Abstract
The presence of low molecular weight surfactants is suspected as one of the causes of poorly foaming milk, as they can interfere with milk proteins in the formation and stabilization of foam. Here, we explore the effect of various surfactants on the foaming properties of reconstituted skim milk powders. Each surfactant is different in electrical charge and molecular weight, including cleaning O- and E-coded chemicals, Tween 80, sucrose stearate, sodium oleate, sodium dodecyl sulfate, cetyltrimethylammonium bromide, benzalkonium chloride, and lecithin. The results showed that surfactants had different effects on foamability, foam stability, and structure, due to their varied effects on milk properties (e.g., pH, zeta potential, and surface tension). E-coded chemicals and sucrose stearate markedly decreased milk foamability, while the impact of Tween 80 and lecithin was considered detrimental to foam stability, as they mostly induced the production of large air bubbles in the foam.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Foam is a colloidal system that is created by trapping millions of gas bubbles in a liquid or solid. Liquid foam is promoted by the dispersion of the hydrophobic phase (gas/air) in hydrophilic liquid (Bhandari & Roos, 2012). Milk foam is most commonly seen as a key component of cappuccino-style and latte-style beverages in coffee shops. Satisfactory foamability and foam stability of milk are quality attributes desired by all baristas to serve drinks to consumers. The foam layer of cappuccino-style beverages is required to be stable over a typical consumption time of 10–15 min.
Milk foam is produced as a result of the presence of surfactant molecules. Milk contains two groups of surfactants, high molecular weight surfactants (e.g., proteins) and low molecular weight (LMW) surfactants (e.g., monoglycerides and diglycerides, phospholipids, and free fatty acids), both of which can stabilize milk foam. Milk foam is stabilized by protein molecules due to their intermolecular interaction ability to form a highly viscoelastic film on the interface. On the other hand, LMW surfactants stabilize foam through the “Gibbs-Marangoni effect” in which they restore the thickness and equilibrium surface tension of thinning films, against destabilization of air bubbles, by their migration to the thinnest region of the film. The foaming properties of the system containing both proteins and LMW surfactants are inferior to those containing only one of the two due to the mutually incompatible interaction between proteins and LMW surfactants at the interface (Ho et al., 2022; Huppertz, 2010).
The displacement of proteins from the interface by LMW surfactants depends on the concentration of LMW surfactants in which a progressive displacement occurs as the concentration of LMW surfactants increases (Ho et al., 2022). The negative effects of LMW surfactants, especially free fatty acids and fat on the foaming properties of milk, are well reported. Kamath et al. (2008a) showed that milk with free fatty acid content of as much as 1.0 µ.equiv/mL resulted in smooth and stable foam. However, as free fatty acid content was increased to 1.5–5.0 µ.equiv/mL, the creamy foam was produced during the steaming process and foam became coarse as soon as the steam frothing was ceased. The coarseness of foam became greater as the amount of free fatty acid increased. Investigation into the effects of different fat contents (0.5, 0.9, 1.5, 2.6, and 3.5%, w/w) on foaming properties of milk by Munchow et al. (2015) found that foam obtained from milk with a higher amount of fat was less stable and consistent than that from milk with a lower fat content.
LMW surfactants not only are natural constituents of milk, but also originate from residual cleaning agents or sanitizers used in milk processing. In some cases, surfactants can be added to improve the foaming properties of milk. Surfactants are classified based on the charge of their hydrophilic head groups. The polar head groups can be nonionic, anionic, cationic, and zwitterionic. A nonionic surfactant has no charge groups on its polar head, while an ionic surfactant carries a net charge either positive (cationic surfactant) or negative (anionic surfactant). Zwitterionic surfactant carries both positive and negative charges on its hydrophilic heads (Rosen & Kunjappu, 2012). Some researchers have investigated the foaming properties of protein-surfactant systems, but have been limited in the types of surfactants used in their studies (Coke et al., 1990; Rouimi et al., 2005; Wei & Liu, 2000; Wilde et al., 1997). Dickinson (1997) reported that water-soluble and nonionic surfactants were superior for protein displacement compared to oil-soluble and ionic surfactants. Additionally, the molecular weight of surfactants might also affect milk foaming properties. It was mentioned that the addition of butyric acid (C4:0) resulted in better foaming properties of milk. Adversely, an addition of caprylic acid (C8:0) diminished the foaming properties to a small extent, whereas sodium salt of stearic acid (C18:0) greatly reduced the foaming properties (Huppertz, 2010). Dandigunta et al. (2021) reported that anionic (i.e., sodium dodecyl sulfate) and cationic (i.e., cetyltrimethylammonium bromide) surfactants at concentrations of 0.1–1.5 g/mL improved the foaming properties of milk while nonionic surfactants (i.e., Tween 80) at the same concentration had a minimal or neutral effect. Deotale et al. (2021) found that the addition of sucrose stearate and Tween 80 at their critical micelle concentrations (0.1 g/L for sucrose stearate and 0.2 g/L for Tween 80) to the instant soluble coffee powder solution (4%, w/v) significantly improved its foamability and foam stability. It is noticed that different foaming methods and conditions were employed in these studies, which possibly makes the comparison and conclusion about the roles of different surfactants on milk foaming to be inconclusive.
As the type and molecular weight of surfactants are considered important factors causing protein displacement from the interface, it is essential to perform studies using all types of surfactants (e.g., nonionic, anionic, cationic, and zwitterionic) with varied molecular weight concerning the foamability and foam stability of milk. The knowledge gained from this study provides a better understanding of how the different types of surfactants affect the foaming properties of milk. This could reveal possible causes of poor foamability and foam stability of milk. In this study, we investigated the effect of various surfactants, which are different in electrical charges and molecular weights (e.g., cleaning O- and E-coded chemicals which are Oxysan ZS and Envirosan sanitizers, respectively, Tween 80, sucrose stearate, sodium oleate, sodium dodecyl sulfate, cetyltrimethylammonium bromide, benzalkonium chloride and lecithin), on foaming properties of reconstituted skim milk powder (SMP). Unlike most previous studies which employed the air injection foaming method to evaluate the effects of surfactants on milk foaming, we employed the steam injection which is the most widely used foaming approach in coffee shops to generate the foam. Therefore, our study will be a complementary document to the reported studies, providing the readers with many fundamental aspects of milk foaming.
