Abstract
The addition of ultrafiltered (UF) milk retentate is known to impact milk properties during mozzarella and cheddar cheese production, but the effect during cream cheese production is less well understood. Little is known about the impact of UF retentate on the intermediate stages of manufacture, such as protein assembly and the formation of hydrated acid gel structures. Here, milk prepared for cream cheese manufacture using a concentration factor of 2.5 or 5 had a similar particle size distribution to unconcentrated cheese milk after homogenization but increased viscosity and a slower rate of acidification, which could be altered by increasing starter culture concentration. The acid gels formed contained more protein and fat, resulting in a higher storage modulus, firmness, and viscosity. A denser microstructure was observed in acid gels formed with UF retentate addition, and quantitative two- or three-dimensional analysis of confocal images found a greater volume percentage of protein and fat, decreased porosity, and increased coalescence of fat. The mobility of water, as assessed by proton nuclear magnetic resonance, was reduced in the dense UF gel networks. Water movement was partially obstructed, although diffusion was possible between interconnected serum pores. These insights improve our understanding of acid gel formation. They can be used by manufacturers to further optimize the early and intermediate stages of cream cheese production when using concentrated milk to reduce acid whey production and lay the foundation for larger pilot scale studies of intermediate and final cream cheese structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ultrafiltered (UF) milk is commonly added during cheese production, as it can increase yield by as much as ~20% and reduce or eliminate the volume of acid whey produced (Mistry & Maubois, 2017). While this is common practice and has been well studied for mozzarella and cheddar cheese (Mistry & Maubois, 2017), the impact of adding UF retentate during cream cheese production is less well understood.
The concentration factor is a key variable when adding UF retentate to cheese. This factor, which indicates the extent of concentration achieved during UF processing, ranges from low (e.g., 1.2) to high (e.g., ≥ 5) and is known to impact the properties of ultrafiltered milk, including milk protein concentration, viscosity, buffer capacity, and calcium concentration (Mistry & Maubois, 2017; Srilaorkul et al., 1989). These changes subsequently alter the properties of the standardized milk preparations that are used for cheese manufacture. The extent to which these properties are altered depends on the composition used for cheese making. In the case of double-fat cream cheese, the composition is typically ~3% protein and ~11–12% fat, i.e., a protein to a fat ratio of ~0.25–0.28 (Brighenti et al., 2018; Ong et al., 2018). For cheddar and mozzarella production, the fat concentration used for cheesemaking is lower, resulting in a much higher protein to a fat ratio of ~0.85 and ~1.31, respectively (Govindasamy-Lucey et al., 2005; Ong et al., 2013).
The use of UF retentate is expected to impact on several stages during the production of cream cheese from the milk preparation, the formation of acid gels, the separation of whey, and final production of curd. Previous studies adding UF retentate with a concentration factor of 4 and ~6 during cream cheese production have shown that the texture and sensory properties of the final cream cheese product are altered, with an increase of 50% in cheese viscosity or the presence of a bitter flavor (Covacevich & Kosikowski, 1977; Salhab, 1999). Less is known about the effect of addition of UF retentate and the concentration factor on the intermediate stages of manufacture and how UF preparations alter the fundamental properties and structuring of ingredients during processing.
In the early stages of cream cheese production, the protein and fat molecules within the standardized milk preparation assemble to form a structure known as an acid gel, in a process induced by lactic acid–producing bacteria, or the addition of acids (e.g., lactic acid or hydrochloric acid). This process stage is critical, as the network structure formed can affect subsequent processability (Brighenti et al., 2018; Ong et al., 2020). The structure formed also rearranges into a characteristic corpuscular cluster in the following process steps, affecting the final product structure and performance (Ong et al., 2018, 2020).
The impact of UF concentration factor on the structure of the acid-gel network and its fundamental properties, including the protein and fat density, the crosslinking of the protein network, structure hydration, and water motility is unclear. The role of water in the protein network is particularly interesting, as the use of UF retentate increases the concentration of protein, fat, and minerals without the accompanying water from milk, altering the balance between the solids content and water during processing. Adjustments to cream cheese processing conditions, such as fermentation or whey separation conditions that are required with the use of UF retentate, are also not documented in the literature. In other dairy systems with a different composition, such as yoghurt, a higher concentration of starter culture is required for effective acidification after addition of UF retentate (Gupta, 2016). Adjustments in rennet coagulation temperature or coagulation time have also been reported as necessary to improve the quality of curd during the production of cheddar cheese with addition of UF retentate (Green, 1987; Ong et al., 2013). A greater understanding of the changes induced by addition of UF retentate during cream cheese production and the factors controlling the fundamental properties of the acid gel structure, including the impact of protein concentration, total solids, and calcium elevated by addition of UF retentate, will potentially improve acid gel formation and may provide a route for further optimization of cream cheese production.
In this study, UF concentration factors of 2.5× and 5× were selected to assess the impact of UF retentate addition on milk preparations and acid gel formation with a focus on the intermediate stages of cream cheese production. The study aims to provide greater insights into the behavior of the initial milk ingredients and their interaction and assembly during the formation of acid gels. A further aim was to assess the characteristics and properties of acid gels formed and to identify possible process optimizations that may be relevant to the initial stages of cream cheese production including milk concentration and acid gel formation with UF retentate addition.
Materials and Methods
Preparation of Milk for Cream Cheese Production
Skim bovine milk (3.7% protein, 0.1% fat, w/v, pasteurized and homogenized) and double cream (1.6% protein, 56% fat, w/v, pasteurized) were purchased from local supermarkets (Woolworths, VIC, Australia). Skim milk was ultrafiltered using a bench-scale cross-flow cell (SEPA Cell, Sterlitech Corporation, Kent, WA, USA) and polyethersulfone (PES) flat sheet membranes (190 × 140 mm, 10 kDa MWCO, Microdyn-Nadir, Wiesbaden, Germany). The milk was ultrafiltered to a volumetric concentration factor (VCF) of 2.5× and 5×, where the VCF is defined as:
and Vt is the total feed volume and Vp is the permeate volume.
The ultrafiltration experiments were conducted in a batch mode. The milk was circulated by a positive displacement pump (Hydra-Cell, Wanner Engineering, MN, USA) fitted with an inverter (E510(IP66), TECO, Melbourne, VIC, Australia) at a flow rate of 3.2 L min−1 (cross-flow velocity of 0.43 ± 0.01 m s−1) and a transmembrane pressure of a ~4 bar and maintained at 50 °C by passing through a water bath. A processing volume of 1.5 L was used to make VCF2.5 and 2 L used to make VCF5 retentate with a filtration time of ~3 h and ~5 h, respectively. The membrane was cleaned-in-place (CIP) at 50 °C after each run. The cleaning process consisted of three steps: (1) circulation of sodium hydroxide solution (~pH 11) for 30 min; (2) circulation of hydrochloric acid (~pH 3) for 30 min; (3) a repeat step (1) following the process described by the manufacturer and in the literature (Koh et al., 2013; Sterlitech Corporation, 2022).
Approximately ~650 mL of milk, defined as cream cheese milk, was prepared for each batch of the experiment by combining the cream with skim milk or retentate to a protein to a fat ratio of ~0.23 ± 0.02. Fat standardized cream cheese milk (CCM) prepared from skim milk concentrated by 1×, 2.5×, and 5× is referred to as CCM1, CCM2.5, and CCM5, respectively. The cheese milk was heated to ~60 °C in a water bath and passed through a pre-warmed Panda homogenizer (NS1001L 2 K, GEA Australia, Thomastown, VIC, Australia) with the pressure (first stage) at 15 MPa. After the first pass, the sample was immediately homogenized a second time with a lower pressure of 5 MPa.
A total of 9 batches of CCM (three replicates of CCM1, CCM2.5, and CCM5, respectively) were prepared randomly on different days. After homogenization and pasteurization (at 65 °C in a water bath for 30 min), the CCM was cooled to 4 °C and stored until needed. CCM samples were divided into three portions and inoculated with 0.05% (w/v), 0.1% (w/v), or 0.2% (w/v) direct vat set (DVS) starter culture containing Lactococcus lactis subsp. cremoris and Lactococcus lactis subsp. lactis (R-704, Chr. Hansen, Melbourne, VIC, Australia) in a random order for rheological, textural, and microstructural analysis. These steps of acid gel formation form the first part of the cream cheese production processes, which is outlined in Supplementary information (SI) Fig. 1. All samples were used within 1 week.
Chemical Analysis of Milk Samples and Acid Whey
The total solids of the milk samples were determined by oven-drying at ~100 °C for 16 h. The Babcock method was used to analyze fat content and nitrogen content determined using a LECO TruMac CNS analyzer (LECO Corporation, St. Joseph, MI, USA). The protein value was then determined by multiplying the nitrogen content by 6.38. All measurements were performed in duplicate for each sample and averaged for further analysis.
