Analysis
Microbial counts were performed in single on a single experiment. Each condition in the experiment was performed at least in duplicate. At least two PPO activity and color measurements were performed on each sample.
Kinetic modeling of PPO residual activity during storage was performed using the nonlinear fit function of OriginPro 2021 (OriginLab, Northampton, MA, USA). Goodness of fit was evaluated based on R2adj and normalized root-mean-squared error (NRMSE). The latter was calculated by dividing the root-mean-squared error by the highest value on the residual activity curve scale. Data from color measurements were expressed as mean ± standard deviation and were subjected to one-way analysis of variance (ANOVA) and Tukey’s honest significant differences test (p < 0.05) using R v. 4.2.2 for Windows (the R foundation for statistical computing).
Results and Discussion
Effect of HS on PPO in Model Systems
A model solution containing 2 U of mushroom PPO in 0.10 M, pH 7 phosphate buffer was analyzed for enzymatic activity during hyperbaric storage at 100 and 200 MPa at room temperature (Fig. 1). An analogous control solution was stored at ambient pressure (0.1 MPa).
Independently on pressure, PPO activity progressively decreased during storage in all samples. Many enzymes, including PPO, are known to lose activity when solubilized in aqueous media (Anese et al., 1994; Liu & Cheng, 2000; Rosenthal et al., 2002; Sadana, 1988). In fact, enzyme molecules in diluted aqueous environments are highly mobile and easily undergo structural modifications that hamper their catalytic activity (Zaks & Russell, 1988). In the case of PPO, this decay was made significantly faster by the application of HS. In fact, PPO complete inactivation occurred in 48 h at environmental pressure (control), while only 24 and 12 h were needed when samples were stored at 100 and 200 MPa, respectively. This indicates that the application of pressure during storage promoted PPO inactivation, which spontaneously occurs in diluted solution at environmental pressure. Samples showing complete loss of PPO activity were further checked for possible reactivation upon refrigeration (4 °C, 0.1 MPa) for up to 4 days. However, no enzyme activity recovery was detected, indicating that PPO inactivation was not only complete but also irreversible.
PPO activity data (Fig. 1) were subjected to kinetic elaboration according to zero-, first-, second- and nth-order kinetic equations. The first-order kinetic model well fitted the experimental data and showed the lowest NRSME and the highest R2adj (data not shown). Based on this result and in agreement with the literature (Henley & Sadana, 1985; Illera et al., 2019; Sadana, 1988), the first-order kinetic model was used to estimate PPO inactivation rate during storage (Table 1).
Kinetic elaboration confirmed that PPO inactivation rate increased with storage pressure level (Table 1). Inactivation rate data were then used to estimate PPO decimal reduction time under pressure (Dp), which was defined as the time (h) required to achieve a 90% decrease in the enzyme activity. Dp values resulted 23.5, 5.6 and 0.2 h at 0.1, 100 and 200 MPa, respectively. Based on these results, the pressure sensitivity (zp) of PPO, defined as the pressure increase needed to cause a 90% reduction of Dp, was computed by linear regression of the log10(Dp) versus pressure (R2adj = 0.9088, p < 0.05, NRMSE = 0.019). The obtained zp was 140.8 MPa, which was comparable with the one reported in the literature (156.3 MPa) for PPO solutions subjected to brief HHP treatments (750–900 MPa) (Guerrero-Beltrán et al., 2005).
Further tests were performed by hyperbaric storage of model solutions having higher initial PPO activity (Table 1). In particular, samples containing 6, 14, and 26-U PPO were considered. According to the kinetic rates calculated using the first-order model, the increase in initial PPO concentration decreased the inactivation rate at 0.1 MPa reaching a minimum average value for the 14-U sample. This stabilization is likely due to enzyme self-crowding (Helm & Miiller, 1991; Liu & Cheng, 2000; Manzocco et al., 2013), which limits conformational changes of protein molecules in close proximity with each other (Minton, 2005; van den Berg et al., 1999). As a consequence, structure-dependent enzymatic inactivation may be significantly hampered (Manzocco et al., 2013). Samples containing more than 2-U enzymes stored at 200 MPa showed inactivation values comparable or even lower than the control sample (0.1 MPa). This result indicates that self-crowding made PPO structure more stable and pressure resistant (Table 1) (Minton, 2005; van den Berg et al., 1999).
Effect of HS in Apple Juice
Based on the interesting results observed in model systems, the effect of HS on PPO was evaluated in fresh apple juice, taken as an example of a perishable food matrix affected by the browning action of this enzyme.