Materials and Methods
Materials
Commercial low heat (72 °C for 20 s) SMP was obtained from Tatura Milk Industries Limited (Victoria, Australia). According to the manufacturer, SMP contained 48.5% lactose, 36.5% protein, 3.5% moisture, 0.5% fat, and 8.8% (w/w) minerals. Cleaning O-coded and E-coded chemicals (which are Oxysan ZS and Envirosan—food contact surface sanitizers used in dairy processing factories) were supplied by a local laboratory (New South Wales, Australia). Oxysan ZS is a mixture of acetic acid, peroxyacetic acid, hydrogen peroxide, and octanoic acid while Envirosan consists of dodecylbenzene sulfonic acid and lactic acid. Tween 80 and cetyltrimethylammonium bromide were obtained from Sigma-Aldrich (New South Wales, Australia). Sucrose stearate and benzalkonium chloride were purchased from Alfa Aesar (Victoria, Australia). Sodium oleate was purchased from Tokyo Chemical Industry (Tokyo, Japan). Sodium dodecyl sulfate was obtained from BDH Chemicals (Pennsylvania, USA). Ultralec® deoiled lecithin was obtained from Archer Daniels Midland (South Australia, Australia). Milli-Q water and deionized water were used to prepare all solutions.
Preparation of Skim Milk Solutions with Added Surfactants
SMP was reconstituted in Milli-Q water at 8.5% total solids (w/w) (Kamath, 2007), which enabled the protein content in SMP solution to be similar to that in raw milk (i.e., 3.1% w/w). Different surfactants were added at the following concentrations to skim milk solutions while stirring, including nonionic surfactants (Tween 80, 0.1%; and sucrose stearate, 0.1%), anionic surfactants (sodium oleate, 0.2%; and sodium dodecyl sulfate, 0.05%), cationic surfactants (benzalkonium chloride, 0.01%; and cetyltrimethylammonium bromide, 0.04%), zwitterionic surfactant (lecithin, 0.2%), and cleaning surfactants (O-coded chemical, 0.02% and 0.1%; and E-coded chemical, 0.05% and 0.5%). All surfactants used in this study represented those potentially used in the milk supply chain either as typically added food-grade components (i.e., Tween 80, sucrose stearate, and lecithin) or as surface cleaning agents (i.e., sodium dodecyl sulfate, sodium oleate, benzalkonium chloride) which can potentially remain in the processed milk. The concentrations of O- and E-coded chemicals were selected based on the estimated residual amounts of each chemical during surface cleaning/sanitization and were suggested by a local milk processing factory. For other types of surfactants (i.e., Tween 80, sucrose stearate, sodium dodecyl sulfate, cetyltrimethylammonium bromide, sodium oleate, benzalkonium chloride, and lecithin), their concentrations were chosen based on the preliminary testing on the foamability and foam stability at various concentrations, and literature (Dandigunta et al., 2021; Deotale et al, 2021). The selected concentrations showed the highest foamability and foam stability. After surfactants were added, all solutions were stirred at 1000 rpm for 10 min using an overhead stirrer (IKA RW20 Digital, Missouri, USA). All solutions were stored overnight in a cold room at 4 °C before use. The classification of surfactants and their concentrations used in this study are shown in Table 1.
Determination of Milk Properties
pH Measurement
The pH of milk samples at 25 °C was measured by a pH meter (TPS pH Cube, Queensland, Australia). The pH probe was calibrated at 25 °C before measurement using phosphate buffer at pH 6.8 and phthalate buffer at pH 4.0.
Particle Size Measurement
Particle size and its distribution of milk samples were determined using an integrated light scattering analyzer (Malvern Mastersizer 2000, Malvern Instruments Ltd, Worcestershire, UK). A few drops of each solution were added into the water-contained beaker while stirring at 2000 rpm to reach an obscuration level of 10%. For particle size calculation, the refractive indexes of milk protein and water were selected at 1.41 and 1.33, respectively, while the absorption index of milk proteins was at 0.01.
Zeta Potential
The determination of the electrical charge (zeta potential) of milk samples was carried out using the Nano Series ZS Zetasizer (Malvern Instruments Ltd, Worcestershire, UK). Milk samples were placed inside a disposable polycarbonate cuvette (ATA scientific, DTS1061). All samples were diluted 100 times with Milli-Q water before use to avoid multiple scattering effects.
Surface Tension
The Wilhelmy plate method using a NIMA ST9000 tensiometer (Nima technology, Coventry, UK) and a platinum Wilhelmy plate (10.25 × 0.16 mm) supplied by Nima technology were used to measure the surface tension of milk samples. The platinum plate was rinsed with absolute ethanol and was flamed before each measurement. The potentiometer was calibrated using Milli-Q water at 25 °C in which a surface tension reading of 72.8 ± 0.5 mN/m was considered accurately standardized. The cold milk samples (e.g., 5 °C which is the initial temperature of milk used for foaming) were poured into a clean container where the platinum plate was immersed in the liquid and slowly lifted. The surface tension of the samples was a maximum force acting on the plate as it was raised out of the samples.