The soluble protein concentration in the acid whey was determined using a Bradford protein assay. The concentrated dye reagent (Bio-Rad Protein Assay Kit II, Bio-Rad Laboratories, Gladesville, NSW, Australia) was diluted with deionized water at a ratio of 1:4, and the diluted solution was filtrated before use. An aliquot of 50-µL sample solution was mixed with 2.5-mL dye solution and was incubated at room temperature for at least 5 min before the measurement. Samples were transferred into micro-cuvettes with a 10-mm path length and were analyzed at 595 nm using a NanoDrop Spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). Bovine serum albumin (Bio-Rad) was used as standard. Measurements were performed in duplicate for each sample and averaged for further analysis.
The fat content in acid whey was determined using the method described by Nguyen et al. (2014). Triolein (T-7140, Sigma-Aldrich, NSW, Australia) was used as the standard solution, which was dissolved in isopropanol with a concentration ranging from 0.25–4 mg mL−1. Each sample was analyzed in duplicate, and the values were averaged for analysis.
Total calcium was determined using inductively coupled plasma mass spectrometry (Agilent Technologies 8900 Series ICP-QQQ-MS, Mulgrave, VIC, Australia). Two tune modes were used for elemental analysis. Helium (He) collision gas at a flow rate of 5 mL min−1 and a combination of nitrous oxide (15%) and hydrogen reaction gases at a flow rate of 7 mL min−1 were used in tune modes 1 and 2, respectively. The instrument was calibrated for calcium (Ca) and zinc (Zn) using a mixed 8-point calibration curve (0, 10, 50, 100, 250, 500, 2000, and 3000 ppb) from a stock multi-element calibration standard-2A (Agilent Technologies). The milk sample was diluted with purified water (Direct-Q UV Water Purification System, Merck, VIC, Australia), and 0.1-mL diluted sample was transferred into an Eppendorf tube and dried in the oven at 100 °C for ~4 h. An aliquot of 50 µL 65% (v/v) HNO3 (Suprapur, Merck, Bayswater, VIC, Australia) was then mixed with a dried pellet for digestion at 95 °C for 30 min using a thermomixer. After digestion, the sample was added with 950-µL purified water to 1 mL and centrifuged at 20,000 g for 30 min. A hundred microliter of supernatant was then taken out and mixed with 900 µL purified water in a 1.5-mL tube and used for injection. Measurements were performed in duplicate, and the isotope 40Ca was selected for analysis.
Particle Size Analysis by Laser Diffraction
The size distribution of suspended solids in the cheese milk was obtained using a Mastersizer 3000 (Malvern Panalytical Ltd, Worcestershire, UK). Pure water was used as a dispersant (refractive index: 1.333). The refractive indices for cheese milk samples were set to be 1.460 and 1.458 at 466 nm and 633 nm, respectively, and the absorption coefficients 1.7 × 10−5 at 466 nm and 0.5 × 10−5 at 633 nm (Michalski et al., 2001), with a laser obscuration of ~5% and stirring speed of 2400 rpm. The measurements were repeated in triplicate for each sample and averaged for analysis.
Rheological and Textural Measurement
Flow Properties of Cream Cheese Milk
The flow curve of CCM was determined using an HR-2 Discovery Hybrid Rheometer (TA Instruments, New Castle, DE, USA) with a cone plate geometry (angle: 2°). The measuring gap was set at 100 µm and the interval time between each shear rate was set to 120 s to give enough time for the sample to equilibrate (Mezger, 2014). The shear rate ranged from 0.1 s−1 to 100 s−1 with 5 data points collected per decade. The temperature was controlled at 31 °C to match the fermentation temperature. Each sample was analyzed in duplicate.
Gelation Dynamics of Cream Cheese Milk
The storage modulus (G′) was recorded during the gelation process using the same rheometer with a standard concentric cylinder tool. The milk sample (50 mL) was inoculated with R-704 mesophilic starter culture (Chr. Hansen) at a concentration of 0.05%, (w/v), 0.1%, (w/v), or 0.2% (w/v). A 20 mL aliquot of the sample was then put into the cup and a solvent trap was used to reduce evaporation. Measurement parameters were set as below: shear strain, 0.1%; frequency, 0.8 Hz; temperature, 31 °C. An aliquot of 20 mL of sample was also transferred to a 50 mL sterile container and incubated in a water bath (31 °C) to monitor changes in pH in parallel to the gelation experiment. A pH meter (SevenCompact, Mettler Toledo, Melbourne, VIC, Australia) with a glass electrode (IJ-44C, Ionode, Tennyson, QLD, Australia) was used to record the pH value every 2.5 min until the pH value dropped to 4.6. The gelation time (Tgel) and gelation pH (pHgel) were arbitrarily defined as the time and pH when the increase in G’ surpassed 1 Pa from the baseline.
Gel Firmness Measurement
The gel firmness was determined using a texture analyzer (Stable Micro Systems, Godalming, England), and a 5-mm cylindrical probe. Cheese milk (50 mL) inoculated with the starter culture was transferred to a cylindrical container (70 mL, Thermo Fisher Scientific) and incubated at 31 °C in parallel with the dynamic gelation measurement. All samples were analyzed immediately at the end of fermentation at pH 4.6. The measuring parameters were: contact force, 0.5 g; test speed, 1 mm s−1; measuring depth, 30 mm. The maximum force shown in the force-distance curve was used as the gel firmness value (Ong et al., 2010). The measurements were repeated three times on different surface areas of the sample and averaged for analysis.
Temperature Sweep and Flow Curve Analysis of Cream Cheese Acid Gel
The temperature sweep and flow curve were performed following the methods described by (Ong et al., 2018) with some modifications. Temperature sweep tests for acid gel were conducted using a flat parallel plate with the gap set at 1.5 mm. The temperature sweep ranged from 20–90 °C, with an increment of 5 °C in 90 s. The strain and frequency values were set at 0.1% and 0.8 Hz for the analysis. A cone plate (angle: 2°) was used for the flow curve analysis with the gap set at 1 mm. The shear rate increased logarithmically from 0.1 to 100 s−1 with 5 data points recorded per decade. The interval time at each shear rate was set to 60 s to allow the sample to equilibrate. The acid gel was stirred gently with a spatula and placed in the fridge at 4 °C for ~1 h before measurement on the rheometer set to 70 °C. A solvent trap was used to reduce evaporation and measurements were performed in duplicate.
Acid Whey Separation by Centrifugation
The acid gel was heated and centrifuged to separate the acid whey. Approximately 1 g acid gel was weighed into a 1.5-mL Eppendorf tube on an electronic balance (Mettler Toledo). Each sample was prepared in triplicate. The sample tubes were then heated at 70 °C with a mixing speed of 500 rpm for 10 min in a Thermomixer (Thermomixer comfort, Eppendorf, Hamburg, Germany), followed by centrifugation at 5000 g for 10 min at room temperature. The acid whey separated from the curd after centrifugation was removed from the tube with a pipette tip. The percentage of whey released was calculated based on the remaining mass in the tube, and each sample was analyzed in triplicate (Ong et al., 2018).
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was conducted to analyze the whey proteins in the acid whey. The reagent and equipment used were from Thermo Fisher Scientific Australia unless specified otherwise. Acid whey diluted with pure water and mixed with a sample buffer (4× Bolt™) and a reducing agent (10× Bolt™) was heated at 95 °C for 5 min and loaded into a protein gel (12-well, Bolt™ 4–12% Bis–Tris Plus Gels). The gel was accommodated in a Mini Gel tank connected to a PowerEase power supply. The running condition for the gel was set at 150 V for 50 min. After destaining, the gel image was captured and processed using the ChemiDoc™ MP Imaging System (Bio-Rad).
Confocal Laser Scanning Microscopy (CLSM) of Cream Cheese Milk and Acid Gel
Images of cheese milk and gel samples were taken using a confocal microscope (Leica SP8, Leica Microsystems, Heidelberg, Germany). Nile red and Fast green FCF (both from Sigma-Aldrich), prepared respectively in dimethyl sulfoxide (DMSO) and distilled water to 1 mg mL−1 as a stock solution, were used for the staining of a fat globule and protein.
To stabilize milk particles for better observation under CLSM, agarose (Standard Molecular Biology Grade Agarose, Scientifix, Melbourne, VIC, Australia) at a concentration of 0.5% (w/v) in water was prepared and boiled before use. The cheese milk sample (0.5 mL) was first stained with 10 µL Nile red and 10 µL fast green FCF in an Eppendorf tube. An aliquot of a 50 µL stained milk sample was then mixed with the 200 µL agarose solution that had been cooled down to ~45 °C. The mixture was vortexed for ~3 s and an aliquot of ~5 µL was pipetted on a cavity slide and immediately sealed with a coverslip before the sample solidified (Ong et al., 2010).
A slice of gel (~5 mm × 5 mm × 2 mm, L × W × H) was cut with a surgical blade and put on a glass slide for staining. Nile red and Fast green FCF were diluted with pure water to 0.1 mg mL−1 and added into the gel sample sequentially. The samples were immediately analyzed within 2 h of preparation. The CLSM images were taken with a 63× oil immersion objective at 2× or 4× digital magnification. The emission filters for Nile red and fast green were set at 520–590 nm and 660–750 nm, respectively (Ong et al., 2010). Two-dimensional (2D) and three-dimensional (3D) images were collected for further analysis.