As widely demonstrated in the literature, HS can promote up to 5 log reductions of a wide spectrum of microbial species in several fresh foods (Basso et al., 2021; Basso et al., 2022a; Bermejo-Prada et al., 2017; Duarte et al., 2022a, b; Duarte et al., 2022b; Fidalgo et al., 2019; Otero & Pérez-Mateos, 2021; Pinto et al., 2018, 2019; Santos et al., 2020;). Based on this evidence, microbiological analyses of apple juice were performed only to confirm the well-known antimicrobial efficacy of HS. In particular, TBC, LAB, and YM were counted in samples stored at 0.1 (control), 100 and 200 MPa. Apple juice stored at 0.1 MPa (control) showed microbial counts increasing from approximately 2 to more than 6 log CFU mL−1 within 4 days. At this point, the microbiological trials were interrupted, since samples were considered unacceptable for consumption. Differently, HS at 100 MPa inhibited the growth of all the considered microbial indexes, which resulted below the detection limit (1 log CFU mL−1) throughout storage for up to 6 days. By increasing storage pressure up to 200 MPa, complete and irreversible inactivation of TBC, LAB, and YM was obtained within 24 h. These results agree with the remarkable bacteriostatic and bactericidal effect of HS reported in the literature for various foods, including apple, watermelon, and strawberry juices (Fidalgo et al., 2014, 2019; Lemos et al., 2017; Otero & Pérez-Mateos, 2021; Pinto et al., 2019; Segovia-Bravo et al., 2012). Given the antimicrobial efficacy of HS and in agreement with the aims of this work, the attention was focused on the effect of HS on apple juice PPO. The initial PPO activity in apple juice was 1.76 ± 0.13 U in the tested conditions, equal to 0.76 ± 0.04 U mg dry matter−1.
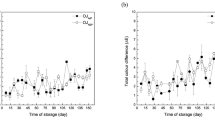
Figure 2 shows that apple juice PPO activity progressively decreased during storage, reaching the complete inactivation after 2 and 6 days at 100 and 200 MPa, respectively. By contrast, at 0.1 MPa, the complete inactivation of PPO was not observed since the experiment was interrupted due to sample microbial spoilage. Data shown in Fig. 2 were further elaborated according to a first-order kinetic model (Table 2).
Kinetic elaboration confirmed the faster inactivation of PPO under 100 and 200-MPa HS as compared to control conditions and yielded a zp value equal to 227.3 MPa (R2 = 0.9429, p < 0.05), indicating apple PPO to be less pressure-sensitive than the mushroom one (140.8 MPa). This could be attributed to the different enzyme structures and to the presence in apple juice of sugars, salts, polysaccharides, and other proteins. Such components are likely to have stabilized the conformation of apple juice PPO by allowing a larger number of interactions with the close environment (Weemaes et al., 1998).
It must be highlighted that, as indicated by the fitting parameters (R2adj = 0.7147, Table 2), the first-order model was less efficacious in describing PPO inactivation data obtained at 200 MPa than those relevant to storage at 0.1 and 100 MPa (R2adj > 0.93). In fact, PPO decay at 200 MPa (Fig. 2) clearly showed a discontinuity point at about 1-h storage, suggesting a biphasic inactivation trend at these pressure conditions. This might be attributed to the presence in apple juice of at least two PPO fractions (Benito-Román et al., 2019; Illera et al., 2019; Siguemoto et al., 2018; Zawawi et al., 2022) with different pressure sensitivity (Weemaes et al., 1998). This hypothesis is supported by literature data showing that PPO occurs in apple in at least two different isoenzymatic forms (Richard-Forget et al., 1994; Vamos-Vigyázó et al., 1981, Wang et al., 1991). To estimate the relative abundance of the two PPO fractions, data fitting was performed using a biphasic first-order model (R2adj = 0.9727; NRMSE = 0.053). The latter is also known as the two-fraction model (Eq. 2) and is often applied to describe heat- or pressure-induced enzymatic inactivation (Benito-Román et al., 2019; Illera et al., 2019; Siguemoto et al., 2018; Weemaes et al., 1998; Zawawi et al., 2022). In particular, the model accounts for the coexistence of isozymes by grouping them into two fractions: a labile one, which is fast inactivated, and a stable one, whose activity is longer retained (Illera et al., 2019). kf and ks account for the inactivation rates of the two PPO fractions and resulted equal to 3.458 ± 1.623 and 0.038 ± 0.004 h−1, respectively. This clearly indicated a difference in the pressure stability between apple PPO fractions. Based on the value of \({RA}_{0}^{f}\), the relative abundance of the pressure sensitive PPO fraction was estimated to be about 40% of the overall apple PPO.