Foaming Methods
Foaming of milk by steam injection was carried out using a commercial coffee machine (Sunbeam Espresso Machine EM6910®, Australia) following the procedure published by Ho et al. (2019). The espresso machine was turned on about 30 min before the experiment. Approximately 100 mL of milk sample was transferred into a 250-mL graduated jug (Genetics Australia Cooperative Ltd., Australia). The milk sample was then chilled to 5 °C using an ice bath. The steam wand was flushed several times to dispose of any condensed water before the foaming experiment. The jug was placed on an adjustable stage that could be steadily moved up and down. The tip of the steam wand was positioned at an angle of 45° relative to the milk surface and 2 mm below the milk surface. During foaming, due to a rapid change of foam-liquid interface, the jug was moved accordingly using the adjustable stage to ensure a proper contact point between the milk surface and the tip of the steam wand. The speed of movement was based on the hissing sound which resulted from the milk stretching by gently letting air be incorporated into the milk. The temperature was recorded using a Testo 720 thermometer (Testo, Victoria, Australia) during steaming. After the milk temperature reached 45 °C, the tip of the steam wand was lowered deeper below the milk surface to stop letting air into the milk and to texturize the foam. The steaming process was terminated when the temperature reached 65 °C.
Determination of Foam Properties
Foamability and Foam Stability
The total volume of foam and liquid phase (VT, mL) and the volume of liquid phase (VL, mL) obtained immediately after foaming were documented to calculate the volume of foam (VF, mL). The foamability was expressed as the initial volume of foam and calculated using Eq. (1):
Foam stability was determined by measuring the percentage of foam volume reduction after 10 min of foam destabilization. All foam bubbles were allowed to collapse at room temperature (25 °C). Ten minutes was selected for the foam stability study by considering the average time to consume the foam-based beverages after they are served by baristas at the coffee shops. Foam stability was calculated using Eq. (2) with VF10 representing foam volume (mL) measured after 10 min of the destabilization process.
Foam Structure
Images of the foam surface at 0 and 10 min after foaming were captured using a light microscope (Prism Optical, Eagle Farm, Queensland, Australia) integrated with a 5.0 MP CCD camera and TSView 7 software (Fuzhou Tucsen Image Technology Co., Ltd., China). For a better image of the foam, a light source unit (Olympus LG-PS2 lamp) was used to illuminate the foam. The captured images were analyzed using ImageJ 1.49 (NIH, Maryland, USA) to measure the size of air bubbles. Because the images of air bubbles in the sub-surface layer showed through air spaces in the surface layer, the measurement of each air bubble diameter could not be done automatically, but manually using the ImageJ software. The longest diameter of air bubbles was measured considering the nonspherical shape of most bubbles. For each foaming condition, the diameter of at least 1500 air bubbles chosen from three foam images was determined. The size distribution of the measured air bubbles was summarized in terms of the smoothed distributions (histogram with fit and group function) of the log-diameter of air bubbles using Minitab 17® software (Minitab Inc., USA) (Ho et al. 2021; Kamath et al., 2008a, b).
Statistical Analysis
The experiments were performed following a completely randomized design with three replications and three measurements performed for each replicate. A statistical software Minitab 17® (Minitab Inc., USA) was used for the statistical analysis. The data were analyzed using one-way ANOVA with Tukey’s method and Dunnett’s method for multiple comparisons. The significant difference in the analysis was set at 5% (p < 0.05).
Results and Discussion
Properties of Milk Samples Added with Various Surfactants
pH
The pH of milk samples added with various surfactants was in a range of 5.98–6.80 (Table 2). As compared to pH of the control samples, the addition of O-coded chemicals (Oxy 1 and 2) and E-coded chemicals (Envi 1 and 2) resulted in a marked decrease in pH values. The presence of acid substances, such as acetic acid and peroxyacetic acid in O-coded chemicals and lactic acid in E-code chemical, was responsible for the reduced pH values of milk samples added with these surfactants. However, due to the alkaline properties of sodium oleate (SO), sodium dodecyl sulfate (SDS), and cetyltrimethylammonium bromide (CTAB), an addition of these into milk samples led to a significant increase in pH values. No physical changes were observed for all milk samples as their pH values were still far higher than the isoelectric pH of proteins (~ pH = 4.6). Several studies report an effect of pH on the foaming properties of milk. Foamability and foam stability of skim milk were less affected by pH ranging from 6.0 to 7.0 (Augustin & Clarke, 2008; Ward et al., 1997). There was little effect on the size of air bubbles in skim milk foam over a pH range of 6.4–7.0 (Borcherding et al., 2009). In addition, Huppertz (2010) mentioned that the volume of foam produced from the reconstituted skim milk at pH 6.2, 6.5, 6.7, and 7.0 was virtually similar. However, when the pH of skim milk was reduced to 4.5–5.0, poor foaming characteristics could be obtained due to the precipitation of caseins (Zhang et al., 2004).
Zeta Potential
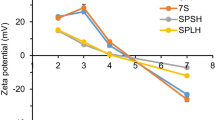
As shown in Table 2, the zeta potential of control milk samples was highly negative with a value of − 24.73 mV and was not significantly altered upon adding surfactants with two possible exceptions: Oxy 2 and SO. While a less negative zeta potential was observed for the milk samples containing Oxy 2 (− 22.43 mV), the SO addition made the zeta potential to be more negative (− 28.07 mV). The effects of the added surfactants on zeta potential could be explained by changes in pH. A reduction in absolute zeta potential values was observed when the pH decreased (Table 2). Another possible reason is the concentrations of added surfactants, and the threshold concentration inducing an alteration of zeta potential is dependent on the types of added surfactants. The addition of anionic surfactants such as SO and SDS to milk samples is expected to increase the negative zeta potential of milk, but only SO did. This could be because SO (0.2%) was added at a higher concentration than SDS (0.05%) (Table 2). Similar results were observed for Oxy surfactant which only reduced the absolute zeta potential of milk at high concentration (i.e., 0.1%). Mellema et al. (2009) also reported that the zeta potential of UHT milk samples added with 0.2% of various surfactants (i.e., unsaturated monoglycerides, diacetyl tartaric esters of monoglycerides, citric acid esters of monoglycerides, and sodium stearoyl lactylates) was not significantly different while the samples added with saturated monoglycerides at the same concentration had more negative zeta potential value.