Image Analysis of CLSM Images
The analysis of fat and protein content was conducted with 3D images (92.3 (x) × 92.3 (y) × 8.95 (z) µm), which were constructed from 30 layers of 2D images. Two sets of 3D image data were collected for each sample. Images were first deconvolved with Huygens software (Scientific Volume Imaging B.V., Hilversum, Netherlands) to reduce noise and improve contrast and resolution. Fat and protein channels were background subtracted at an intensity level of 30, and then the images were deconvolved with CLME algorithm for 40 iterations. The deconvolved images were further processed in Imaris software (Bitplane, South Windsor, CT, USA) for surface segmentation. The fat channel was manually thresholded based on “background subtraction (local contrast)” to account for less even intensity of the fat particles. The green channel, which was homogenous, was thresholded by “absolute intensity.” The volume of the unstained area was obtained by subtracting the fat and protein surface volume from the total volume. The porosity was defined as the unstained volume divided by the total volume.
Analysis of fat particle size distribution in 2D images (92.3 (x) × 92.3 (y) µm) was performed using the public domain software ImageJ (version 1.58a, National Institutes of Health, Bethesda, MD, USA). The 2D micrographs were split into grayscale channels, in which the red (fat) channel was of interest and applied with deconvolution using the plugin Iterative Deconvolve 3D. The deconvolved images were then further processed with “unsharp mask” and “median” filters in ImageJ to sharpen and enhance edges. Afterward, the images were thresholded automatedly using the “Mean” thresholding algorithm, and a watershed was performed to separate connected fat particles. After setting the scale, the “analyze particles” function was used to obtain the counts and fat particle area. Based on stereology, the average cross-sectional area of fat globule (\(\overline{s}\)) approaches the value of 2/3 \(\pi {r}^{2}\) (Fullman, 1953), where \(r\) is the sphere size of the particle. Thus, the \(r\) can be obtained by the equation below:
The volume of the particle size (4/3* \(\pi {r}^{3}\)) was further calculated and used to construct a volume-based size distribution. Three images were analyzed for each sample.
NMR Measurements
Cheese milk was inoculated with 0.05% (w/v) starter culture. An aliquot of a ~0.5 mL inoculated sample was transferred into a 3-mm micro-NMR tube using a syringe, and an aliquot of a 20 mL sample was transferred to a 50-mL container. Both the micro-NMR tube and the container were put in a waterbath at 31 °C for fermentation, and the pH of the cheese milk in the container was monitored using a pH meter. Once the pH of the sample in the container dropped to 4.6, the micro-NMR tube was transferred to a fridge at 4 °C for NMR measurement. The micro-NMR tube containing the acid gel was then inserted into a standard 5-mm NMR tube filled with deuterated chloroform for NMR measurement. All measurements were carried out on a Bruker Avance III 600 spectrometer using a TCI cryoprobe fitted with a single-axis field-gradient (Gz) (Bruker BioSpin GmbH, Germany) at 20 °C. Standard RF pulse sequences (t1ir, cpmg and stegp1s, Bruker pulse sequence library) of inversion recover (IR), Carr-Purcel-Meiboom-Gill (CPMG), and pulsed-filed gradient stimulated echo (PFG-STE) were used for measuring longitudinal relaxation time (T1), transverse relaxation time (T2), and translational diffusion coefficient of water (Dw) in cream cheese acid gel, respectively. Specifically, the IR sequence was collected with 16 variable delays (τ), ranging from 50 ms to 25 s; the CPMG sequence was recorded with 18 echo delays (τ) varied from 4.8 to 242 ms, and, for the PFG-STE sequence, 32 datapoints were collected with an effective duration (δ e) of 1.273 ms, a diffusion delay (Δ) of 100 ms and the gradient strength ranging from 2.87 to 54.44 G cm−1. All measurements for CCM1, CCM2.5, and CCM5 acid gels were performed in duplicate with the samples prepared independently. In addition, the diffusion coefficient of deionized water was also measured as a reference. All data were processed using TOPSPIN (Version 3.6, Bruker BioSpin) with 1H chemical shifts referenced to CDCl3 at 7.24 ppm and analyzed using the T1/T2 relaxation module within TOPSPIN. Longitudinal relaxation time (T1), transverse relaxation time (T2), and translational diffusion coefficient of water (Dw) were determined by the following equations:
where, in Eq. (5), γ is the proton gyromagnetic ratio with g, δe, and Δ being the amplitude, effective duration, and diffusion delay, respectively (Yao et al., 2018).
Statistical Analysis
A split-plot design with whole plots in completely randomized design (CRD) was used to analyze the effects of concentration factor and inoculation concentration on the gelation properties of acid gels, gel firmness, acid whey volume, and protein and fat contents in the whey. The concentration factor was set as the whole plot factor, and the inoculation concentration was set as the subplot factor. A general linear model in Minitab (Minitab Inc., State College, PA, USA) was used to analyze significant differences between factor level means, and a mixed-effects model was used for pairwise comparisons (Kowalski et al., 2007). One-way ANOVA (analysis of variance) was used in all other statistical analyses with a significance level (p-value) set at 0.05. Data plotting was conducted using Origin 2019 software (OriginLab Corporation, Northampton, MA, USA).
Results and Discussion
Properties of UF-Concentrated Milk
Cheese milk with varying levels of UF retentate was prepared by combining skim milk or skim milk concentrated 2.5- or 5-fold (volumetric concentration factor (VCF) 2.5 or 5) with cream, producing CCM1, CCM2.5, and CCM5 (Table 1). CCM2.5 and CCM5 had significantly higher concentrations of protein, fat, total solids, and calcium than CCM1 but retained the same target protein to a fat ratio (0.21–0.24), which is needed for the production of double-fat cream cheese with a final fat and protein content of ~33% and ~7.5%, respectively (Brighenti et al., 2018). Milk pH was similar between preparations. The UF process was typical of prior studies; whey proteins were retained in the UF retentate for VCF2.5 and VCF5, with no protein bands visible in the permeate using SDS-PAGE (results not shown) and only ~0.02–0.04% non-protein nitrogen (NPN) detected in the permeate. The protein content of skim milk, which was slightly lower than indicated by the manufacturer’s label (3.4%, w/v vs. ~3.7%, w/v), increased to 8.2% (w/v) in VCF2.5 and 14.3% in VCF5, consistent with past reports of ~13–15% for VCF5 (Erdem, 2005; Marella et al., 2015). The calcium content also increased to ~261 mg 100 mL−1 (VCF2.5) and ~476 mg 100 mL−1 (VCF5) after concentration, similar to the increases previously noted for VCF5 retentate (~450 mg 100 mL−1) (Luo et al., 2015), as colloid calcium is retained, while soluble calcium passes through the membrane in the permeate.
Homogenization was equally effective on CCM1, CCM2.5, and CCM5 milk preparations, producing a similar particle size distribution, despite the ~1.5- to 2-fold differences in total solids (Fig. 1a). The volume-weighted mean diameter (D[4, 3]) was also similar between preparations and a characteristic of milk used for cream cheese production (1.14 ± 0.2 µm, 1.38 ± 0.26 µm, and 1.43 ± 0.29 µm for CCM1, CCM2.5, and CCM5, respectively, P > 0.05). Standard homogenization conditions were likely effective due to the similar protein to a fat ratio for all milk treatments (Table 1), as reductions in this ratio (~0.58–0.08) are known to result in larger particle sizes (Tomas et al., 1994).
Properties of UF-concentrated milk CCM2.5 (
 ) and CCM5 (
) and CCM5 (
 ) compared to CCM1 (
) compared to CCM1 (
 ). Particle size distribution (a), representative CLSM images of b CCM1, c CCM2.5, d CCM5 and apparent viscosity (e). The fat in CLSM images stained by Nile red appears red, and protein stained by Fast Green FCF appears green. The scale bars are 10 µm in length. White circles indicate protein-fat clusters. Error bars in (a) and (e) indicate standard deviation of the mean (n = 3)
). Particle size distribution (a), representative CLSM images of b CCM1, c CCM2.5, d CCM5 and apparent viscosity (e). The fat in CLSM images stained by Nile red appears red, and protein stained by Fast Green FCF appears green. The scale bars are 10 µm in length. White circles indicate protein-fat clusters. Error bars in (a) and (e) indicate standard deviation of the mean (n = 3)
All three homogenized preparations contained characteristic homogenization clusters, where particles contain both fat and casein proteins (Ogden et al., 1976; Ong et al., 2018), indicated by the white circles in Fig. 1b–d, where the particle size was consistent with measurements by laser diffraction (Fig. 1a). The significance of these clusters is that they form one of the building blocks that assemble to generate the network structure during the later stages of acid gel formation, so their consistency is important for potentially generating similar microstructure. As expected, CCM5 had the highest density of particles, followed by CCM2.5. This difference in the particle number is also important for microstructure generation, as it is expected to impact the density and interconnectivity of the network formed.
