Abstract
A modified atmosphere packaging (MAP) system in pallets was developed for ‘Padrón’ peppers as a way to extend their shelf-life while maintaining good fruit quality. Peppers were stored at 6 °C in cardboard boxes arranged on pallets wrapped in micro-perforated low-density polyethylene (LDPE) bags. Physico-chemical (moisture, firmness, color, chlorophylls, carotenoids, ascorbic acid, and total phenolic content) and sensory analysis were carried out after 0, 7, 14, and 21 days of storage. An initial mixture of 11.8% O2–8.5% CO2 prevented anaerobic conditions and kept suitable CO2 levels throughout the entire storage period. Silica gel was tested as a moisture absorbent and considerably reduced water vapor condensation inside packaging bags. The MAP system developed maintained the shelf-life of peppers until the end of the 21-day storage period. The fruit always showed a good appearance and color. No rotting or other types of undesirable alterations were observed. MAP markedly reduced the fraction of peppers with water loss as evidenced by a minimum percentage of fruit with wrinkles (12.5%) as compared to unpackaged samples (75%). Good pigment stability was also observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Of the more than 200 varieties, Capsicum annuum is one of the five main species of peppers (Hernández-Ortega et al., 2012) that belong to the genus Capsicum. The combination of color, flavor, and nutritional value of bell peppers has made them popular in recent years (Toledo-Martín et al., 2016). The pepper is a carotenogenic fruit, and during its maturation, the transformation of the chloroplast into chromoplast occurs. Chlorophylls disappear and carotenoids are formed; as a consequence, a considerable color change takes place (Bonaccorsi et al., 2016; Figeroa Cares et al., 2015; Hernández-Pérez et al., 2020). Peppers contain a wide variety of phytochemicals (phenolics, vitamin C, and carotenoids) with healthy effects for humans (Ozgur et al., 2011). In some studies carried out, the antimicrobial activity of peppers has been demonstrated (Gurnani et al., 2016; Wahba et al., 2010). In addition, high antioxidant activity and significant analgesic and anti-inflammatory properties were found in carotenoid extracts of dried guajillo chiles, which indicates that they might be beneficial in reducing inflammation and pain(Hernández-Ortega et al., 2012).
The bell pepper is a very perishable vegetable and therefore requires proper handling after harvest. The optimum storage temperature for peppers is 7 to 10 °C. Temperatures of 5 °C can be used in ripe red fruit (Escalona et al., 2019). The quality loss of bell peppers during their prolonged storage is mainly due to the development of rot, produced mostly by Botrytis cinerea and Alternaria alternata, chilling injury (CI), and rapid loss of water (Sakaldaş & Kaynaş, 2010). Botrytis can grow even when the recommended storage temperatures for peppers are used. Botrytis can be controlled using high levels of CO2 (> 10%) but damage to peppers can occur (Singh et al., 2014). Rhizopus stolonifer may also cause these peppers to rot, and the bacterium Erwinia carotovora (Escalona et al., 2019) may cause soft rot, another important type of decay.
Loss of water is responsible for wilting, shriveling, softening of tissues, and physiological disorders of fruits and vegetables. This is why the relative humidity (RH) of the air above the surface of the fruit should be maintained at 90–95%. In this way, the passage of water from the interior of the tissues, where RH of the air between the cells is close to 100%, towards the outside by the humidity gradient is reduced. The exposure of the pepper for prolonged periods under conditions of RH greater than 95% or the condensation of water on its surface can favor the growth of pathogenic organisms (Fornaris, 2005). CI is manifested with symptoms of depressions or sunken areas on the surface of the fruit (pitting), darkening of the calyx, discoloration of the seed cavity, browning of the seeds, weight loss, and greater susceptibility to fungal attacks such as Botrytis cinere (Escalona et al., 2019; Fornaris, 2005; Lim et al., 2007). Another symptom of CI is the presence of black rot at the stem end due to Alternaria (Singh et al., 2014).
Controlled atmosphere (CA) and modified atmosphere packaging (MAP) have been found to be rather suitable techniques that can be used to extend the shelf-life and maintain the quality of fresh-cut and fresh fruit and vegetables during their postharvest storage (Belay et al., 2018; Caleb et al., 2013; Esturk et al., 2012; Fan et al., 2021; Mangaraj et al., 2014; Muftuoǧlu et al., 2012; Saxena et al., 2013; Zhong et al., 2022).
Commodity respiration and gas permeation through the packaging film are two important components of the MAP system. In the passive MAP system, the initial concentrations of O2 and CO2 inside the package are the same as those in the outside atmosphere. Due to fruit respiration, O2 decreases and CO2 begins to accumulate because of O2 consumption and CO2 production in the metabolic process. This causes concentration gradients between the gases inside and outside the package which induce O2 entry as well as allow CO2 to exit through film. These processes continue until an equilibrium state is established in which the amounts of O2 entering the package and CO2 leaving the package equal the amount of O2 consumed and CO2 produced by the packaged fruit, respectively (Mangaraj et al., 2014). In the active MAP system, the process is similar, but a steady stage is reached more quickly. The interaction between the commodity respiration rate and the passage of gases through the packaging film results in an environment poorer in O2 and richer in CO2. The exposure of commodities to the environment developed inside a package as a consequence of the interaction of the external atmosphere, package, and product is known as MAP (Dwi Anggono et al., 2022). MAP creates an atmosphere of low O2 and high CO2 concentrations in the headspace of the package which prevents microbial growth and oxidative reactions that require free oxygen thus delaying respiration, maturation, and senescence (Sakaldaş & Kaynaş, 2010).