The determination of zeta potential is a common practice to assess the stability of colloidal dispersions (Duan et al., 2011; Morrison & Ross, 2002). Particles in a suspension with high absolute zeta potential generally result in a highly stable system due to strong repulsion between particles. Zeta potential values from about − 16 to − 30 mV are generally considered a threshold of either coagulation of dispersion, respectively (Tiller et al., 2009). Zeta potential is dependent on not only pH, but also surfactant, particle size and shape, ionic strength, and surface electrical conductivity (Xiaodi & Shuntang, 2016). Wang et al. (2005) observed the effects of different types of surfactants on the zeta potential of SiO2 colloids with a negative charge (− 54 mV). They found that the anionic surfactant (SDS) increased the magnitude of the negative zeta potential of SiO2 particles. In contrast, cationic surfactant (CTAB) decreased the zeta potential, while nonionic surfactant (polyoxyethylene(4) dodecyl ether) exhibited little effect on changes in the zeta potential of SiO2.
Particle Size
The particle size parameters of milk samples are shown in Table 2. Milk samples added with various surfactants in this study had a similar mean particle size diameter which was 0.158–0.168 µm and 0.127–0.138 µm for D[4,3] and D[3,2], respectively. The similar particle size among all samples indicated that all surfactants were completely dissolved in skim milk solutions and did not destabilize proteins in milk. The particle size distribution of skim milk samples derived from the light scattering data was made up primarily of the casein micelles (Figure S1, supplementary material), which were also reported to be about 0.02–0.6 µm in diameter (Kamigaki et al., 2018).
Surface Tension
As illustrated in Table 2, milk samples added with various surfactants had surface tension ranging from 42.69 to 56.14 mN/m. The average surface tension of the control sample at 5 °C was 53.36 mN/m, which is slightly higher than that reported by McCarthy (2011), with a value of 52.0 mN/m at 20 °C. This difference could be due to the different measurement temperatures. The surface tension of milk decreases with increased measurement temperatures (Sherbon, 1988). Most surfactants in the present study significantly depressed the surface tension of skim milk (p < 0.05), except for O-coded chemicals 0.02% and 0.1%, sucrose stearate, and lecithin. There was an insignificant difference in surface tension (p > 0.05) between control and milk samples added with O-coded chemicals 0.02% and 0.1%, sucrose stearate, and lecithin. The experimental results demonstrated that the addition of LMW surfactants into milk solutions did not always reduce surface tension.
Foaming Properties of Milk Samples Added with Various Surfactants
Foamability and Foam Stability
The foamability of milk samples with various surfactants added is shown in Fig. 1a. Except for the E-coded chemical and sucrose stearate, which decreased the foamability considerably, other surfactants did not markedly affect the foamability. Wei and Liu (2000) showed that foamability and foam stability of protein solution (bovine serum albumin) were reduced as the bulk concentration of Triton X‐100 (a nonionic surfactant) increased over a range of 0–0.25 mM. Stancil (2013) also revealed that the addition of a clean-in-place (CIP) cleaning solution containing a blend of surfactants (oxirane, methyl-, polymer with oxirane) at 1.0% and 5.0% significantly reduced the foaming capacity of milk.
Foamability (a) and foam stability (b) of skim milk added with different groups of surfactants. Refer to Table 1 for sample codes
For foam stability, the higher percentage of foam volume reduction after 10 min (%VF reduction) indicated lower foam stability. As shown in Fig. 1b, as compared to the control sample, only three surfactants—benzalkonium chloride, Tween 80, and lecithin—exhibited a significant reduction of foam stability of milk samples (p < 0.05). The milk samples with added benzalkonium chloride produced the least stable foam with a %VF reduction of more than 80%, followed by milk samples with added Tween 80 and lecithin with a %VF reduction of about 50%. The foam produced from milk samples added with other surfactants was more stable and similar to the control one, with the %VF reduction in a range of 28–35%. In this study, 0.1% (w/w) of Tween 80 was used, approximately 100 times higher than its critical micelle concentration (CMC) in pure water (0.0013–0.0015%, w/v) as reported by Harris and Angal (1990). CMC is defined as the concentration of surfactant at which surfactant molecules form aggregates called micelles. It should be noticed that the actual CMC of Tween 80 in milk might be different from that in pure water, although the reconstituted skim milk solution was constituted of more than 90% of water. As much as 0.1% (w/w) of Tween 80 was likely to able to interfere with the stabilization of air bubbles by milk protein molecules. Conversely, the presence of benzalkonium chloride in milk significantly decreased the foam stability despite its use below its CMC. The CMC of benzalkonium bromide is 0.17% as reported by Tadros (2014). It should be emphasized that further research is still required to figure out the effect of surfactant concentration below and above CMC on the foaming behavior of mixed polymer-surfactant solutions. Concerning the deoiled lecithin used in this study, there is no information available regarding its CMC. This may be because lecithin contains various phospholipids, and the CMC of phospholipids varies depending on the phospholipid’s head group and acyl chain length (Zhang et al., 2017). Several studies have reported that phospholipids negatively affect the foaming properties of milk. Sodini et al. (2006) and Huppertz (2010) observed very low stability of foam obtained from buttermilk, which has a high content of phospholipids.
Researchers have proposed that the foaming properties of a mixed solution containing both protein and LMW surfactants relied on the ratio of surfactant to protein. At low concentrations of surfactants, they can form complexes with less surface active protein molecules through either hydrophobic or electrostatic interactions, or by the combination of the two forces. Thus, the air/water interface was still dominated by protein molecules (Grigoriev et al., 2007). With increased surfactant concentration, more complexes were formed until they were saturated. At sufficiently high concentrations of surfactants, the interface was dominated by surfactant molecules. As a result, the foam behavior was dictated by the sole surfactant (Coke et al., 1990; Kelley & McClements, 2003; Wilde et al., 1997). These trends are consistent with several reported experiments on a mixture of protein with Tween 20 (Maldonado-Valderrama et al., 2007), Triton X‐100 (Wei & Liu, 2000), and Tween 80 (Grigoriev et al., 2007). Moreover, Wei and Liu (2000) and Lech et al. (2015) showed that the foam stability of a mixed solution of protein and LMW surfactants was lower than that of protein used alone. In addition, Kamath et al. (2008a) also reported that the foam stability of milk decreased as the level of free fatty acids was increased. This tendency is because of the disruption of cohesive interactions between protein molecules as well as the displacement of protein molecules from the interface by LMW surfactants (Coke et al., 1990; Maldonado-Valderrama & Patino, 2010). It was found that the foam stability of a mixed protein-surfactant solution at a high concentration of surfactant is similar to that of surfactant alone, and that foam stabilized by protein is much more stable than that stabilized by LMW surfactant (Bos & Vliet, 2001; Malcolm et al., 2006). In addition, the changes of protein structure induced by surfactants were also important to the foaming properties of protein-surfactant mixtures (Lech et al., 2014).