CCM5 had the highest viscosity as a function of a shear rate, followed by CCM2.5 and CCM1. At 1 s−1, the viscosity of CCM5 was ~10-fold and ~16-fold higher than the viscosity measured for CCM2.5 and CCM1, respectively. CCM5 was also less shear thinning (Fig. 1e). This difference in viscosity was expected due to the higher protein and fat content in UF containing preparations (Table 1), which reduces inter-particle distance, increases particle interactions, and contributes to higher viscosity (Bienvenue et al., 2003). The high viscosity of this concentrated cheese milk can potentially affect pump performance, requiring additional checks and optimization of the viscosity range at manufacturing scale. The shear range examined here (0.1–100 s−1) includes the estimated shear of 4–75 s−1 relevant to cream cheese production, where the pipe is 5 to 6.5 cm in diameter, and the flow rate ranges from 1350–13,500 L h−1 (Ong et al., 2018).
A further physical change induced by concentration of the milk preparations is a reduction in thermal conductivity, which is determined by milk composition. The higher solids and fat content and reduced water content of UF cheese milk will reduce thermal conductivity (k) by ~7–13% to 0.50 Wm−1 K−1 and 0.47 Wm−1 K−1 for CCM2.5 and CCM5, respectively, at 25 °C, compared to 0.54 Wm−1 K−1 for CCM1 (Munir et al., 2016). This leads to a lower heat transfer coefficient (h), which is proportional to the thermal conductivity k (h = 3.66 k/d, where d is the diameter of the pipe as described by Sparrow et al. (2013)) under laminar flow through a pipe at a uniform wall temperature, resulting in a less-effective heat transfer. For example, heating CCM5 preparations in a 2-m-long double pipe heat exchanger between 40 and ~73 °C, assuming a 15 Lh−1 flowrate, is predicted to heat the milk 1 °C less than unconcentrated milk preparations. While the thermal conductivity of the UF preparations is higher than that of the existing 56% double-fat cream product, the impact for the reduced heat transfer should be considered, together with changes in viscosity, when optimizing processing of CCM milk preparations.
The UF concentrated milk preparations produced using the bench scale filtration unit allow the effect of milk concentration (CCM1, CCM2.5, and CCM5) to be examined in the early stages of cream cheese production, when an acid gel is formed. This study next focused on the production of these acid gels and characterization of their fundamental physico-chemical properties.
Acid Gel Formation
An acid gel was formed from each of the CCM1, CCM2.5, and CCM5 milk preparations by fermentation, generating a key intermediate within the production of cream cheese. The UF retentate in the CCM2.5 and CCM5 increased the buffering capacity of the milk, and these preparations took ~1.8 and ~3 times longer to reach pH 4.6 (i.e., greater TpH4.6; Fig. 2) using the same starter concentration (0.05% w/v; Table 2). Milk proteins, soluble minerals, and colloidal calcium phosphate (CCP) are the main components affecting the buffering capacity of milk (Salaün et al., 2005). Though a large proportion of soluble minerals is lost in the permeate, the retention of milk proteins and CCP leads to a greater buffering capacity in the UF retentate compared with the unconcentrated skim milk (Salaün et al., 2005).
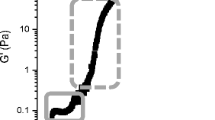
Changes in pH (
 ) and storage modulus (G′) (
) and storage modulus (G′) (
 ) as a function of time during the acidification of CCM1 (a), CCM2.5 (b), and CCM5 (c), where all samples had a starter culture concentration of 0.05% (w/v). The graphs of samples with 0.1% (w/v) and 0.2% (w/v) starter culture can be found in the SI Figs. 2 and 3. Curves are representative of three separate experiments
) as a function of time during the acidification of CCM1 (a), CCM2.5 (b), and CCM5 (c), where all samples had a starter culture concentration of 0.05% (w/v). The graphs of samples with 0.1% (w/v) and 0.2% (w/v) starter culture can be found in the SI Figs. 2 and 3. Curves are representative of three separate experiments
The UF retentate also significantly increased the storage modulus of the gel, G′pH4.6, which increased ~ 5-fold for CCM2.5 and ~10-fold for CCM5 (Fig. 2; P < 0.01, Table 2). This increase can be attributed to increased interactions between particles in the network (Lucey et al., 1997) due to the higher concentration of both protein, fat, and total solids in these acid gels (Table 1). These observations confirm that the concentrated cream cheese milk studied here has an increase in storage modulus like other rennet-coagulated UF concentrates of varying composition in previous studies (Dussault-Chouinard et al., 2019).
The time taken for the onset of gelation, Tgel, was not significantly different between milk preparations with the same starter concentration despite the difference in total solids (Fig. 2, P > 0.05, Table 2), perhaps due to the high variability in Tgel observed for some concentrated samples. The pH at the onset of gelation (pHgel) for CCM1 was ~5.2, close to the value obtained for acidified skim milk (pHgel ~5.30) (Kristo et al., 2011); this pH increased to ~5.5–5.7 for the UF cheese milk (Table 2), although the variability was relatively large. The most significant difference in pHgel was observed between CCM5 (with 0.05% (w/v) starter culture) and CCM1. The large variability of Tgel, as well as pHgel, for UF treatments is likely a result of the lower volume of milk and starter culture used at small scale.
The local maximum for the loss tangent (tan δmax) at ~ pH 4.8–5.0, observed in all milk preparations (Table 2), was lower for CCM acid gels prepared from UF retentate compared to CCM1 (Table 2). This difference suggests the rearrangement of casein in the UF-concentrated milk preparations during gelation may be less than in the control, as the presence of tan δmax has been ascribed to the loosening of casein-casein bonds during solubilization of colloidal calcium phosphate (CCP) in yogurt (Lee & Lucey, 2004) and may indicate differences in casein interactions in the acid gels studied here, although the higher number of protein and fat particles may also play a role. The pH at which the tan δmax occurred was similar (Table 2).
The effect of the starter culture concentration (0.05% (w/v), 0.1% (w/v), or 0.2% (w/v)) on acidification and gel properties was examined to determine whether a higher concentration of starter culture could be used to speed the rate of acidification without altering the stiffness of the gel. An increase in starter culture concentration from 0.05% (w/v) to 0.2% (w/v) significantly decreased Tgel for all milk preparations, approximately halving the time for gel formation (P < 0.05, Table 2). This was coincident with a decrease in the time taken for the milk to reach a pH of 4.6 by ~50–60% (Table 2). Despite these changes, the faster acidification did not alter the stiffness, or G′pH 4.6, of the gel. This is in contrast to studies of skim milk yoghurt gels, where faster acidification (4% cf 0.5% (w/w) starter culture) led to a stiffer gel network (Lee & Lucey, 2004). Unlike the yoghurt gels, fat is a key determinant of gel stiffness in the acid gels formed here. The presence of high concentrations of fat (~13–31%; Table 1) in the gel network likely limits the influence of starter culture concentration on the rearrangement of casein here. As expected, pHgel was not altered, as this is known to depend on the neutralization of net repulsive charge on casein micelles, which is not typically affected by different concentrations of starter culture.
These experiments show that concentrated cream cheese milk (CCM2.5 and CCM5) can be used to make acid gels with a higher storage modulus (G′pH4.6). While such gels are slower to reach pH 4.6, the productivity of the acidification process can be increased by using a higher concentration of starter culture, without significantly affecting the stiffness, or G′pH4.6, of the gel.
Acid Gel Firmness and Viscosity
The firmness of the acid gels formed by the CCM1, CCM2.5, and CCM5 milk preparations after fermentation was further assessed using a penetration test. This test involves large deformations leading to gel disruption, imitating the forces involved in the handling of gels during food processing (Angioloni & Collar, 2009).
UF concentration significantly increased gel firmness (P < 0.01), as shown in Fig. 3a, resulting in a ~4- and 8-fold increase in gel firmness for CCM2.5 and CCM5, respectively. This elevated firmness can again be attributed to the higher concentration of protein, fat, and total solids in these gels (Table 1), making these structures more resistant to physical deformation. A higher concentration of starter culture did not alter the gel firmness (P > 0.05, Fig. 3a), consistent with the similar G′pH4.6 measured for these gels (Table 2). The consistency between gel textural properties measured by penetration and small deformation (G′pH4.6), as in other studies (Angioloni & Collar, 2009) also indicates both methods are effective for monitoring gel strength for intermediates formed during cream cheese production.