In addition to the effect of modified atmosphere on the product, MAP notably restricts its loss of water which can have a greater influence on quality maintenance than O2 and CO2 concentrations (Akbudak, 2008). For this to occur, the packaging material must provide an adequate transmission of water vapor to prevent moisture loss (Cerit & Demirkol, 2020). The packaging film also reduces the exposure of the produce to contaminants and pathogens because it separates the fruit from the external environment (Akbudak, 2008). Keeping the packaged product at low temperatures results in better fruit quality. Therefore, MAP in combination with refrigeration temperature could provide a substantial extension of the shelf-life of peppers at the same time that it maintains good nutritional, sensory, and microbiological quality.
Sharma et al. (2018) found that the use of MAP completely inhibited the decomposition of bell peppers kept at 7 °C and no CI symptoms were observed in both packaged and unpackaged fruit. However, MAP did not significantly prevent CI observed at 1.5 °C. The results of Manolopoulou et al. (2010) showed that MAP notably reduced the fraction of green bell peppers with CI symptoms when stored at 5 °C when compared to unpackaged fruit. In addition, the fraction of fruit with CI was markedly reduced at 10 °C in both types of samples when compared to fruit maintained at 5 °C. The modified atmosphere created within packaging might increase fruit tolerance to low temperatures and reduce CI (Sharma et al., 2018).
Frans et al. (2021) consider that MAP might be a useful tool to reduce the development of internal rot in bell peppers during their postharvest storage at conventional temperatures of 7–16 °C. Silica gel sachets were used by Singh et al. (2014) for bulk packaging of green bell peppers, and the shelf-life of the fruit (49 days) was extended compared to that of the fruit without the absorbent (42 days), thereby showing a 97% marketability rate. ) reported that passive MAP extended the shelf-life of green chillies stored at 8 °C to 28 days which is much longer than the 15 days they showed for unpackaged samples.
The ability of MAP to extend the shelf-life and preserve the postharvest quality of fruit and vegetables was enhanced when used in combination with other technologies such as LED illumination (Zhang et al., 2022), ultraviolet radiation (Franczuk et al., 2021; Vunnam et al., 2014), ozone (Pinto et al., 2020), ultrasonic treatment (Chen & Fan, 2021; Zhang et al., 2019), and coatings like chitosan (Zhong et al., 2022) and chitosan carbon-dot (Fan et al., 2021). The benefits of using MAP in combination with gamma irradiation and modified chitosan-based coating containing the nanoemulsion of an essential oil have been reported (Severino et al., 2015). A study by Feng et al. (2018) suggested the combined use of ultrasound and CA as a much effective preservation method than CA alone for fruit and vegetables. The combined use of MAP + methyl jasmonate was also assessed (Ozturk et al., 2019).
Taheri et al. (2020) impregnated filter papers with chitosan nanoparticles containing Heracleum persicum fruit essential oil (HPEO-CSNPs) and put them into PE bags containing bell pepper fruit. This treatment extended fruit shelf-life when compared to untreated MAP fruit. In MAP organic chilli peppers stored refrigerated and then at room temperature, sequential pretreatment of samples by hot water dipping (HWD) and coating with carboxymethyl cellulose or chitosan maintained fruit quality by slowing physico-chemical changes and improving the antioxidant system (Krongyut & Duangsi, 2021). Ranjitha et al. (2015) reported that the use of MAP in fresh-cut pepper pretreated with 2% calcium propionate extended its marketability up to 9 days when stored at 8 °C. However, the microbiological quality was at the best level until the sixth day of storage. MAP in combination with several disinfection technologies has been also evaluated in peppers. The shelf-life of MAP green chillies treated with ozone was extended up to 36 days which contrasted with the periods of 28 and 22 days in the case of chlorine-washed and untreated MAP fruit, respectively; furthermore, the postharvest quality was better retained (). Similar results were obtained by Horvitz and Cantalejo (2015) in partially dehydrated ready-to-eat pepper strips. Chitravathi et al. (2020) reported that the combined use of MAP + gamma irradiation in green chillies extended the shelf-life of fruit up to 42 days and retained better postharvest quality.
A very convenient way to save space when storing fruits and vegetables is the use of pallets, which also facilitates the transport of the commodities. However, most of the studies reported in the scientific literature for fruit and vegetables packaged under modified atmosphere or stored under CA, like those mentioned above, do not use a palletized MAP system. Peano et al. (2017) evaluated the use of a palletized storage system under MAP in plums and reported that it extended shelf-life longer and retained better postharvest quality when compared to air-palletized samples. This storage method proved advantageous in that it allows different fruit and vegetables to be stored in the same cold storage chamber by providing different atmosphere compositions for individual pallets. Additionally, storage units could also be used to transport commodities throughout the supply chain to reach markets farther away from production areas. Furthermore, with a palletized MAP system, the use of plastics is reduced, making it more sustainable. A disadvantage of using palletized MAP with respect to individual MAP is that once the packaging bags that wrap the pallets are opened, the gaseous composition inside them changes. However, this MAP system can be successfully applied to package, and store or transport, only the quantity of produce that is really going to be marketed at any given time. Therefore, it is necessary to plan the amount of fruit to be packaged based on the estimated volume of sales.
Recently, our research group (Olveira-Bouzas et al., 2021) developed a simple and easy way to use the palletized MAP system for tomatoes. This method delayed color evolution and reduced loss of firmness and rate of decay in tomatoes; shelf-life was also extended to 21 days. According to our investigation, no scientific literature has been found that studies the effect of pallet storage under MAP on the postharvest quality and shelf-life of peppers and, specifically, on ‘Padrón’ peppers. Therefore, the objective of this work was to optimize the MAP system developed by our group for ‘Padrón’ peppers and evaluate its effectiveness to maintain postharvest quality and extend the shelf-life of the fruit. Physico-chemical parameters were determined over 21 days of storage at 6 °C in unpackaged and packaged samples. Sensory analysis was also performed by a panel of trained tasters during the same time period.