Milk samples with added Tween 80, benzalkonium chloride, and lecithin exhibited high foamability (similar to the control), but low foam stability. Meanwhile, milk samples added with E-coded chemical and sucrose stearate exhibited very low foamability, but high foam stability. It is widely known that the rate at which surfactants diffuse to the air–liquid interface, which is highly dependent on the properties of the surfactant itself (e.g., size, surface hydrophobicity, and structural flexibility), determines foamability, whereas foam stability is determined by properties of interfacial adsorbed layers. Solutions that possess higher foamability may have lower foam stability and vice versa (Petkova et al. 2012).
When grouping the various surfactants based on the charge of their hydrophilic head groups (Fig. 1), it was less likely that the foamability was determined by the type of surfactant as milk samples added with the surfactants from different groups resulted in a similar volume of foam in comparison to the control. Also, two different surfactants under the same group showed varied effects on foamability. Two nonionic surfactants, Tween 80 and sucrose stearate, with molecular weights of 1310 and 608.77 Da, respectively, had different types of foamability. The foamability of milk samples added with Tween 80 was similar to that of the control, and both resulted in a higher foamability than sucrose stearate. When it comes to anionic surfactants, sodium oleate and sodium dodecyl sulfate have a similar molecular weight (288.38 and 304.44 Da, respectively), and both produced similar foam volumes to the control. A similar trend was also observed for cationic surfactants–cetyltrimethylammonium bromide and benzalkonium chloride. However, milk samples with added lecithin produced less foam than the control. Rouimi et al. (2005) reported that in milk protein–surfactant systems, the nature, structure, and especially concentration of surfactants relative to protein concentration determined the surface elasticity of the protein film, which subsequently impacts the amount of incorporated air—the higher the surface elasticity of the protein film, the higher foamability of the system.
Similar to the foamability results, the stability of foam might not be determined by the charge of the surfactant’s hydrophilic head group. As shown in Fig. 1, different types of surfactants produced foam with similar stability to the control foam. These results are not in agreement with previously reported studies (Alahverdjieva et al., 2008; Kotsmar et al., 2009) which found that different types of surfactants (ionic or nonionic) influenced the adsorption behavior of proteins and surfactants at the air–water interface in different ways. Ionic surfactants are known to interact with protein molecules both electrostatically and hydrophobically, whereas nonionic surfactants can only interact with protein via hydrophobic interactions. However, these researchers did not conduct their experiments on foam stability. Hence, the correlation of the adsorption of protein/surfactant in a mixed solution with the stability of foam relative to different types of surfactant seems to be inconclusive. Bureiko et al. (2015) also mentioned that the foaming properties of protein/surfactant mixtures cannot be solely explained by electrostatic and hydrophobic interactions. Under the same group of surfactants, two different chemicals (Tween 80 and sucrose stearate) distinctly affected foam stability. This is likely to be caused by their large differences in molecular weight. On the other hand, milk samples with added anionic surfactants (sodium dodecyl sulfate and sodium oleate) produced foam with similar stability (p > 0.05). This is probably because of their similarity in molecular weight. This suggests that different molecular weights impact foam stability. Nevertheless, this trend was not observed in cationic surfactants. Milk samples with added cetyltrimethylammonium bromide and benzalkonium chloride, which are similar in molecular weight, had significantly different foam stability. For zwitterionic surfactant, only lecithin was used in this study, and it was detrimental to foam stability. Pugh (2016) mentioned that the molecular structure of the surfactants determined the cohesive bonding and packing of the molecules at the air/liquid interface which might affect the foaming behavior.
Correlation Between Foaming Properties and Properties of Milk Samples
No correlation between either zeta potential or pH and foaming properties was observed in the present study. A previous study found that the foam stability of protein solutions (pH 7) was improved as zeta potential decreased from − 31.7 ± 0.8 to − 50.5 ± 0.8 mV (Wang et al., 2015). However, the foam produced from protein solution (pH 6.2) with a lower zeta potential (− 45.5 ± 0.8 mV) was much more stable. This indicates that zeta potential is not the only parameter determining the stability of foam (Wang et al., 2015). As all milk samples added with various surfactants in this study had a similar particle size distribution, it was expected that their foaming properties were unaffected by particle size. Furthermore, the surface tension was not correlated with the foamability. For instance, the foamability of control and milk samples with added sodium dodecyl sulfate was similar despite their significant difference in surface tension. Meanwhile, control and milk samples with added sucrose stearate differed in foamability, although they shared a similarity in surface tension. These results are in the agreement with previous studies which mentioned that the surface tension value of milk was not indicative of its foamability (Kamath, 2007; Kamath et al., 2008a). The rate constant of surface tension reduction was instead that which closely corresponded with the foamability. Moreover, Rouimi et al. (2005) emphasized the great influence of surface elasticity on foamability rather than considering surface tension as the only criterion in the prediction of foamability.
A similar trend of a weak relationship between foam stability and surface tension was observed in this study. Except for Tween 80 and lecithin, the other surfactants did not significantly affect the foam stability (p > 0.05) despite their differences in surface tension. According to Rouimi et al. (2005), the reduction of surface tension is not a strong predictor for foam stability. Instead, the viscoelastic characteristics and its gas permeability are considered to be the dominant factors greatly affecting the final stability of foam (Dutta et al., 2002).