Acid gel properties for CCM1, CCM2.5 and CCM5 acid gels. Gel firmness as a function of skim milk concentration factor in cream cheese acid gels inoculated with 0.05% (w/v) (
 ), 0.1% (w/v) (◇) and 2% (w/v) (
), 0.1% (w/v) (◇) and 2% (w/v) (
 ) starter culture (a). The viscosity of CCM1 (
) starter culture (a). The viscosity of CCM1 (
 ), CCM2.5 (
), CCM2.5 (
 ) and CCM5 (
) and CCM5 (
 ) acid gels as a function of temperature (b) and shear rate (c). All samples had a starter culture concentration of 0.05% (w/v). Error bars represent the standard deviation of the mean (n = 3)
) acid gels as a function of temperature (b) and shear rate (c). All samples had a starter culture concentration of 0.05% (w/v). Error bars represent the standard deviation of the mean (n = 3)
The viscosity of the acid gels was measured as a function of temperature and a shear rate as heat treatment and shear forces are essential components of effective acid whey separation during cream cheese production (Ong et al., 2018). All three acid gels displayed similar temperature sweep curves, although the complex viscosity was higher for the more concentrated preparations CCM2.5 and CCM5 (~4- and 8-fold higher at 70 °C), where the greater number of molecules is expected to lead to increased molecular interaction and a more viscous gel (Fig. 3b). The curves contained a number of features, which were most evident for CCM5, where the solids content was highest. These were a decline in viscosity with increasing temperature due to increased hydrophobic interactions and decreased casein voluminosity, which are thought to lead to whey expulsion (Lucey et al., 1997), a drop in viscosity at ~40 °C due to fat liquefaction and a slight increase in viscosity at temperatures > 75 °C due to the formation of denatured whey protein clusters and whey-casein aggregates, which increase interactions within the gel (Lucey et al., 1999). These features indicate that a standard heating temperature of ~70 °C can potentially be used for whey separation for these acid gels, as the fat will be liquid and viscosity will be relatively low.
All CCM acid gels displayed shear-thinning behavior when the flow properties were evaluated at 70 °C (Fig. 3c). As expected, the gels formed from CCM2.5 and CCM5 UF acid gel were more viscous. The viscosity of the CCM1 acid gels was ~10-fold lower than observed previously, possibly due to different processing conditions (e.g., formulation and homogenization pressures) (Ong et al., 2018). The difference in viscosity between acid gels is expected to alter the flow properties of the acid gels; while all are expected to have laminar flow, the Reynolds numbers are estimated to be ~1124, ~393, and ~137, respectively (assuming a flow rate of 1350 L h−1 and a diameter of 5 cm) (Madlener et al., 2009). An elevated viscosity can also increase fouling during heat treatment, as observed previously in other milk preparations with added carrageenan (Prakash et al., 2010).
Acid Whey Analysis
The volume and composition of acid whey generated from the acid gels were assessed next, as reduced production of acid whey is a potential advantage of acid gels made from UF-concentrated milk and high-fat cream. The concentrated gels CCM2.5 and CCM5 produced ~40% and ~61% less acid whey, respectively (Fig. 4a), showing the potential advantage of these preparations. Further optimization of the centrifugation conditions, including speed and duration, may be required to achieve the ideal moisture content for further processing to make cream cheese. Using standard conditions, the CCM5 gels generated ~22% acid whey, which is higher than the target value of ~7–16% (Brighenti et al., 2018; Ong et al., 2018), suggesting a shorter centrifugation or lower speed will be optimal. Concentrated gels made with a higher concentration of starter culture produced a similar volume of whey, consistent with their similar mechanical properties (Fig. 4a, Table 2, and Fig. 3). Previously, a higher degree of spontaneous whey separation has been observed in yogurt with a lower concentration of starter culture (1.93% w/w vs. 1.69% w/w whey separation for 0.5% w/w and 4% w/w starter culture, respectively (Lee & Lucey, 2004)), but the solid concentrations were significantly lower than those tested here (10.7% vs. ~20–42% here), potentially explaining these differences.
The percentage of acid whey (a) and the protein (b) and fat (c) content in acid whey as a function of skim milk concentration factor in cream cheese acid gels inoculated with a concentration of 0.05% (w/v) (
 ), 0.1% (w/v) (◇), and 2% (w/v) (
), 0.1% (w/v) (◇), and 2% (w/v) (
 ) starter culture. Error bars represent the standard deviation of the mean (n = 3). SDS-PAGE of acid whey separated from CCM1, CCM2.5, and CCM5 acid gels (d). Lanes 1, 4, and 7: 0.05% (w/v) starter inoculation concentration. Lanes 2, 5, and 8: 0.1% (w/v) starter inoculation concentration. Lanes 3, 6, and 9: 0.2% (w/v) starter inoculation concentration. α-LA: α-lactalbumin, β-LG: β-lactoglobulin
) starter culture. Error bars represent the standard deviation of the mean (n = 3). SDS-PAGE of acid whey separated from CCM1, CCM2.5, and CCM5 acid gels (d). Lanes 1, 4, and 7: 0.05% (w/v) starter inoculation concentration. Lanes 2, 5, and 8: 0.1% (w/v) starter inoculation concentration. Lanes 3, 6, and 9: 0.2% (w/v) starter inoculation concentration. α-LA: α-lactalbumin, β-LG: β-lactoglobulin
A higher concentration of protein was present in the whey produced from the concentrated gels, with ~2-fold and ~4-fold greater protein occurring for the CCM2.5 and CCM5 acid gels, respectively (Fig. 4b), reflecting the higher protein concentrations in these preparations (Table 1). Whey protein α-lactalbumin (α-LA) increased 1.4-fold and 2.1-fold, respectively, and β-lactoglobulin (β-LG) 1.6-fold and 2.1-fold, respectively, as measured by SDS-PAGE densitometric analysis (Fig. 4d). The relative intensities of β-LG and α-LA (~2.9:1) reflected the proportions found in bovine milk (Ng-Kwai-Hang & Kroeker, 1984) and were similar for acid whey expelled from all preparations. A higher concentration of fat was also present in the acid whey separated from CCM2.5 and CCM5 acid gels, reflecting the higher concentration of fat in these concentrated samples (Fig. 4c, Table 1). The total protein and fat loss to the acid whey were estimated to be ~0.15 g per 100 g cheese milk for CCM1; this value was slightly lower at ~0.12 g per 100 g of cheese milk for UF treatments; the gels made from UF-concentrated milk had a higher concentration of protein and fat but a lower volume of acid whey, leading to similar levels of overall loss. It is also worth noting that the concentration of whey proteins in the acid whey is relatively low compared to the concentration of whey protein in unconcentrated milk or UF retentate, where whey proteins account for ~20% of the total protein. This very low whey protein content is likely the result of multiple heat treatments made during the cream cheese processing, e.g., homogenization, batch pasteurization, and heating of acid gel, which lead to the whey protein being captured within the acid gel. The reduction of acid whey expulsion in the production of UF-concentrated acid gels for cream cheese offers potentially valuable environmental benefits, as this can reduce water and energy consumption and emissions associated with whey treatment.
Microstructure Analysis of Cream Cheese Acid Gel
The microstructure of the acid gels prepared from CCM1, CCM2.5, and CCM5 was assessed by CLSM (Fig. 5), as this technique can provide key insights into the formation of gel structure, which can influence gel properties. The structure of the CCM2.5 and CCM5 gels, including the protein network, was dense. CCM5 contained the densest protein and fat network with the fewest pores, while the CCM1 control was less dense and more porous, as shown in the 2D gel cross-sections (Fig. 5a–c), where the pores appear as dark unstained areas. These structural differences can be attributed to the higher concentration of protein, fat, and total solids in UF-concentrated samples (Table 1), noted also in the greater number of particles in Fig. 1, which also led to higher G′pH4.6 and gel firmness (Table 2, Fig. 3). Similar differences in the structure were also observed for the CCM samples in the 3D cross-sections reconstructed from imaging deeper into each acid gel sample (Fig. 5d–f).
Representative 2D (a–c) and 3D reconstructed (d–f) CLSM images of acid gels made from CCM1 (a, d), CCM2.5 (b, e), and CCM5 (c, f), where the starter culture concentration was 0.05% (w/v) for all samples. Fat stained by Nile red appears red, and protein stained by Fast Green FCF appears green. Scale bars are 10 µm in length. CCM1, CCM2.5, and CCM5 represent fat standardized cream cheese milk prepared from skim milk, 2.5- and 5-fold UF retentate, respectively. White arrows indicate serum pores, and the white circles indicate fat globules
Fat particles appeared integrated within the protein network, a characteristic of the acid gels formed during cream cheese production (Ong et al., 2018, 2020). One notable difference between samples was the large fat particles also visible within the CCM5 gel (Fig. 5c), which were not commonly present in the other gels nor observed in the CCM5 milk preparation (Fig. 1b–d) where protein and fat clusters were visible. These large droplets may form in the concentrated milk preparations during cold storage or during pasteurization. The formation of fat crystals during cold storage can promote coalescence, as these crystals can impair the interfacial layer surrounding the fat (Lopez et al., 2007). Fusion of fat droplets could also occur during pasteurization, as the casein pesudomembranes could be disrupted when preparations are heated (65 °C, 30 min) (Darling & Butcher, 1978). Acid fermentation destabilizes casein proteins that can coat fat droplets, but this process is less likely to lead to fat coalescence, as the fermentation process is relatively static, and the chances of rupture of the fat globule membranes are minimal. This hypothesis is supported by the findings by Lopez et al. (2017), who found the size of the fat droplets of the milk emulsion (20% fat (w/w), pH 6.7) did not change after acidification to pH 4.6 in ~12 h.