In accordance with the inactivation effect of HS on apple PPO (Fig. 2; Table 2), pressurized storage allowed to better maintain the visual appearance of the juice (Table 3). In particular, storage at 200 MPa significantly limited the decrease in luminosity (L*) and the increase in redness (a*) (p < 0.05), which are typically associated with the formation of brown polymers upon PPO-catalyzed oxidation of phenols (Bermejo-Prada & Otero, 2016). Differently, the application of HS caused apple juice yellowness (b*) to increase significantly more than under room pressure conditions.
This result apparently contradicts the observed decrease in PPO activity during pressurized storage. Nevertheless, the increase in yellowness was likely due to disruption of apple cell organelles (e.g., plastids, chromoplasts) upon pressurization with release of yellow pigments (e.g., lutein, zeaxanthin) in the juice (Gonzalez & Barrett, 2010; Saini et al., 2015) and potential increase of its overall appeal to consumers.
Conclusions
This work shows that hyperbaric storage accelerates the spontaneous inactivation of PPO in aqueous model systems and apple juice. The effect of HS on PPO seems to depend on enzyme nature and concentration. The acquired results clearly indicate that HS can contemporarily guarantee not only food microbiological stability but also the control of enzymatic browning in apple juice. It can be hypothesized that similar HS effects could be achieved even considering catalytic proteins other than PPO, as well as their action in a wide range of liquid and solid foods. However, due to the remarkable variability of the pressure sensitivity of enzymes, the effect of HS should be carefully studied in each matrix.
Finally, the possibility of using HS to achieve targets beyond microbial and enzymatic inhibition appears fascinating. In this context, the release of pigments upon pressurization could be actually regarded as a potential strategy to improve sensory properties and availability of bioactive compounds in foods by storing them at near-zero energetic cost.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Anese, M., Nicoli, M. C., & Dall’Aglio, G., & Lerici, C. R. (1994). Effect of high pressure treatments on peroxidase and polyphenoloxidase activities. Journal of Food Biochemistry, 18(4), 285–293. https://doi.org/10.1111/j.1745-4514.1994.tb00503.x
Basso, F., Maifreni, M., Innocente, N., Manzocco, L., & Nicoli, M. C. (2022a). Raw milk preservation by hyperbaric storage: effect on microbial counts, protein structure and technological functionality. Food Research International, 156, Article 111090. https://doi.org/10.1016/j.foodres.2022.111090
Basso, F., Manzocco, L., & Nicoli, M. C. (2022b). Hyperbaric storage of food: Applications, challenges, and perspectives. Food Engineering Reviews, 14, 20–30. https://doi.org/10.1007/s12393-021-09296-7
Basso, F., Manzocco, L., Maifreni, M., & Nicoli, M. C. (2021). Hyperbaric storage of egg white at room temperature: Effects on hygienic properties, protein structure and technological functionality. Innovative Food Science and Emerging Technologies, 74, Article 102847. https://doi.org/10.1016/j.ifset.2021.102847
Benito-Román, Ó., Teresa Sanz, M., Melgosa, R., de Paz, E., Escudero, I., & Beltrán, S. (2019). Studies of polyphenol oxidase inactivation by means of high pressure carbon dioxide (HPCD). Journal of Supercritical Fluids, 147, 310–321. https://doi.org/10.1016/j.supflu.2018.07.026
Bermejo-Prada, A., & Otero, L. (2016). Effect of hyperbaric storage at room temperature on color degradation of strawberry juice. Journal of Food Engineering, 169, 141–148. https://doi.org/10.1016/j.jfoodeng.2015.08.030
Bermejo-Prada, A., Colmant, A., Otero, L., & Guignon, B. (2017). Industrial viability of the hyperbaric method to store perishable foods at room temperature. Journal of Food Engineering, 193, 76–85. https://doi.org/10.1016/j.jfoodeng.2016.08.014