Foam Structure
Representative microscopic images of the foam surface can be seen in Fig. 2. These images show that the number of air bubbles decreased as destabilization time progressed from 0 to 10 min. All milk samples produced foam with polyhedral-shaped air bubbles, except the foam obtained from milk samples added with benzalkonium chloride which had spherical-shaped air bubbles at t = 10 min. The results at t = 0 indicated that the surfactant addition led to the increased size of air bubbles. As indicated in Figs. 2 and 3, during 10 min of destabilization, except benzalkonium chloride which induced a smaller size of air bubbles, the other surfactants promoted the growth of air bubble size. As mentioned in the “Foamability and Foam Stability” section, the foam of milk samples added with benzalkonium chloride was very unstable (Fig. 1b). Immediately after being generated, air bubbles of these samples started to coalesce and collapse rapidly, which has evident from the pronounced areas of liquid observed on the foam surface of the BAC sample at t = 10 min (Fig. 2). For this sample, more than 80% of foam including mainly large air bubbles had collapsed after 10 min of destabilization, which could explain for smaller air bubble size in the foam of this sample at t = 10 min. Similarly, Kamath et al. (2008a) reported that the combined effects of coalescence/rupture of large air bubbles and decrease in the size of smaller bubbles as a result of disproportionation (where large air bubbles grow at the expense of smaller air bubbles) were the reasons for the preponderance of smaller air bubbles at the half-life of the foam and the shift in size distribution towards the smaller size for whole milk foam.
Images of the surface of foam produced by milk samples added with various surfactants at 0 and 10 min of the destabilization process. Scale bar = 1000 µm. Refer to Table 1 for sample codes
Distribution of air bubble size of foam produced by skim milk added with various surfactants. A—foam at t = 0 min; B—foam at t = 10 min. Refer to Table 1 for sample codes. The distribution was generated via the smoothed histogram with fit and group function of the log diameter of air bubbles using Minitab 17® software (Minitab Inc., USA). For each foam condition, the longest diameter of at least 1500 air bubbles from three foam images was determined and used for plotting the size distribution
For the milk samples added with the other surfactants, the size distribution curve of air bubbles became broader and shifted to a larger size compared to those of the corresponding fresh foams (Fig. 3), indicating the slow coalescence of air bubbles during the foam destabilization. The trend of the increasing size of air bubbles during the storage of foam was also observed by Ho et al. (2019) and Xiong et al. (2020). The foam produced from milk samples added with Tween 80 and lecithin exhibited the largest air bubbles at both t = 0 min and t = 10 min. It is noticed that the data in Fig. 3 were the air bubbles size constructed via the smoothed histogram distribution of the log-diameter of air bubbles. The accurate quantification of air bubble size is very difficult because the images of the sub-surface bubble layer showed through the air spaces in the bubbles of the surface layer, which makes distinguishing between the individual bubbles impossible. In addition, the data are represented for air bubbles only on the foam surface layer, not the entire column of the foam, and as a result, the differentiation among different types of air bubble instabilities (i.e., disproportionation, coalescence, or drainage) is very difficult. However, the changes in air bubble size distribution curves of foam over time provide insight into the stability of foam and foam appearance. The most attractive foam to consumers should have a small size of air bubbles and narrow size distribution, which is especially preferred for cappuccino-style beverages. In this study, the broadest distribution of air bubble size was observed for milk samples added with lecithin and cetyltrimethylammonium bromide while other samples had similar size distribution. The size of air bubbles in foam was found to be correlated with foam stability (with Pearson correlation coefficient between air bubble size at t = 0 and %VF reduction, r = 0.7). The larger air bubbles provide lower foam stability (e.g., benzalkonium chloride, Tween 80, and lecithin).
It is noticed that the size of air bubbles is well correlated to their total surface area—the smaller size of air bubbles is the larger the total surface area of foam. In film thinning where two or more air bubbles approach closely together, the liquid films separating them are very thin, but the air bubbles do not actually touch each other, and there is no change in total surface area. In coalescence where two or more air bubbles fuse to form a single larger air bubble, the thin liquid films rupture and the total surface area reduces (Schramm & Wassmuth, 1994). Therefore, foam stability can be evaluated based on the total surface area of foam, but its determination is very challenging due to the nonspherical shape and highly overlapping of air bubbles in foam.
Conclusions
We investigated the effects of different types of surfactants on the foaming properties of reconstituted skim milk solutions. All milk samples with added surfactants showed similarity in particle size in terms of D[4,3] and D[3,2], but exhibited differences in pH values, zeta potential, and surface tension. The surface tension had a weak correlation with the foamability and foam stability of milk. The presence of various surfactants had different effects on the foaming characteristics of milk. E-coded chemical and sucrose stearate in this study were found to decrease the foamability while benzalkonium chloride, Tween 80, and lecithin were detrimental to foam stability. Moreover, it was less likely that the foamability and foam stability were determined by the charge of a surfactant’s hydrophilic head group. Instead, the molecular structure of the surfactants is possibly the factor determining the effect on the foaming properties of skim milk.
Data Availability
Research data are not shared.
References
Alahverdjieva, V. S., Grigoriev, D. O., Miller, V. B., Mbhwald, E. V., Fainerman, R., & Aksenenko, H. (2008). Competitive adsorption from mixed hen egg-white lysozyme/surfactant solutions at the air-water interface studied by tensiometry, ellipsometry, and surface dilational rheology. Journal of Physical Chemistry B, 112(7), 2136–2143.
Augustin, M. A., & Clarke, P. T. (2008). Skim milk powders with enhanced foaming and steam-frothing properties. Dairy Science and Technology, 88(1), 149–161.
Bhandari, B., & Roos, Y. H. (2012). Food materials science and engineering: An overview. In B. Bhandari & Y. H. Roos (Eds.), Food Materials Science and Engineering (pp. 1–25). Blackwell Publishing Ltd.