Stereology was used to estimate the particle size of fat droplets in acid gels from 2D images using ImageJ (Fig. 6). This method was selected due to the close-packed nature of the fat within the concentrated UF acid gels, which makes the separation of fat droplets by segmentation difficult to perform when using 3D images. While the distribution of the fat droplet sizes determined by ImageJ analysis of 2D images was similar for the three acid gels (Fig. 6a), larger droplets were also detected in CCM5 samples (Fig. 6a insert). The majority of fat droplets were estimated to be 0.5–10 µm in diameter, but more droplets with 5–13 µm in diameter were found in CCM5, consistent with visual observation of the size of these droplets in CLSM images (Fig. 5). The D[4, 3] diameter also increased from ~2.2 µm for CCM1 and CCM2.5 gels to ~3.2 µm for CCM 5 samples (P < 0.05). These data suggest that the aggregation and the coalescence of fat were greater during the preparation of the high-fat CCM5 acid gel. This hypothesis is supported by previous observations of renneted coagulated UF retentate, where a high-fat content leads to a gel with larger fat globules (Ong et al., 2010).
Particle size distribution obtained from image analysis of 2D CLSM images of CCM1 (
 ), CCM2.5 (
), CCM2.5 (
 ), and CCM5 (
), and CCM5 (
 ) acid gels where the starter culture concentration was 0.05% (w/v) for all samples (a). The insert highlights the volume density differences between fat droplets for CCM1, CCM2.5, and CCM5 gels within the size range of 5–13 µm. The percentage of fat (b) and protein (c) on a volume basis and the porosity of CCM acid gels (d) as a function of skim milk concentration factor obtained from the analysis of 3D CLSM images. CCM1, CCM2.5, and CCM5 represent fat standardized cream cheese milk prepared from skim milk, 2.5- and 5-fold UF retentate, respectively
) acid gels where the starter culture concentration was 0.05% (w/v) for all samples (a). The insert highlights the volume density differences between fat droplets for CCM1, CCM2.5, and CCM5 gels within the size range of 5–13 µm. The percentage of fat (b) and protein (c) on a volume basis and the porosity of CCM acid gels (d) as a function of skim milk concentration factor obtained from the analysis of 3D CLSM images. CCM1, CCM2.5, and CCM5 represent fat standardized cream cheese milk prepared from skim milk, 2.5- and 5-fold UF retentate, respectively
The 3D CLSM images were used to assess the volume percentage of fat and protein, which increased significantly as a function of UF concentration, with CCM2.5 having a higher volume than CCM1 and CCM5 having the highest volume for these components (P < 0.01, Fig. 6b and c), with an approximate 2-fold increase across the concentration range. Conversely, porosity decreased significantly with UF concentration (P < 0.01, Fig. 6d), with an approximate 2-fold decrease across the concentration range. There was a correlation between the volume of fat measured on the basis of dry weight for CCM samples and the volume determined by image analysis (SI Fig. 4, 0.896). The volume of protein also correlated well with protein concentration (SI Fig. 4, 0.934), but the imaged volume of the protein phase was ~6–7 times greater than the actual measured protein concentration, which could be attributed to the hydration of the milk proteins. This illustrates the limitation of applying image analysis to determine composition, although analysis of fat is more reliable. In another study on the agarose-canola oil gels, with much lower oil concentrations (1–11%), the volume of the canola oil phase analyzed by image analysis also closely matched the experimental concentration (Mhaske et al., 2019) and the distinct boundary between fat and water components may allow better assessment of fat volume.
NMR Relaxation Times and Self-Diffusion Coefficient of the Water Component
The mobility of water within the CCM gels was assessed by proton NMR relaxometry, as this method can provide further insight into the microstructure of the gels, as the relationship between volume concentration factor, or solid content and the diffusion of water within such gels is not completely understood. Two characteristic NMR parameters of a water component, the T1 (longitudinal relaxation time) and T2 (transverse relaxation time), can be readily measured, and their values strongly correlate with water mobility (Roefs & Vliet, 1989). Generally, longer 1H T1 or T2 times correspond to greater water mobility (Kuo et al., 2001).
The proton NMR spectra of cream cheese acid gels used in the present study are shown in Fig. 7a–c. The intensity of a water signal at 4.94–4.98 ppm decreased, while the intensity of the acyl chains of the fat between 0.94 and 2.34 ppm increased, as the volume concentration factor increased in CCM1 to CCM5 acid gels. These spectral characteristics are consistent with visual and quantitative observations of solid density in CLSM images (Figs. 5 and 6).
The mobility of water in cream cheese acid gels. 1H NMR spectra of cream cheese acid gels CCM1 (c), CCM2.5 (b), and CCM5 (a). The insert in (c) shows a signal arising from the acyl chains of fat. The T1 (d), T2 (e) relaxation time and water self-diffusion coefficient Dw (f) of the water component in cream cheese acid gels as a function of skim milk concentration factor. Values are expressed as the mean ± the standard deviation (n = 2)
A decrease of 1H water relaxation time was observed in CCM acid gels as a function of volume concentration factor (Fig. 7 d and e) or as a function of total solid content between 20 and 42.5% (SI Fig. 5), a trend that was most apparent for T2. This observation extends our understanding of water mobility in acid dairy gels across a wider range of solid contents with lower mobility occurring for acid gels from more concentrated preparations. Previously, T1 values have been found to decrease from 1.273 s to 0.936 s (measured at 25 °C at a low field strength of 42.5 MHz), when fat and nonfat milk solids were added to increase solids in the manufacture of yogurt (Yu et al., 2016). The trend was consistent here, but the values of T1 were higher, which could be due to the lower protein content in the gels and higher field strength, 600 MHz, used in this study.
A slight deviation from a monoexponential decay was observed for T2 here, possibly reflecting the presence of different forms of water (e.g., free water and bound water) in milk gels, as reported previously by Tellier et al. (1993). This previous study also observed a single T2 in renneted skim milk, as occurred here, which is likely the result of the fast exchange between different proton components in the gels. The decrease in T2 as a function of the solid content also indicates water mobility decreases in CCM2.5 and CCM5 acid gels.
The self-diffusion coefficient of water (Dw) was measured as a further measure of the state of a water component within the three acid gels. As expected, the Dw of cream cheese acid gels (Fig. 7f) was lower than that of free water (2.09 × 10−9 m2 s−1 at 20 °C), reflecting reduced diffusion in all gels compared to free water. The value of Dw also decreased with the concentration factor, exhibiting a linear decay with solid content (SI Fig. 5), indicating reduced mobility in the acid gels made from UF retentate. This decrease accorded with the observation of the microstructure, where fewer and smaller serum pores were present in the UF acid gels, in which water molecule movement was restricted (Fig. 5).
The Dw data reported in this study also suggest that the relationship between Dw and solid content may not be linear for the entire range of solid concentrations. Here, the intercept of the linear fit with y-axis (solid content at 0, SI Fig. 5) gives a Dw of 1.87 × 10−9 m2 s−1, which is lower than that of free water. This suggests the linearity observed might not extend to very low concentrations of solids. This linearity may be valid, however, for dairy products with solids between 3–50% based on observations for milk protein mixtures and cream cheese that have displayed lower water self-diffusion with higher dry matter (Métais et al., 2004; Møller et al., 2012). Dry matter is also known to be the main factor affecting diffusion in cream cheese (Møller et al. (2012). The value of Dw of CCM acid gels observed here is higher than observed previously for cream cheese with a similar solid content, which has been reported to vary between ~0.85 and 1.1 × 10−9 m2 s−1 at 25 °C (Møller et al., 2012). A further decrease in water mobility can, therefore, be expected, as these acid gels are further processed into the final cream cheese product, where factors such as the presence of food hydrocolloids lower Dw.
Obstruction, where large molecules reduce diffusion, may be the primary factor reducing the movement of water in the UF acid gels observed here. Obstruction is one of the two factors proposed by Mariette et al. (2002) to influence water diffusion in dairy dispersions and gels. Hydration, which influences water-protein interactions, can also restrict water movement, reducing diffusion and lowering Dw. The changes in Dw observed here cannot be attributed to the effect of hydration alone, however, as the values of Dw (1.0–1.4 × 10−9 m2 s−1) are below those observed (~2.1–1.9 × 10−9 m2 s−1 at 25 °C) for casein dispersions with a similar protein content to the acid gels examined here (3–7%) (Table 1) (Gottwald et al., 2005; Mariette et al., 2002) where the protein is expected to be similarly hydrated. The close packing of protein and fat aggregates within the acid gels (Fig. 5) will lead to obstruction. The addition of fat, for instance, can change Dw from ~1.3 × 10−9 m2 s−1 to ~0.8 × 10−9 m2 s−1 (at 20 °C) when the solid content is increased from ~17% in fat-free UF retentate to 51% in fat-containing counterparts (Métais et al., 2004), illustrating the impact of increased solid content.
The estimated root mean square displacement, (2Dw Δ)1/2, is ~14–17 µm for the diffusion time (Δ) of 100 ms used in the present study, which is larger than the pore size of the gels observed from the 2D CLSM images (Fig. 5). This suggests the water molecules are not restricted to move within a single serum pore but are able to move through the channels and interconnected serum pores that are expected to be present within the 3D gel network. The significance of this finding is that this particular structure could facilitate the transport of water and other aqueous solutes (e.g., lactose and lactic acid) throughout the gel matrix, which is critical for acid whey separation and could also impact sensory properties of the final cheese.