Duarte, R. V., Casal, S., da Silva, J. A. L., Gomes, A., Delgadillo, I., & Saraiva, J. A. (2022a). Nutritional, physicochemical, and endogenous enzyme assessment of raw milk preserved under hyperbaric storage at variable room temperature. ACS Food Science and Technology, 2(6), 961–974. https://doi.org/10.1021/acsfoodscitech.2c00027
Duarte, R. V., Pinto, C. A., Gomes, A. M., Delgadillo, I., & Saraiva, J. A. (2022b). A microbiological perspective of raw milk preserved at room temperature using hyperbaric storage compared to refrigerated storage. Innovative Food Science & Emerging Technologies, 78, Article 103019. https://doi.org/10.1016/j.ifset.2022.103019
Eisenmenger, M. J., & Reyes-De-Corcuera, J. I. (2009). High pressure enhancement of enzymes: A review. Enzyme and Microbial Technology, 45(5), 331–347. https://doi.org/10.1016/j.enzmictec.2009.08.001
Fidalgo, L. G., Castro, R., Trigo, M., Aubourg, S. P., Delgadillo, I., & Saraiva, J. A. (2019). Quality of fresh Atlantic salmon (Salmo salar) under hyperbaric storage at low temperature by evaluation of microbial and physicochemical quality indicators. Food and Bioprocess Technology, 12(11), 1895–1906. https://doi.org/10.1007/s11947-019-02346-3
Fidalgo, L. G., Delgadillo, I., & Saraiva, J. A. (2020). Autolytic changes involving proteolytic enzymes on Atlantic salmon (Salmo salar) preserved by hyperbaric storage. LWT - Food Science and Technology, 118, Article 108755. https://doi.org/10.1016/j.lwt.2019.108755
Fidalgo, L. G., Santos, M. D., Queirós, R. P., Inácio, R. S., Mota, M. J., Lopes, R. P., Gonçalves, M. S., Neto, R. F., & Saraiva, J. A. (2014). Hyperbaric storage at and above room temperature of a highly perishable food. Food and Bioprocess Technology, 7(7), 2028–2037. https://doi.org/10.1007/s11947-013-1201-x
Gonzalez, M. E., & Barrett, D. M. (2010). Thermal, high pressure, and electric field processing effects on plant cell membrane integrity and relevance to fruit and vegetable quality. Journal of Food Science, 75(7), R121–R130. https://doi.org/10.1111/j.1750-3841.2010.01763.x
Guerrero-Beltrán, J. A., Barbosa-Cánovas, G. V., & Swanson, B. G. (2005). High hydrostatic pressure processing of fruit and vegetable products. Food Reviews International, 21(4), 411–425. https://doi.org/10.1080/87559120500224827
Helm, V. J., & Miiller, B. W. (1991). Stability of the synthetic pentapeptide thymopentin in aqueous solution: Effect of pH and buffer on degradation. International Journal of Pharmaceutics, 70(1–2), 29–34. https://doi.org/10.1016/0378-5173(91)90160-P
Henley, J. P., & Sadana, A. (1985). Categorization of enzyme deactivations using a series-type mechanism. Enzyme and Microbial Technology, 7(2), 50–60. https://doi.org/10.1016/0141-0229(85)90013-4
Illera, A. E., Chaple, S., Sanz, M. T., Ng, S., Lu, P., Jones, J., Carey, E., & Bourke, P. (2019). Effect of cold plasma on polyphenol oxidase inactivation in cloudy apple juice and on the quality parameters of the juice during storage. Food Chemistry: X, 3, Article 100049. https://doi.org/10.1016/j.fochx.2019.100049
Janovitz-Klapp, A. H., Richard, F. C., Groupy, P. M., & Nicolas, J. J. (1990). Inhibition studies on apple polyphenol oxidase. Journal of Agricultural and Food Chemistry, 38(4), 926–931. https://doi.org/10.1021/jf00094a002
Lemos, Á. T., Casal, S., Barba, F. J., Phimolsiripol, Y., Delgadillo, I., & Saraiva, J. A. (2022). Preservation of high pressure pasteurised milk by hyperbaric storage at room temperature versus refrigeration on inoculated microorganisms, fatty acids, volatile compounds and lipid oxidation. Food Chemistry, 387, Article 132887. https://doi.org/10.1016/j.foodchem.2022.132887
Lemos, Á. T., Ribeiro, A. C., Fidalgo, L. G., Delgadillo, I., & Saraiva, J. A. (2017). Extension of raw watermelon juice shelf-life up to 58 days by hyperbaric storage. Food Chemistry, 231, 61–69. https://doi.org/10.1016/j.foodchem.2017.03.110
Liu, H. S., & Cheng, Y. C. (2000). Stability enhancement of α-amylase by supercritical carbon dioxide pretreatment. Progress in Biotechnology, 16, 149–154. https://doi.org/10.1016/S0921-0423(00)80028-3