Materials and Methods
Plant Material
This study was carried out in ‘Padrón’ peppers (Capsicum annuum L. cv. ‘Padrón’) grown in Ourense (Galicia, northwest Spain; geographic coordinates: 42°17′40″N; 159 m above sea level) and supplied by the agricultural cooperative Hortoflor 2, S.C.L. (Ourense). Peppers were cultivated in a greenhouse. Three assays were performed with fresh peppers harvested in two consecutive years: the first year (first assay) in September, and the second year in June and September for the second and third assay, respectively. The freshly harvested peppers were immediately taken to the facilities of the cooperative for their packaging and storage.
Reagents
The neoxanthin and violaxanthin used were purchased from ChromaDex (Irvine, CA, USA). The chlorophyll a, chlorophyll b, lutein, β-carotene, and the 2 N Folin–Ciocalteau reagent were supplied from Sigma-Aldrich (St. Louis, MO, USA). The HPLC-grade acetonitrile, ethyl acetate, metaphosphoric acid, potassium di-hydrogen phosphate (KH2PO4), anhydrous sodium carbonate (Na2CO3), 85% phosphoric acid, and 99% L-( +)-ascorbic acid were purchased from Panreac (Barcelona, Spain). The acetone was from Scharlau (Barcelona, Spain) and 98% gallic acid from Acros (Geel, Belgium). The ultrapure water was obtained by a Milli-Q water purification system from Millipore (Bedford, MA, USA).
Packaging, Storage, and Sampling
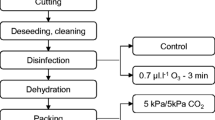
The packaging of the peppers was carried out by the technicians of the agricultural company according to the MAP system developed by Olveira-Bouzas et al. (2021) for tomatoes. Peppers were distributed in cardboard boxes (50 × 30 × 17 cm) which were placed on four pallets. Each of the boxes with peppers weighed approximately 5 kg and each pallet contained around ten boxes distributed in two levels. Three of the loaded pallets were subjected to a modified atmosphere (packaged pallets or MAP pallets) containing a O2–CO2 mixture while the remaining pallet was air-stored (N2: 78.1%; O2: 21.0%; CO2: 0.035%) and used as a control (unpackaged or control pallet). Micro-perforated low-density polyethylene (LDPE) bags (Ref. 6A2F) 60 µm thick and 24.30 × 13.55 × 26.00 m (length × width × height) big were used for packaging and supplied by Adrados Envase y Embalaje SL (Madrid, Spain). The permeabilities of this packaging material to O2 and CO2 were 3300 and 16,600 cm3 m−2 day−1 atm−1, respectively, and the water vapor transmission 1.67 g m−2 day−1 atm−1 (23 °C), as per the data provided by the supplier. The packaging bag was placed on each MAP pallet before depositing the boxes with the product, and once the bags were hermetically closed with a flange, the MAP pallets, together with the control pallet, were introduced into a cooling chamber at 6 °C and 91% RH and stored for 21 days. A gaseous mixture composed of N2–CO2 (“Freshline 20—Carburos Metálicos Company”, Coruña, Spain) was injected into PE bags displacing the air until the required O2 and CO2 concentrations were reached.
Three assays were carried out to find the optimal composition of the gases in the modified atmosphere. In the first assay, the convenience of using a certain initial gas composition of O2–CO2 was studied and, based on the results obtained, the decision was made to modify it using more O2 and less CO2. Therefore, a second assay was carried out with a different initial gaseous atmosphere. The possibility of extending the storage time of the samples to 28 days was also studied. Since the results obtained in this assay were also unsatisfactory, we tested a new gaseous composition in a third assay. In addition, the effectiveness of silica gel as a moisture absorber was evaluated to prevent the condensation of water vapor inside the bags and the deterioration of the peppers during storage. Therefore, in this last assay, a cardboard box containing 2.5 kg of silica gel and orange indicator (WG-2) was placed on each of MAP pallets as previously done by Olveira-Bouzas et a. (2021). The samplings in the first and last of the assays were carried out after 0, 7, 14, and 21 days of storage whereas the 7-day sampling was not performed in the second assay because the storage time was meant to be extended.
For each of the assays, the sampling on day 0 was carried out on the control pallet; subsequent samplings consisted of two sub-samplings, one from the control pallet and the other from a MAP pallet. A different MAP pallet was opened and used for each sampling day. Once a sampling had been carried out, the pepper samples were immediately taken to the Veterinary Faculty of the University of Santiago de Compostela (Galicia, Spain) for their sensory and physico-chemical analysis in the Tasting Room and the Laboratory of Food Technology, respectively.
Gaseous Composition
The differential mass balance equations that describe the changes that take place in the composition of O2 and CO2 inside a package containing a respiring commodity (Mangaraj et al., 2014) are:
-
(i)
For O2: rate of O2 entry into package space − rate of O2 consumed by product = rate of O2 accumulation inside package space
-
(ii)
For CO2: rate of CO2 generated by the fruit − rate of CO2 leaving the package space = rate of accumulation CO2 inside package space
The evolution of the gas composition inside the packaging bags was measured throughout the 21 days of storage. Technicians from the agricultural company monitored the gas composition daily using a portable Witt-Gasetechnik gases-meter model Oxibaby® M + for O2/CO2. Gas analysis was performed with a needle attached to the gas analyzer through a septum pasted on the packaging film. The gas requirement for sampling is minimal. The measurements of CO2 are based on IR-absorption and an electrochemical cell is used to measure O2.
Determination of the CIE L*a*b* Color Parameters
The color of fruit was measured using the ColorFlex colorimeter (HunterLab, Reston, VA, USA) appropriately calibrated with a standard tile (L* = 93.41; a* = − 1.07; b* = + 1.13). Measurements were performed using D65 illuminant and a 10° observation angle as references. The color measurements (25 ± 1 °C) of peppers were made at four random points on their surface. The data are presented as means of 10 bell ‘Padrón’ peppers.