Differences in Milk and Acid Gel Structure Formed from Skim Milk or Addition of UF Retentate
A schematic illustration (Fig. 8) highlights the differences introduced to the structure of acid-coagulated gels with addition of UF retentate. Cream cheese milk is formulated by standardizing skim milk or retentate with cream to a protein to a fat ratio of ~0.21–0.24 (Fig. 8a and d). When UF retentate is used, there is a higher concentration of fat and protein (Fig. 8d). Homogenization is equally effective, and clusters of protein and fat can be observed in all cream cheese milk samples (Fig. 8b and e). After fermentation, the acid gel produced from UF retentate shows a denser structure, with fewer serum pores and a lower porosity (Fig. 8f). This leads to a reduced diffusion coefficient for water, as the dense structure of interconnected fat and protein particles obstructs passage (Fig. 8f), although water molecules are still able to pass through the serum pores. In contrast, the acid gels made from skim milk have a porous protein-fat network structure (Fig. 8c), where water diffuses faster than in the UF acid gel but is reduced relative to a casein dispersion. The UF acid gel also displays noticeably larger fat globules (Fig. 8f), which is attributed to the higher concentration of fat and greater coalescence of fat during processing. These differences in the microstructure (Fig. 8), together with the higher storage modulus of the acid gels (Fig. 2), may also alter the subsequent processing steps and the final texture of the cream cheese. While the use of a bench scale membrane filtration unit limited the volume of milk concentrate produced in the current study, future studies at a larger pilot scale could seek to examine the link between the intermediate stages of acid gel formation and the structure of the final cream cheese product.
Conclusion
This study highlights how the addition of UF retentate influences the behavior of milk preparations used for cream cheese making. While homogenization was effective with 2.5× and 5× concentrated milk and similar characteristic homogenization clusters were observed in all preparations, milk viscosity increased with the use of UF retentate. Changes to the buffering capacity reduced the rates of acidification, but this could be addressed by increasing starter culture concentration. Ingredient assembly produced characteristic protein networks, in which the fat was well integrated. The increased number of protein and fat particles from the solids in UF cheese milk led to a denser protein and fat network, where greater bonding between protein and fat is expected to occur. There was a greater concentration of protein in these acid gels (~1.7- to 2.5-fold), greater volume of protein and fat by image analysis (~2-fold) and a significant reduction in porosity (~2-fold). The increase in solids within UF acid gels, at the expense of water, reduced diffusion. Water molecules were still able to move between interconnected serum pores but experienced some obstruction in UF acid gels. These physicochemical changes in gel density led to changes in physical properties, including an increase in gel storage modulus (up to ~10-fold), gel firmness (up to ~ 8-fold), and viscosity (up to ~ 8-fold at 70 °C). In general, addition of UF retentate is expected to lower thermal conductivity, increase milk and acid gel viscosity, and increase the propensity for fouling. Small process changes may, therefore, be needed at either pilot or manufacturing scale to optimize heat exchange, pumping, and cleaning during cream cheese production. These insights increase our understanding of how addition of UF retentate alters the structure and properties of acid gels will assist the future development and optimization of cream cheese processes for reduced production of acid whey.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Angioloni, A., & Collar, C. (2009). Small and large deformation viscoelastic behaviour of selected fibre blends with gelling properties. Food Hydrocolloids, 23(3), 742–748. https://doi.org/10.1016/j.foodhyd.2008.04.005
Bienvenue, A., Jiménez-Flores, R., & Singh, H. (2003). Rheological properties of concentrated skim milk: Importance of soluble minerals in the changes in viscosity during storage. Journal of Dairy Science, 86(12), 3813–3821. https://doi.org/10.3168/jds.S0022-0302(03)73988-5
Brighenti, M., Govindasamy-Lucey, S., Jaeggi, J., Johnson, M., & Lucey, J. (2018). Effects of processing conditions on the texture and rheological properties of model acid gels and cream cheese. Journal of Dairy Science, 101(8), 6762–6775. https://doi.org/10.3168/jds.2018-14391
Covacevich, H., & Kosikowski, F. (1977). Cream cheese by ultrafiltration. Journal of Food Science, 42(5), 1362–1364. https://doi.org/10.1111/j.1365-2621.1977.tb14498.x
Darling, D. F., & Butcher, D. W. (1978). Milk-fat globule membrane in homogenized cream. Journal of Dairy Research, 45(2), 197–208. https://doi.org/10.1017/S002202990001637X
Dussault-Chouinard, I., Britten, M., & Pouliot, Y. (2019). Improving rennet coagulation and cheesemaking properties of reverse osmosis skim milk concentrates by pH adjustment. International Dairy Journal, 95, 6–14. https://doi.org/10.1016/j.idairyj.2019.03.008
Erdem, Y. K. (2005). Effect of ultrafiltration, fat reduction and salting on textural properties of white brined cheese. Journal of Food Engineering, 71(4), 366–372. https://doi.org/10.1016/j.jfoodeng.2004.10.040
Fullman, R. L. (1953). Measurement of particle sizes in opaque bodies. JOM, 5, 447–452. https://doi.org/10.1007/BF03398971
Gottwald, A., Creamer, L. K., Hubbard, P. L., & Callaghan, P. T. (2005). Diffusion, relaxation, and chemical exchange in casein gels: A nuclear magnetic resonance study. The Journal of Chemical Physics, 122(3), 034506. https://doi.org/10.1063/1.1825383
Govindasamy-Lucey, S., Jaeggi, J., Johnson, M., Wang, T., & Lucey, J. (2005). Use of cold ultrafiltered retentates for standardization of milks for pizza cheese: Impact on yield and functionality. International Dairy Journal, 15(6–9), 941–955. https://doi.org/10.1016/j.idairyj.2004.08.017
Green, M. L. (1987). Effect of manipulation of milk composition and curd-forming conditions on the formation, structure and properties of milk curd. Journal of Dairy Research, 54(2), 303–313. https://doi.org/10.1017/S0022029900025449
Gupta, V. K. (2016). Quality of plain set yoghurt as affected by levels of ultrafiltration concentration of milk and inoculum of yoghurt culture. Turkish Journal of Agriculture-Food Science and Technology, 4(6), 508–514. https://doi.org/10.24925/turjaf.v4i6.508-514.685
Koh, L., Ashokkumar, M., & Kentish, S. (2013). Membrane fouling, cleaning and disinfection. In A. Y. Tamime (Ed.), Membrane processing: Dairy and beverage applications (pp. 73–106). Blackwell Publishing Ltd.
Kowalski, S. M., Parker, P. A., & Geoffrey Vining, G. (2007). Tutorial: Industrial split-plot experiments. Quality Engineering, 19(1), 1–15. https://doi.org/10.1080/08982110601057179
Kristo, E., Miao, Z., & Corredig, M. (2011). The role of exopolysaccharide produced by Lactococcus lactis subsp. cremoris in structure formation and recovery of acid milk gels. International Dairy Journal, 21(9), 656–662. https://doi.org/10.1016/j.idairyj.2011.02.002
Kuo, M. -I., Gunasekaran, S., Johnson, M., & Chen, C. (2001). Nuclear magnetic resonance study of water mobility in pasta filata and non-pasta filata Mozzarella. Journal of Dairy Science, 84(9), 1950–1958. https://doi.org/10.3168/jds.S0022-0302(01)74637-1
Lee, W. J., & Lucey, J. A. (2004). Structure and physical properties of yogurt gels: Effect of inoculation rate and incubation temperature. Journal of Dairy Science, 87(10), 3153–3164. https://doi.org/10.3168/jds.S0022-0302(04)73450-5
Lopez, C., Camier, B., & Gassi, J. -Y. (2007). Development of the milk fat microstructure during the manufacture and ripening of Emmental cheese observed by confocal laser scanning microscopy. International Dairy Journal, 17(3), 235–247. https://doi.org/10.1016/j.idairyj.2005.12.015
Lopez, C., Cauty, C., Rousseau, F., Blot, M., Margolis, A., & Famelart, M. -H. (2017). Lipid droplets coated with milk fat globule membrane fragments: Microstructure and functional properties as a function of pH. Food Research International, 91, 26–37. https://doi.org/10.1016/j.foodres.2016.11.025
Lucey, J., Munro, P., & Singh, H. (1999). Effects of heat treatment and whey protein addition on the rheological properties and structure of acid skim milk gels. International Dairy Journal, 9(3–6), 275–279. https://doi.org/10.1016/S0958-6946(99)00074-6
Lucey, J., Van Vliet, T., Grolle, K., Geurts, T., & Walstra, P. (1997). Properties of acid casein gels made by acidification with glucono-δ-lactone. 1. Rheological properties. International Dairy Journal, 7(6–7), 381–388. https://doi.org/10.1016/S0958-6946(97)00027-7
Luo, X., Ramchandran, L., & Vasiljevic, T. (2015). Lower ultrafiltration temperature improves membrane performance and emulsifying properties of milk protein concentrates. Dairy Science & Technology, 95, 15–31. https://doi.org/10.1007/s13594-014-0192-3
Madlener, K., Frey, B., & Ciezki, H. (2009). Generalized reynolds number for non-newtonian fluids. Progress in Propulsion Physics, 1, 237–250. https://doi.org/10.1051/eucass/200901237
Marella, C., Salunke, P., Biswas, A., Kommineni, A., & Metzger, L. (2015). Manufacture of modified milk protein concentrate utilizing injection of carbon dioxide. Journal of Dairy Science, 98(6), 3577–3589. https://doi.org/10.3168/jds.2014-8946
Mariette, F., Topgaard, D., Jönsson, B., & Soderman, O. (2002). 1H NMR diffusometry study of water in casein dispersions and gels. Journal of Agricultural and Food Chemistry, 50(15), 4295–4302. https://doi.org/10.1021/jf0115948
Métais, A., Cambert, M., Riaublanc, A., & Mariette, F. (2004). Effects of casein and fat content on water self-diffusion coefficients in casein systems: A pulsed field gradient nuclear magnetic resonance study. Journal of Agricultural and Food Chemistry, 52(12), 3988–3995. https://doi.org/10.1021/jf035383+
Mezger, T. G. (2014). Rotational tests. The Rheology Handbook (4th ed., pp. 33–85). Vincentz Network.