Borcherding, K., Lorenzen, P. C. H. R., & Hoffman, W. (2009). Effect of protein content, casein–whey protein ratio and pH value of the foaming properties of skimmed milk. International Journal of Dairy Technology, 62(2), 161–169.
Bos, M. A., & van Vliet, T. (2001). Interfacial rheological properties of adsorbed protein layers and surfactants: A review. Advances in Colloid and Interface Science, 91(3), 437–471.
Bureiko, A., Trybala, A., Kovalchuk, N., & Starov, V. (2015). Current applications of foams formed from mixed surfactant–polymer solutions. Advances in Colloid and Interface Science, 222, 670–677.
Coke, M., Wilde, P. J., Russell, E. J., & Clark, D. C. (1990). The influence of surface composition and molecular diffusion on the stability of foams formed from protein/surfactant mixtures. Journal of Colloid and Interface Science, 138(2), 489–504.
Dandigunta, B., Karthick, A., Chattopadhyay, P., & Dhoble, A. S. (2021). Impact of temperature and surfactant addition on milk foams. Journal of Food Engineering, 299, 110509.
Deotale, S. M., Dutta, S., Moses, J. A., & Anandharamakrishnan, C. (2021). Comparative study of stabilization of coffee bubbles at the air-water interface through different surfactants. Applied Food Research, 1(2), 100012.
Dickinson, E. (1997). Properties of emulsions stabilized with milk proteins: Overview of some recent developments. Journal of Dairy Science, 80(10), 2607–2619.
Duan, R. L., Sun, X., Liu, J., Gong, T., & Zhang, Z. R. (2011). Mixed micelles loaded with silybin-polyene phosphatidylcholine complex improve drug solubility. Acta Pharmacologica Sinica, 32, 108–115.
Dutta, A., Chengara, A., Nikolov, A., Wasan, D. T., Chen, K., & Campbell, B. (2002). Effect of surfactant composition on aeration characteristics and stability of foams in aerated food products. Journal of Food Science, 67(8), 3080–3086.
Grigoriev, D. O., Derkatch, S., Krägel, J., & Miller, R. (2007). Relationship between structure and rheological properties of mixed BSA/Tween 80 adsorption layers at the air/water interface. Food Hydrocolloids, 21(5), 823–830.
Harris, L. V., & Angal, S. (1990). Protein purification methods: A practical approach, 317 p. Oxford University Press.
Ho, T. M., Bhandari, B. R., & Bansal, N. (2022). Functionality of bovine milk proteins and other factors in foaming properties of milk: A review. Critical Reviews in Food Science and Nutrition, 62(17), 4800–4820.
Ho, T. M., Dhungana, P., Bhandari, B., & Bansal, N. (2021). Effect of the native fat globule size on foaming properties and foam structure of milk. Journal of Food Engineering, 291, 110227.
Ho, T. M., Le, T. H. A., Yan, A., Bhandari, B. R., & Bansal, N. (2019). Foaming properties and foam structure of milk during storage. Food Research International, 116, 379–386.
Huppertz, T. (2010). Foaming properties of milk: A review of the influence of composition and processing. International Journal of Dairy Technology, 63(4), 477–488.
Kamath, S. (2007). Foaming of milk. PhD Thesis, School of Land, Crop and Food Sciences, University of Queensland, Australia.
Kamath, S., Wulandewi, A., & Deeth, H. (2008a). Relationship between surface tension, free fatty acid concentration and foaming properties of milk. Food Research International, 41(6), 623–629.
Kamath, S., Huppertz, T., Houlihan, A. V., & Deeth, H. C. (2008b). The influence of temperature on the foaming of milk. International Dairy Journal, 18, 994–1002.
Kamigaki, T., Ito, Y., Nishino, Y., & Miyazawa, A. (2018). Microstructural observation of casein micelles in milk by cryo-electron microscopy of vitreous sections (CEMOVIS). Microscopy, 67(3), 164–170.
Kelley, D., & McClements, D. J. (2003). Interactions of bovine serum albumin with ionic surfactants in aqueous solutions. Food Hydrocolloids, 17(1), 73–85.
Kotsmar, C., Pradines, V., Alahverdjieva, V. S., Aksenenko, E. V., Fainerman, V. B., Kovalchuk, V. I., Krägel, J., Leser, M. E., Noskov, B. A., & Miller, R. (2009). Thermodynamics, adsorption kinetics and rheology of mixed protein–surfactant interfacial layers. Advances in Colloid and Interface Science, 150(1), 41–54.
Lech, F. J., Meinders, M. B. J., Wierenga, P. A., & Gruppen, H. (2015). Comparing foam and interfacial properties of similarly charged protein–surfactant mixtures. Colloids and Surfaces a: Physicochemical and Engineering Aspects, 473, 18–23.
Lech, F. J., Steltenpool, P., Meinders, M. B. J., Sforza, S., Gruppen, H., & Wierenga, P. A. (2014). Identifying changes in chemical, interfacial and foam properties of β-lactoglobulin–sodium dodecyl sulphate mixtures. Colloids and Surfaces a: Physicochemical and Engineering Aspects, 462, 34–44.
Malcolm, A. S., Dexter, A. F., & Middelberg, A. P. J. (2006). Foaming properties of a peptide designed to form stimuli-responsive interfacial films. Soft Matter, 2(12), 1057–1066.
Maldonado-Valderrama, J., Martin-Molina, A., Martin-Rodriguez, A., Cabrerizo-Vilchez, M. A., Galvez-Ruiz, M., & Langevin, D. (2007). Surface properties and foam stability of protein/surfactant mixtures: Theory and experiment. Journal of Physical Chemistry C, 111(6), 2715–2723.
Maldonado-Valderrama, J., & Patino, J. M. R. (2010). Interfacial rheology of protein–surfactant mixtures. Current Opinion in Colloid & Interface Science, 15(4), 271–282.