Determination of Moisture
For the determination of moisture, the samples were crushed and then lyophilized using the Coolsafe Superior PRO 90–80 lyophilizer (SCANVAC, Stockholm, Sweden). Moisture was determined in triplicate and expressed as a percentage.
Determination of Firmness
Firmness was determined by measuring the puncture force with the TA.XTplus Texture Analyzer (Stable Micro System, Surrey, UK) equipped with a 50 N load cell. Puncture tests were performed by a probe with cylindrical tip (P/0.255) of 6.35 mm diameter, measuring the force necessary for the probe to move 10 mm at a speed of 2 mm s−1. The peppers were cut into 5 × 2 cm pieces and placed with the inside surface facing up in the plane of the texturometer support. Puncture force was measured in triplicate. The data are presented as means of 10 fruit and expressed as N.
Determination of Chlorophylls and Carotenoids
The pigment extraction (neoxanthin, violaxanthin, lutein, β-carotene, chlorophyll a, and chlorophyll b) was performed according to the method by Nishiyama et al. (2005) with slight modifications. The edible portion of a pepper was crushed in a mixer for 1 min with Na2CO3 (20 g kg−1) which was added to correct the acidity and prevent the chlorophylls from degrading to pheophytins. A portion of the homogenate (10 g) was ground for 1 min in a mortar with 15 mL of cold acetone (− 20 °C). The extract was filtered under vacuum and then transferred to a 50 mL volumetric flask. The extraction procedure was repeated twice more with 15 mL of cold acetone. After all the filtrate was collected in the volumetric flask, it was brought to volume with cold acetone. Solution was rapidly vortexed and filtered twice through a 0.20 µm nylon syringe filter from Waters Corporation (Milford, MA, USA). All extraction procedures were carried out under dim light and using containers covered with foil to prevent pigment photodegradation. Filtrate was subjected to HPLC analysis or stored at − 20 °C in an amber bottle until subsequent analysis. Twenty microliters of the filtrate was injected in duplicate into an HPLC apparatus equipped with a UV–VIS diode array detector, degasser, temperature stabilizer, and manual injector. Chromatographic separation was performed on a Sun Fire C18 (250 × 4.6 mm, 5 μm) column from Waters Corp. A Sun Fire C18 (20 × 4.6 mm, 5 μm) precolumn (Waters) was coupled to the analytical column. Gradient elution was performed with 90% (v/v) acetonitrile (solvent A) and ethyl acetate (solvent B). The gradient was initiated at 0% B and linearly increased to 60% over 15 min; then, this composition was maintained for 10 min before returning to the initial conditions. The flowrate was 1.5 mL min−1 and the column thermostated at 25 °C. Chromatographic peaks were identified by comparing the retention times with those of authentic standards. Neoxanthin and chlorophyll a were detected at 430 nm, violaxanthin, lutein, and β-carotene at 450 nm, and chlorophyll b at 460 nm. For quantification, the external calibration method was used which provided Pearson’s linear correlation coefficients (r) between 0.9959 and 0.9999. Samples were analyzed in triplicate. Chlorophyll and carotenoid contents are given as mg kg−1 in fresh weigh (FW).
The limit of detection (LD) of method for each pigment was calculated as the concentration corresponding to the average signal of the blank plus 3 times the standard deviation. The values obtained were 0.016, 0.011, 0.009, 0.078, 0.012, and 0.016 mg L−1 for chlorophyll a, chlorophyll b, lutein, β-carotene, neoxanthin, and violaxanthin, respectively. The measurement and method coefficients of variation were 0.96–4.71% and 2.12–4.06%, respectively. Analytical recoveries were higher than 97.84% in all cases.
Determination of Ascorbic Acid (AA)
AA was extracted from samples following the procedure that Olveira-Bouzas et al. (2021) made by slightly modifying the procedure previously made by Baardseth et al. (2010). Then, the mixture was vortexed and filtered through a Ø 110 mm filter paper and filtered again through a 0.2 µm nylon filter, while it was kept away from direct sunlight. Twenty microliters of the filtrate was manually injected in duplicate into the HPLC system describe above. The Spherisorb ODS2 C18 column (250 × 4.6 mm, 5 µm) and Spherisorb ODS2 C18 precolumn (20 × 4.6 mm, 5 µm) used were thermostated at 25 °C. The mobile phase (isocratic mode) consisted of a 0.2 M KH2PO4 solution adjusted to a pH value of 2.4 with 85% phosphoric acid (Gökmen et al., 2000). The flowrate of the mobile phase was 0.8 mL min−1. AA was detected at 254 nm and quantified using external calibration (r = 0.9999). The chromatographic peak was identified by comparing the retention time with that of an AA standard. All samples were analyzed in triplicate. Results are expressed as mg kg−1 FW. LD was 0.0122 mg L−1, the analytical recovery 98.00%, and the measurement and method coefficients of variation were 4.16 and 5.52%, respectively.
Determination of Total Phenolic Content (TPC)
Phenolics were extracted from samples following the procedure that Olveira-Bouzas et al. (2021) made by slightly modifying the procedure previously made by Oboh (2005). TPC was determined in the filtered extract (in triplicate) using the Folin–Ciocalteau reagent according to method that Olveira-Bouzas et al. (2021) implemented by making slight modifications to the method previously used by Singleton et al. (1999). Absorbance at 760 nm was measured with a UV–VIS spectrophotometer and TPC was determined using a calibration curve (r = 0.9977) obtained with gallic acid standards. All samples were analyzed in triplicate. TPC was expressed as mg of gallic acid equivalents (GAE) kg−1 FW. The method and measurement coefficients of variation were 2.76 and 2.77%, respectively.