Mhaske, P., Condict, L., Dokouhaki, M., Katopo, L., & Kasapis, S. (2019). Quantitative analysis of the phase volume of agarose-canola oil gels in comparison to blending law predictions using 3D imaging based on confocal laser scanning microscopy. Food Research International, 125, 108529. https://doi.org/10.1016/j.foodres.2019.108529
Michalski, M. -C., Briard, V., & Michel, F. (2001). Optical parameters of milk fat globules for laser light scattering measurements. Le Lait, 81(6), 787–796. https://doi.org/10.1051/lait:2001105
Mistry, V. V., & Maubois, J. L. (2017). Application of membrane separation technology to cheese production. In P. L. H. McSweeney, P. F. Fox, P. D. Cotter, & D. W. Everett (Eds.), Cheese: chemistry, physics and microbiology (4th ed., pp. 677–697). Elsevier.
Møller, S. M., Hansen, T. B., Andersen, U., Lillevang, S. K., Rasmussen, A., & Bertram, H. C. (2012). Water properties in cream cheeses with variations in pH, fat, and salt content and correlation to microbial survival. Journal of Agricultural and Food Chemistry, 60(7), 1635–1644. https://doi.org/10.1021/jf204371v
Munir, M., Zhang, Y., Yu, W., Wilson, D., & Young, B. (2016). Virtual milk for modelling and simulation of dairy processes. Journal of Dairy Science, 99(5), 3380–3395. https://doi.org/10.3168/jds.2015-10449
Ng-Kwai-Hang, K., & Kroeker, E. (1984). Rapid separation and quantification of major caseins and whey proteins of bovine milk by polyacrylamide gel electrophoresis. Journal of Dairy Science, 67(12), 3052–3056. https://doi.org/10.3168/jds.S0022-0302(84)81671-9
Nguyen, H. T. H., Ong, L., Lefèvre, C., Kentish, S. E., & Gras, S. L. (2014). The microstructure and physicochemical properties of probiotic buffalo yoghurt during fermentation and storage: A comparison with bovine yoghurt. Food and Bioprocess Technology, 7, 937–953. https://doi.org/10.1007/s11947-013-1082-z
Ogden, L. V., Walstra, P., & Morris, H. (1976). Homogenization-induced clustering of fat globules in cream and model systems. Journal of Dairy Science, 59(10), 1727–1737. https://doi.org/10.3168/jds.S0022-0302(76)84430-X
Ong, L., Dagastine, R., Kentish, S., & Gras, S. (2010). The effect of milk processing on the microstructure of the milk fat globule and rennet induced gel observed using confocal laser scanning microscopy. Journal of Food Science, 75(3), E135–E145. https://doi.org/10.1111/j.1750-3841.2010.01517.x
Ong, L., Dagastine, R., Kentish, S., & Gras, S. (2013). Microstructure and composition of full fat Cheddar cheese made with ultrafiltered milk retentate. Foods, 2(3), 310–331. https://doi.org/10.3390/foods2030310
Ong, L., Kentish, S. E., & Gras, S. L. (2018). Small scale production of cream cheese: A comparison of batch centrifugation and cloth bag methods. International Dairy Journal, 81, 42–52. https://doi.org/10.1016/j.idairyj.2018.01.008
Ong, L., Pax, A. P., Ong, A., Vongsvivut, J., Tobin, M. J., Kentish, S. E., & Gras, S. L. (2020). The effect of pH on the fat and protein within cream cheese and their influence on textural and rheological properties. Food Chemistry, 332, 127327. https://doi.org/10.1016/j.foodchem.2020.127327
Prakash, S., Huppertz, T., Karvchuk, O., & Deeth, H. (2010). Ultra-high-temperature processing of chocolate flavoured milk. Journal of Food Engineering, 96(2), 179–184. https://doi.org/10.1016/j.jfoodeng.2009.07.008
Roefs, S., & Vliet, T. V. (1989). Pulse NMR of casein dispersions. Journal of Food Science, 54(3), 704–708. https://doi.org/10.1111/j.1365-2621.1989.tb04686.x
Salaün, F., Mietton, B., & Gaucheron, F. (2005). Buffering capacity of dairy products. International Dairy Journal, 15(2), 95–109. https://doi.org/10.1016/j.idairyj.2004.06.007
Salhab, H. H. (1999). The application of ultrafiltration in the manufacture of cream cheese. ProQuest Dissertations & Theses Global. Publication Number 304466815, Master's thesis, University of Alberta.
Sparrow, E., Gorman, J., & Abraham, J. (2013). Quantitative assessment of the overall heat transfer coefficient U. Journal of Heat Transfer, 135(6), 061102. https://doi.org/10.1115/1.4023566
Srilaorkul, S., Ozimek, L., Wolfe, F., & Dziuba, J. (1989). The effect of ultrafiltration on physicochemical properties of retentate. Canadian Institute of Food Science and Technology Journal, 22(1), 56–62. https://doi.org/10.1016/S0315-5463(89)70302-3
Sterlitech Corporation. (2022). Membrane chemical cleaning: Why is it required and how is it performed? Retrieved November 15, 2022 from https://www.sterlitech.com/blog/post/membrane-chemical-cleaning
Tellier, C., Mariette, F., Guillement, J. P., & Marchal, P. (1993). Evolution of water proton nuclear magnetic relaxation during milk coagulation and syneresis: Structural implications. Journal of Agricultural and Food Chemistry, 41(12), 2259–2266. https://doi.org/10.1021/jf00036a007
Tomas, A., Paquet, D., Courthaudon, J. -L., & Lorient, D. (1994). Effect of fat and protein contents on droplet size and surface protein coverage in dairy emulsions. Journal of Dairy Science, 77(2), 413–417. https://doi.org/10.3168/jds.S0022-0302(94)76967-8
Yao, S., Meikle, T. G., Sethi, A., Separovic, F., Babon, J. J., & Keizer, D. W. (2018). Measuring translational diffusion of 15N-enriched biomolecules in complex solutions with a simplified 1H–15N HMQC-filtered BEST sequence. European Biophysics Journal, 47, 891–902. https://doi.org/10.1007/s00249-018-1311-5
Yu, H. Y., Wang, L., & McCarthy, K. L. (2016). Characterization of yogurts made with milk solids nonfat by rheological behavior and nuclear magnetic resonance spectroscopy. Journal of Food and Drug Analysis, 24(4), 804–812. https://doi.org/10.1016/j.jfda.2016.04.002
Acknowledgements
The authors thank Dr. Katherine Ganio and Dr. Swati Varshney from the Mass Spectrometry and Proteomics Facility at The Bio21 Molecular Science and Biotechnology Institute at The University of Melbourne for conducting the calcium analysis. The authors also thank the Advance Microscopy Facility (AMF), The Biological Optical Microscopy Platform (BOMP) at The Bio21 Molecular Science and Biotechnology Institute for access to equipment. Prof. Sally Gras and Dr. Lydia Ong are supported by The Dairy Innovation Hub.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
Qihui Wu: conceptualization, methodology, investigation, data curation, formal analysis, visualization, writing–original draft. Lydia Ong: conceptualization, methodology, validation, visualization, writing, review, editing, and supervision. Shenggen Yao: methodology, data curation, visualization, formal analysis, writing, review, and editing. Sandra Kentish: writing, review, editing, and supervision. Sally Gras: conceptualization, resources, validation, visualization, writing, review, editing, and supervision.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, Q., Ong, L., Yao, S. et al. Effect of Ultrafiltered Milk on the Rheological and Microstructure Properties of Cream Cheese Acid Gels. Food Bioprocess Technol 16, 1728–1745 (2023). https://doi.org/10.1007/s11947-022-02991-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-022-02991-1