Manzocco, L., Ignat, A., Valoppi, F., Burrafato, K. R., Lippe, G., Spilimbergo, S., & Nicoli, M. C. (2016). Inactivation of mushroom polyphenoloxidase in model systems exposed to high-pressure carbon dioxide. Journal of Supercritical Fluids, 107, 669–675. https://doi.org/10.1016/j.supflu.2015.07.029
Manzocco, L., Panozzo, A., & Nicoli, M. C. (2013). Inactivation of polyphenoloxidase by pulsed light. Journal of Food Science, 78(8), E1183–E1187. https://doi.org/10.1111/1750-3841.12216
Manzocco, L., Quarta, B., & Dri, A. (2009). Polyphenoloxidase inactivation by light exposure in model systems and apple derivatives. Innovative Food Science and Emerging Technologies, 10(4), 506–511. https://doi.org/10.1016/j.ifset.2009.02.004
Minton, A. P. (2005). Models for excluded volume interaction between an unfolded protein and rigid macromolecular cosolutes: Macromolecular crowding and protein stability revisited. Biophysical Journal, 88(2), 971–985. https://doi.org/10.1529/biophysj.104.050351
Mozhaev, V. V., Lange, R., Kudryashova, E. V., & Balny, C. (1996). Application of high hydrostatic pressure for increasing activity and stability of enzymes. Biotechnology and Bioengineering, 52(2), 320–331. https://doi.org/10.1002/(SICI)1097-0290(19961020)52:2%3c320::AID-BIT12%3e3.0.CO;2-N
Otero, L. (2019). Hyperbaric storage at room temperature for fruit juice preservation. Beverages, 5(3), 49. https://doi.org/10.3390/beverages5030049
Otero, L., & Pérez-Mateos, M. (2021). Hyperbaric storage of Atlantic razor clams: Effect of the storage conditions. Food and Bioprocess Technology, 14, 530–541. https://doi.org/10.1007/s11947-021-02596-0
Pinto, C. A., Martins, A. P., Santos, M. D., Fidalgo, L. G., Delgadillo, I., & Saraiva, J. A. (2019). Growth inhibition and inactivation of Alicyclobacillus acidoterrestris endospores in apple juice by hyperbaric storage at ambient temperature. Innovative Food Science and Emerging Technologies, 52, 232–236. https://doi.org/10.1016/j.ifset.2019.01.007
Pinto, C. A., Moreira, S. A., Fidalgo, L. G., Santos, M. D., Vidal, M., Delgadillo, I., & Saraiva, J. A. (2017). Impact of different hyperbaric storage conditions on microbial, physicochemical and enzymatic parameters of watermelon juice. Food Research International, 99, 123–132. https://doi.org/10.1016/j.foodres.2017.05.010
Pinto, C. A., Santos, M. D., Fidalgo, L. G., Delgadillo, I., & Saraiva, J. A. (2018). Enhanced control of Bacillus subtilis endospores development by hyperbaric storage at variable/uncontrolled room temperature compared to refrigeration. Food Microbiology, 74, 125–131. https://doi.org/10.1016/j.fm.2018.03.010
Richard-Forget, F., Goupy, P., & Nicolas, J. (1994). New approaches for separating and purifying apple polyphenol oxidase isoenzymes: Hydrophobic, metal chelate and affinity chromatography. Journal of Chromatography A, 667(1–2), 141–153. https://doi.org/10.1016/0021-9673(94)89062-5
Rosenthal, A., Ledward, D., Defaye, A., Gilmour, S., & Trinca, L. (2002). Effect of pressure, temperature, time and storage on peroxidase and polyphenol oxidase from pineapple. Progress in Biotechnology, 19, 525–532. https://doi.org/10.1016/S0921-0423(02)80148-4
Sadana, A. (1988). Enzyme deactivation. Biotechnology Advances, 6(3), 349–446. https://doi.org/10.1016/0734-9750(88)91890-3
Saini, R. K., Nile, S. H., & Park, S. W. (2015). Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Research International, 76, 735–750. https://doi.org/10.1016/j.foodres.2015.07.047
Santos, M. D., Delgadillo, I., & Saraiva, J. A. (2020). Extended preservation of raw beef and pork meat by hyperbaric storage at room temperature. International Journal of Food Science and Technology, 55(3), 1171–1179. https://doi.org/10.1111/ijfs.14540