Sensory Analysis
Sensory analysis was carried out by a panel of ten trained tasters. The basic training for judges was done according to ISO 8586:2012. Each of them evaluated a pair of samples, a control, and a packaged pepper, using the previously elaborated tasting sheet. The samples were presented at random to the panel with random 3-digit codes. The descriptors set was developed according to sensory analysis standards (ISO 11035:1994; ISO 13299:2016) and the panel of tasters met once a week. A list of descriptive terms for the evaluation of sensory quality was drawn up and the most appropriate ones were selected using statistical techniques. Once the terms were selected, the type of scale and, where appropriate, the references used in it were chosen and the form of evaluation of each descriptor was established. Finally, before evaluating the final samples, the panel of tasters conducted five training sessions to ensure their performance or efficacy. The tasting sheet included thirteen sensory descriptors. A two-point scale (presence/absence) was used for color uniformity, surface brightness, wrinkles, spots, internal off-odors, external off-odors, and burning sensation. The data obtained for each descriptor were expressed as a percentage of samples with presence or absence of such a descriptor. A three-point scale was employed for moisture sensation. To evaluate this descriptor, tasters opened the pepper with their hands and touched the inner surface. The tasters subjectively determined the degree of hydration of the pepper and qualified it as dry, slightly hydrated, or quite hydrated. The data obtained were expressed as a percentage of samples that obtained such a qualification. The descriptors external color intensity, external odor intensity, internal odor intensity, turgidity, and number of dark seeds were evaluated subjectively using 10-cm unstructured scales. The references of the scale for the external color intensity were Pantone 367C color (0) and Pantone 364C color (10). The intensities of internal and external odor were scored from weak (1) to high (10). The tasters evaluated turgidity by using their fingers to press from the middle to the top; the scale references were rubber ball and plastic corn cob skewer. The number of dark seeds was expressed as a percentage. The tasting sheet also included the item “other alterations” so that the tasters could indicate any other alteration observed in the samples during their storage.
Statistical Analysis
The results obtained from sensory and physico-chemical analysis were subjected to two-way analysis of variance (ANOVA) with interaction (t*P). Sources of variability were storage time (t) and the use of packaging (P) or not. The means between the first two samplings were compared with the t-Student test. The Pearson’s chi-squared test was applied to each of the descriptors evaluated by two-point scales on the percentages of samples with a presence of such a descriptor. The test allowed us to know if there were significant differences between samples depending on the method of pepper storage. The Mann–Whitney U nonparametric test for two independent samples (unpackaged and packaged pepper) was applied to the sensory descriptor evaluated with a three-point structured scale. All analyses were performed using SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results and Discussion
First Assay
The initial composition of the packaging atmosphere used in this first assay was around 7.9% O2–12.3% CO2, varying slightly between pallets. The changes in the gaseous composition measured in one of the pallets during storage time are shown in Fig. 1. Changes found in the rest of pallets were similar and data are not represented in the plot. Similar trends were reported by other researchers, that is, a decrease in O2 and an increase in CO2 (Akbudak, 2008). In our study, the O2 levels inside packaging bags fell below 2.5% after 12 days of storage but no off-flavors and odors were detected due to fermentation processes (Ospina Meneses & Cartagena Valenzuela, 2008; Rojas-Grau et al., 2009). Imahori et al. (2002) reported that when peppers were exposed to O2 levels below 1%, it enhanced the ethanol fermentation metabolism. The development of anaerobic conditions might also lead to the proliferation of anaerobic microorganism (Lucera et al., 2011). CO2 increased as time advanced to stabilize at around 20.7% after 13 days. CO2 levels during the entire storage period were much higher than the recommended values (2–5%) for peppers (Manolopoulou et al., 2010). These high concentrations of CO2 had been reported to damage bell peppers causing discoloration, pitting, and softening (Devgan et al., 2019). Owoyemi et al. (2021) detected severe fermentative off-flavors in red bell peppers stored in non-perforated packages, which became inedible, due to the anaerobic conditions created (O2 < 1.0%) and the high CO2 levels (CO2 > 9%) reached caused severe peel damage. Devgan et al. (2019) reported that the shelf-life of MAP yellow bell peppers was extended to 28 days by addition of an oxygen absorber. However, the amount of absorbent should be carefully optimized to prevent anaerobic conditions.
Large water vapor condensation was observed inside the bags after the seventh day of storage. This was most likely due to the development of high RH caused by the material (microperforated-LDPE) of the packaging bags that does not have sufficient permeability for water vapor. Studies carried out by ) in MAP green chillies using several non-perforated films showed that the films of LDPE and polyolefin, the latter to a lesser extent, led to condensation in the packets whereas the use of an anti-fog film maintained the freshness of peppers due to its higher water vapor permeability. Sharma et al. (2018) observed the presence of water droplets on bell peppers fruit in PE packaging. However, other researchers did not report the condensation of water vapor when peppers were packaged with a LDPE film (Cerit & Demirkol, 2020; Manolopoulou et al., 2010; Ornelas-Paz et al., 2015; Sakaldaş & Kaynaş, 2010).
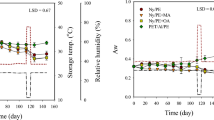
Firmness and color are two of the most important parameters for peppers in terms of consumer acceptance. Fruit and vegetables tend to soften after harvesting resulting in a loss of firmness. Softening is due to the breakdown of the carbohydrates in the cell wall structure and the increase in soluble pectin substances that results in a weakening of the cell walls and a reduction of the cohesive forces binding cells together (Majidi et al., 2014). MAP has a positive effect slowing down the activity of the enzymes involved in the degradation of the cell wall (polygalacturonase and pectinmethylesterase) and thus reducing softening (Artés Calero, 2006). It was also suggested that the high CO2 concentration involved in MAP results in suppression of the degradation of protopectin to soluble pectin thus reducing fruit softening (Majidi et al., 2014). Changes in firmness found over storage time are plotted in Fig. 2A. Statistical results obtained by two-way ANOVA with interaction and the t-Student test for firmness and other physico-chemical parameters are shown in Table 1. At day 0, firmness in peppers was 9.52 N and remained unchanged during storage time in both packaged and unpackaged samples. In agreement with this result, no significant differences were observed in the scores awarded by the tasters for turgidity over time. Turgidity, as well as the rest of the sensory descriptors evaluated, was scored using 10-cm unstructured scales; the corresponding statistical data are shown in Table 2.