McCarthy, O. (2011). Physical and physico-chemical properties of milk. In J. W. Fuquay, P. F. Fox, & P. L. H. McSweeney (Eds.), Encyclopedia of dairy science (pp. 467–477). Academic Press.
Mellema, M., de Groot, P. W. N., & Golding, M. (2009). Impaired or accelerated aggregation of proteins in sterilised milk by adding surfactants. International Dairy Journal, 19(12), 728–736.
Morrison, I. D., & Ross, S. (2002). Colloidal dispersions: Suspensions, emulsions, and foams, 656 p. Wiley.
Munchow, M., Jorgensen, L., Amigo, J. M., Sorensen, K., & Ipsen, R. (2015). Steam-frothing of milk for coffee: Evaluation for foam properties using video analysis and feature extraction. International Dairy Journal, 51, 84–91.
Petkova, R., Tcholakova, S., & Denkov, N.D. (2012). Foaming and foam stability for mixed polymer–surfactant solutions: effects of surfactant type and polymer charge. Langmuir, 28(11), pp.4996–5009.
Pugh, R. J. (2016). Generation of bubbles and foams. In R. J. Pugh (Ed.), Bubble and foam chemistry (pp. 155–188). Cambridge University Press.
Rosen, M. J., & Kunjappu, J. T. (2012). Surfactants and interfacial phenomena (4th ed.). John Wiley & Sons.
Rouimi, S., Schorsch, C., Valentini, C., & Vaslin, S. (2005). Foam stability and interfacial properties of milk protein–surfactant systems. Food Hydrocolloids, 19(3), 467–478.
Sherbon, J. W. (1988). Physical properties of milk. In N. P. Wong (Ed.), Fundamentals of dairy chemistry (pp. 409–460). Van Nostrand Reinhold Company.
Schramm, L. L., & Wassmuth, F. (1994). Foams: Basic principles. In L. L. Schramm (Ed.), Foams: Fundamentals and applications in the petroleum industry, chapter 1 (pp. 3–45). American Chemical Society.
Sodini, I., Morin, P., Olabi, A., & Jimenez-Flores, R. (2006). Compositional and functional properties of buttermilk: A comparison between sweet, sour and whey buttermilk. Journal of Dairy Science, 89, 525–536.
Stancil, S. (2013). A study of the frothing capacity of whole milk- 1. A market survey of conventional and organic milks, 2. The effect of clean in place (CIP) cleaning solutions, Master thesis, Clemson University, Clemson, USA.
Tadros, T. F. (2014). An introduction to surfactants, pp. 10. De Gruyter, Berlin, Germany.
Tiller, F. M., Li, W., & Chen, W. (2009). Solid/liquid separation, In Albright LF (ed.), Albright's chemical engineering handbook, pp. 1605. CRC Press, Florida, USA.
Wang, W., Gu, B., & Liang, L. (2005). Effect of surfactants on the formation, morphology, and surface property of synthesized SiO2 nanoparticles. Journal of Dispersion Science and Technology, 25(5), 593–601.
Wang, Z., Zhang, S., & Vardhanabhuti, B. (2015). Foaming properties of whey protein isolate and λ-carrageenan mixed systems. Journal of Food Science, 80(8), N1893–N1902.
Ward, B. R., Goddard, S. J., Augustin, M.-A., & McKinnon, I. R. (1997). EDTA-induced dissociation of casein micelles and its effect on foaming properties of milk. Journal of Dairy Research, 64(4), 495–504.
Wei, X. F., & Liu, H. Z. (2000). Relationship between foaming properties and solution properties of protein/nonionic surfactant mixtures. Journal of Surfactants and Detergents, 3(4), 491–495.
Wilde, P. J., Nino, M. R. R., Clark, D. C., & Rodríguez Patino, J. M. (1997). Molecular diffusion and drainage of thin liquid films stabilized by bovine serum albumin-Tween 20 mixtures in aqueous solutions of ethanol and sucrose. Langmuir, 13(26), 7151–7157.
Xiaodi, S., & Shuntang, G. (2016). Effect of diluent type on analysis of zeta potential of colloid particles of soymilk protein. Transactions of the Chinese Society of Agricultural Engineering, 32(7), 270–275.
Xiong, X., Ho, M. T., Bhandari, B., & Bansal, N. (2020). Foaming properties of milk protein dispersions at different protein content and casein to whey protein ratios. International Dairy Journal, 109, 104758.
Zhang, H., Dudley, E. G., & Harte, F. (2017). Critical synergistic concentration of lecithin phospholipids improves the antimicrobial activity of eugenol against Escherichia coli. Applied and Environmental Microbiology, 83(21), 1–9.
Zhang, Z., & Goff, H. D. (2004). Protein distribution at air interfaces in dairy foams and ice cream as affected by casein dissociation and emulsifiers. International Dairy Journal, 14, 647–657.
Acknowledgements
The authors gratefully thank the School of Agriculture and Food Sciences at the University of Queensland for the facilities and technical assistance. A sincere appreciation also goes to the Indonesia Endowment Fund for Education (LPDP) scholarship that enabled Andre Tanzil to study at the University of Queensland.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This project was financially supported by the ARC Dairy Innovation Hub, a collaboration between the University of Melbourne, the University of Queensland, and Dairy Innovation Australia Ltd.
Author information
Authors and Affiliations
Contributions
Thao M. Ho, Bhesh R. Bhandari, and Nidhi Bansal designed the experiments. Andre Tanzil and Thao M. Ho conducted the experiments to collect the data. Thao M. Ho, Bhesh R. Bhandari, and Nidhi Bansal interpreted the results. Thao M. Ho and Andre Tanzil drafted and revised the manuscript. Nidhi Bansal as a corresponding author supervised the experiment and corrected the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ho, T.M., Tanzil, A., Bhandari, B.R. et al. Effect of Surfactant Type on Foaming Properties of Milk. Food Bioprocess Technol 16, 1781–1793 (2023). https://doi.org/10.1007/s11947-023-03012-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-023-03012-5