Santos, M. D., Fidalgo, L. G., Pinto, C. A., Duarte, R. V., Lemos, Á. T., Delgadillo, I., & Saraiva, J. A. (2021a). Hyperbaric storage at room like temperatures as a possible alternative to refrigeration: Evolution and recent advances. Critical Reviews in Food Science and Nutrition, 61(12), 2078–2089. https://doi.org/10.1080/10408398.2020.1770687
Santos, M. D., Matos, G., Pinto, C. A., Carta, A. S., Lopes-da-Silva, J. A., Delgadillo, I., & Saraiva, J. A. (2021b). Hyperbaric storage effect on enzyme activity and texture characteristics of raw meat. Food Engineering Reviews, 13, 642–650. https://doi.org/10.1007/s12393-020-09261-w
Segovia-Bravo, K. A., Guignon, B., Bermejo-Prada, A., Sanz, P. D., & Otero, L. (2012). Hyperbaric storage at room temperature for food preservation: A study in strawberry juice. Innovative Food Science and Emerging Technologies, 15, 14–22. https://doi.org/10.1016/j.ifset.2012.02.005
Siguemoto, É. S., Pereira, L. J., & Gut, J. A. W. (2018). Inactivation kinetics of pectin methylesterase, polyphenol oxidase, and peroxidase in cloudy apple juice under microwave and conventional heating to evaluate non-thermal microwave effects. Food and Bioprocess Technology, 11(7), 1359–1369. https://doi.org/10.1007/s11947-018-2109-2
Steele, B. T., Murphy, N., Arbus, G. S., & Rance, C. P. (1982). An outbreak of hemolytic uremic syndrome associated with ingestion of fresh apple juice. Journal of Pediatrics, 101(6), 963–965. https://doi.org/10.1016/S0022-3476(82)80021-8
Vamos-Vigyázó, L., & Haard, N. F. (1981). Polyphenol oxidase and peroxidase in fruits and vegetables. Critical Reviews in Food Science and Nutrition, 15(1), 49–127. https://doi.org/10.1080/10408398109527312
van den Berg, B., Ellis, R. J., & Dobson, C. M. (1999). Effects of macromolecular crowding on protein folding and aggregation. The European Molecular Biology Organization Journal, 18(24), 6927–2933. https://doi.org/10.1093/emboj/18.24.6927
Wang, S. Y., Jiao, H. J., & Faust, M. (1991). Changes in the activities of catalase, peroxidase, and polyphenol oxidase in apple buds during bud break induced by thidiazuron. Journal of Plant Growth Regulation, 10, Article 33. https://doi.org/10.1007/BF02279308
Weemaes, C., Ludikhuyze, L., van den Broeck, I., & Hendrickx, M. (1998). High pressure inactivation of polyphenoloxidases. Journal of Food Science, 63(5), 873–877. https://doi.org/10.1111/j.1365-2621.1998.tb17917.x
Yoruk, R., & Marshall, M. R. (2003). Physicochemical properties and function of plant polyphenol oxidase: A review. Journal of Food Biochemistry, 27(5), 361–422. https://doi.org/10.1111/j.1745-4514.2003.tb00289.x
Zaks, A., & Russell, A. J. (1988). Enzymes in organic solvents: Properties and applications. Journal of Biotechnology, 8(4), 259–269. https://doi.org/10.1016/0168-1656(88)90018-1
Zawawi, N. A. F., Hazmi, N. A. M., How, M. S., Kantono, K., Silva, F. V. M., & Sulaiman, A. (2022). Thermal, high pressure, and ultrasound inactivation of various fruit cultivars’ polyphenol oxidase: Kinetic inactivation models and estimation of treatment energy requirement. Applied Sciences, 12(4), 1–19. https://doi.org/10.3390/app12041864
Acknowledgements
The authors would like to thank Dr. Michela Maifreni and Mr. Marco Zanatta, who carried out microbiological analyses and enzymatic activity measurements, respectively.
Funding
Open access funding provided by Università degli Studi di Udine within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
Lara Manzocco: Conceptualization, supervision, writing—review and editing, and funding acquisition. Federico Basso: Investigation, formal analysis, data curation, visualization, and writing—original draft. Maria Cristina Nicoli: Supervision, writing—review and editing, and funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Manzocco, L., Basso, F. & Nicoli, M.C. Effect of Hyperbaric Storage at Room Temperature on the Activity of Polyphenoloxidase in Model Systems and Fresh Apple Juice. Food Bioprocess Technol 16, 2247–2256 (2023). https://doi.org/10.1007/s11947-023-03025-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-023-03025-0