Several researchers (Akbudak, 2008; Manolopoulou et al., 2010; Sahoo et al., 2014; Sakaldaş & Kaynaş, 2010; Singh et al., 2014) reported a loss of firmness in several cultivars of cold-stored green bell peppers that was slowed down using MAP. In a study by Lwin et al. (2022) on bell peppers (cvs. yellow Volante and red Sirocco) stored at 22 °C, MAP also delayed pericarp firmness loss and reduced fruit shriveling. In our work, firmness was significantly lower in packaged peppers in all samplings which might be attributed to the water vapor condensation inside packaging bags that causes peppers to be slightly softer. However, the tasters did not detect significant differences in turgidity between unpackaged and packaged fruit.
Color in green peppers is related to the total chlorophyll content (Sakaldaş & Kaynaş, 2010). Chlorophylls easily undergo degradation processes and most of them are converted into pheophytin and other derivatives leading to noticeable color changes (Cano et al., 1998). In this first assay, the values of the color parameters showed only small changes during storage (Tables 1 and 3). H° at day 0 was 113.28°, similar to those obtained in the following assays. H° values greater than 90° correspond to a more intense green color while angles close to 90° to a yellow one (Manolopoulou et al., 2010). H° was slightly higher in packaged than unpackaged peppers throughout storage and decreased in both types of samples at the end of storage. In accordance with this result, packaged peppers obtained the highest scores for “external color intensity,” although no significant differences were found between unpackaged and packaged samples. Ornelas-Paz et al., (2015) also reported minor changes in color (L*, a*, and b*) of both unpackaged and MAP green Jalapeño peppers stored for 42 days and they considered the low temperature used for their storage (7 °C) to be responsible for this since chlorophylase only remains active at temperatures between 10 and 75 °C (Arkus et al., 2005). However, Singh et al. (2014) found good retention of color (L*, C*, and H°) in MAP peppers (cv. Swarna) stored at 8 °C whereas the color parameters were reduced in unpackaged samples maintained at the same temperature. Koide and Shi (2007) did not find appreciable changes in the color parameters of MAP green peppers after 7 days of storage at 10 °C nor was the development of yellow color on the surface of the fruit found. Taheri et al. (2020) reported severe color changes (ΔE) and a notable decrease of the L* values in MAP red bell peppers (mature stage) during storage (9 °C/30 days). The red color in peppers, associated with carotenoids, is degraded by oxidation and the presence of oxidative enzymes during storage. Changes were reduced by placing filter papers impregnated with HPEO or HPEO-CSNPs inside the packaging bags. It might be due to the antioxidant properties of HPOE.
The initial contents of chlorophyll a and chlorophyll b in ‘Padrón’ peppers were 123.9 and 27.9 mg kg−1 FW, respectively. The initial content of β-carotene was 18.3 mg kg−1 FW and those of the xanthophylls neoxanthin, violaxanthin, and lutein were 5.6, 6.7, and 12.8 mg kg−1 FW, respectively. All pigments suffered degradation during storage in both control and MAP peppers. For all pigments, slightly higher values were found in control peppers the first 14 days of storage, although, at a statistical level, chlorophyll a and β-carotene were not affected by the use of MAP (Tables 1 and 4). Sakaldaş and Kaynaş (2010) found a continuous decrease of total chlorophyll in cold-stored unpackaged peppers (cv. Maxibell F1). They also indicated that MAP slowed degradation of chlorophylls which was attributed to the low O2 concentration and low respiration. Akbudak (2008) found similar results and a positive correlation between color and total chlorophyll. Carotenoids usually decrease during senescence and increase during the maturation, and these changes can be affected by temperature. Therefore, biosynthesis of carotenoids in fruit and vegetables during ripening, including peppers, is reduced or inhibited by refrigeration (Yahia & Ornelas-Paz, 2010). In our work and, according to what was stated above, the decrease in the amount of β-carotene at the end of storage was attributed to the senescence of peppers. In a study carried out by Ornelas-Paz et al. (2015), the amount of β-carotene was not altered during storage in unpackaged and packaged peppers that had been kept under refrigeration. ) found a continuous increase of total carotenoids in green chili peppers at the same time that the degradation of chlorophyll was taking place; they also found that both processes were slowed down due to a slower respiration rate when MAP was used.
Vitamin C is used as an indicator of quality for fresh vegetables (El-Mogy & Kitinoja, 2019). Red bell peppers are an excellent source of AA (Taheri et al., 2020). Mature peppers generally have the highest AA content when compared to immature peppers (Bae et al., 2014; Howard et al., 2000). Changes in the content in AA throughout the storage time are plotted in Fig. 3A and statistical results are shown in Table 1. The initial AA content in this first assay was 100.1 mg kg−1 FW. No significant effect of the storage time was found, but an increasing trend was observed in the values of AA in both unpackaged and packaged samples. Singh et al. (2014) reported a similar trend in chilled peppers stored in open PP bags (RH 95.0%). These authors associated the increase in AA to pepper dehydration because, when AA was expressed in terms of dry matter, its loss was almost 70% of the initial value. In our work, values of AA were lower in MAP fruit. Koide and Shi (2007) reported only minor changes in AA in green peppers packaged in several films and kept refrigerated at 10 °C for 7 days. Ornelas-Paz et al. (2015) found a similar content of AA in unpackaged and packaged, cold-stored jalapeño peppers; AA maintained quite constant during storage in both types of fruit. However, AA increased continuously in unpackaged peppers stored at 23 °C which was associated with postharvest ripening and dehydration. Sahoo et al. (2014) reported a decrease in AA in peppers stored at room temperature or refrigerated, although it was found to be higher in samples at room temperature. Andrade Cuvi et al. (2011) reported an 5% increase of AA in peppers in the first 7 days of storage at 0 °C and then a reduction in its content.
Phenolic compounds contribute to the color and flavor of fruits and vegetables as well as to the antioxidant system in plant tissues, since some phenolic compounds are powerful antioxidants (Krongyut & Duangsi, 2021). The phenolic compounds are considered to be one of the main phytochemicals related to human health (Devgan et al., 2019).
Initial TPC was 323.2 mg GAE kg−1 FW and decreased in unpackaged fruit to 241.3 mg GAE kg−1 FW at the end of storage (Fig. 4A; Table 1). However, TPC in packaged fruit remained quite constant during the first 14 days of storage and then decreased to 202.6 mg GAE kg−1 FW. Several researchers have reported an increase of TPC in peppers as ripening progresses (Chuah et al., 2008; Howard et al., 2000; Zhuang et al., 2012). According to this, the decrease of TPC in unpackaged samples might indicate senescence of peppers, in which case the use of MAP would slow it down. Ornelas-Paz et al. (2015) did not find changes in TPC in both unpackaged and packaged green jalapeño peppers during storage and Barbosa et al. (2020) only found negligible losses in MAP fresh-cut bell peppers at different maturation stages (green, yellow, and red). However, Devgan et al. (2019) reported an increase of TPC in MAP yellow bell peppers during storage. Other researchers (Chitravathi et al., 2020, 2015a, b) found the same trend as Devgan et al. (2019) in unpackaged and MAP green chillies with highest TPC values in the unpackaged peppers. Taheri et al. (2020) found a linear increase of TPC in MAP red bell peppers (mature stage) up to the first 18 days of storage and then a decrease. Krongyut and Duangsi (2021) reported an increase of TPC in MAP chilli pepper (full-red stage) with cold storage, and at the same time, an increase in the activity of phenylalanine ammonia lyase (PAL) which is a key enzyme in phenolics biosynthesis.
The number of “dark seeds” in this first assay increased after 7 days in both packaged and unpackaged peppers, but significant differences between the two samples were not found. In addition, unpackaged samples scored higher for “internal odor intensity” than packaged samples throughout the entire storage time. As for the sensory descriptors evaluated using two-point scales, differences were found only for “spots” at 14 and 21 days of storage. At the end of storage, 37.5% of the control peppers had spots while none of the MAP peppers presented them. Spots observed in control peppers after 14 days of storage are shown in Fig. 5. In the item “other alterations,” the tasters indicated that, in both control and MAP peppers, the peduncle was dry after 14 days of storage. They also observed presence of rot at the end of storage in both type of peppers due to microbial growth. We attribute the appearance of rot in MAP peppers to the condensation of water vapor on the surface of the fruit, as well as to the gaseous atmosphere created inside the pallets with levels of O2 outside the recommended range. In the second assay, we decided to change the initial gas composition inside PE bags to prevent anaerobic conditions, leaving the study of the effect of using a moisture absorber to prevent water vapor condensation for the third assay.
Second Assay
In this second assay, a gas mixture which contained higher O2 and lower CO2 levels (10.4% O2–10.4% CO2) than those of the previous assay was tested to prevent the development of a hypoxic modified atmosphere. The possibility of extending the time of storage of the samples to 28 days was also studied and we decided to eliminate the 7-day sampling. However, the new gas composition used was not effective in maintaining adequate levels of the gases within the PE bags. O2 fell below the recommended value after 1 week of storage, and in addition, CO2 increased over time to stabilize around 20.1% after 8 days; however, levels this high are not recommended (Fig. 1).
At a sensory level (Table 2), the packaged peppers obtained the highest scores for the descriptor “external color intensity” and, as occurred in the first assay, the tasters gave the highest scores for “internal odor intensity” to the unpackaged peppers. The tasters also observed an increase in the number of “dark-seeds” after 14 days of storage in both packaged and unpackaged peppers with no significant differences as per storage method.
Firmness (9.70 N at day 0) did not change over time or because of the storage method, even though the water vapor condensation continued to persist in the MAP pallets (Fig. 2B; Table 1). Consequently, turgidity (Table 2) was not influenced by packaging the peppers or not and no changes were found in the scores of this descriptor throughout the storage time for both types of samples.
Pepper color was affected by the storage time (Tables 1 and 3). L* tended to increase in both control and MAP peppers as storage time advanced, indicating an increase in lightness of fruit but, at a statistical level, the changes were not significant. C* and b* tended to increase and H° to decrease slightly. The changes in b* and H* indicate a trend of fruit to develop a yellowish-green color. However, the scores awarded by the tasters to the intensity of the external color did not change significantly over time; nevertheless, a decrease in the values of this descriptor in control samples has been observed. ΔE increased in both type of samples with smaller changes in packaged samples which agreed with the highest scores given by tasters to the external color intensity of packaged fruit. No significant differences were found between unpackaged and packaged samples for the rest of the color parameters. In this second assay, only the most abundant pigments were determined, and their content continuously decreased during storage (Tables 1 and 4). β-carotene and the two chlorophylls were not affected by MAP, as occurred with β-carotene and chlorophyll a in the first assay.
AA (150.6 mg kg−1 FW) decreased in control peppers during storage and, at 21 days, was 78.2% of its initial value (Fig. 3B; Table 1). AA is known to decrease in the presence of light, oxygen, and heat (Plaza et al., 2006). AA is very prone to degradation by oxidative processes such as autoxidation and enzymatic oxidation caused by ascorbate-oxydase, polyphenol-oxidase, cytochrome–oxidase, and peroxidase (Sánchez-Mata et al., 2003). Ascorbate-oxidase is known to be the main enzyme responsible for deterioration of AA. Under stress, ascorbate-oxydase levels increase (Taheri et al., 2020). Oxidation of AA and phenolic compounds can result in loss of nutritional value and sensory attributes (Queiroz et al., 2011). The amount of AA was fairly constant during storage in MAP fruit. The low O2 levels might have inhibited the oxidative processes that degrade AA. Several researchers (Akbudak, 2008; Cerit & Demirkol, 2020; Sahoo et al., 2014; Sakaldaş & Kaynaş, 2010) also reported a beneficial effect of MAP on AA; high losses were found in unpackaged cold-stored bell peppers and MAP slowed its degradation. Taheri et al. (2020) found that AA in MAP red bell peppers (mature stage) at day 30 of storage decreased 82.5%, when compared to the initial day; the application of HPEO-CSNPs slowed down its loss. In MAP chilli peppers at the full-red stage that were cold-stored up to 12 days, the AA degradation rate was slower (Krongyut & Duangsi, 2021). Differences found in the several studies might be related to differences in maturity stage and genetics (Castro et al., 2008).
Regarding the sensory descriptors evaluated by two-point scales, no significant relation between each of them and the use or not of MAP was found. In “other alterations” the tasters indicated the presence of rottenness from 14 days of storage in both control and MAP peppers. The presence of rottenness in MAP peppers could be explained by the condensation of water vapor along with an unsuitable packaging atmosphere, as indicated in the first assay. The results of the second assay showed that it was not possible to extend the shelf-life of ‘Padrón’ Peppers for another week. Therefore, the 28-day sampling was not carried out in the last assay.
Third Assay
In the third and final assay, another initial gas composition was tested (11.8% O2–8.5% CO2), containing higher O2 and lower CO2 levels than those used in the previous assays. Therefore, O2 concentration was increased to have more oxygen available and thus avoid anaerobic conditions. We decreased the amount of CO2 so that this gas would not increase as much as in the previous assays. The samplings were maintained at 0, 7, 14, and 21 days of storage as in the first assay. We also decided to use silica gel to reduce excess moisture inside the LDPE bags and prevent water vapor condensation. In general, silica gel is highly effective as a moisture absorbent because it can absorb up to 50% of its own weight in water (Singh et al., 2014).
The behavior of the gases was contrary to what was expected. The initial concentration of O2 in one of the pallets increased to stabilize after 1 week at around 18.7% and initial CO2 decreased over time and stabilized at around 2.2% after 10 days (Fig. 1). Hence, anaerobic conditions did not occur at any time and the CO2 levels in the modified atmosphere at equilibrium were within the recommended values (2–5%). Similar trends, that is, an increase in O2 inside packages and a decrease of CO2, were found by Barbosa et al. (2020) in MAP fresh-cut bell peppers.
The use of silica gel as a moisture absorbent noticeably reduced the condensation of water vapor which was concentrated mainly on the areas of the pallet farthest from the cardboard box that contained the absorbent, as in our group’s prior work (Olveira-Bouzas et al., 2021). Despite this, firmness was lower in packaged peppers in the 14- and 21-day samplings, as it happened in the first assay (Fig. 2C). However, at a sensory level, the tasters did not find significant differences in turgidity (Table 2) between the control and MAP peppers. We attribute the reduction in the average firmness values found in MAP samples to the possible heterogeneity of the peppers in the sampling in terms of how they were affected by the condensation because, in the second assay, when silica gel was not used, the packaged and control samples were found to have the same firmness throughout the 21 days of storage, despite the higher water vapor condensation.
Regarding the color parameters, changes in most of them over storage time in both packaged and unpackaged samples were observed (Tables 1 and 3). b*, C* tended to increase and H° to decrease, as in the second assay. The changes in b* and H° indicated that the green color of the peppers was fading and tending to turn yellowish green. However, the scores for “external color intensity” did not show significant changes. The packaging factor was not significant for any of the color parameters.
The contents of lutein, β-carotene, chlorophyll a, and chlorophyll b at day 0 in control pallet were 13.9, 18.2, 83.6, and 22.8 mg kg−1 FW, respectively (Fig. 6). β-carotene and chlorophyll a were not affected by MAP, as observed in the previous assays (Table 1). The β-carotene and chlorophylls contents were fairly constant throughout the storage time. At the end of storage, retention of β-carotene in packaged fruit was 96.2% and chlorophyll a and chlorophyll b were 92.8 and 87.7%, respectively. Similar retentions were obtained in control fruit at the end of storage. The loss of lutein was 69.1% at the end of storage in both type of samples.
AA (186.9 mg kg−1 FW) showed a decreasing trend during the time of storage in both unpackaged and packaged peppers indicating that its degradation occurred (Fig. 3C; Table 1). However, no significant differences were found for the conservation method of peppers which is most likely related to the fact that the oxygen levels in the modified atmosphere at equilibrium (18.7%) were similar to those in samples stored under air (21%). According to the statistic analysis by ANOVA, the initial value of TPC (354.7 mg GAE kg−1 FW) did not change significantly during storage neither in the control fruit nor in the packaged fruit. In fact, the values at the end of storage were similar to the initial value (Fig. 4B; Table 1).
As for the five descriptors evaluated using 10-cm unstructured scales (Table 2), the number of dark-seeds increased after 7 days of storage in unpackaged fruit, and a week later in packaged fruit. In addition, the scores awarded by the tasters to the intensity of the external odor were lower for the packaged peppers during the entire storage time, as was the case with the intensity of internal odor in the first two assays. Regarding the sensory descriptors evaluated with two-point scales, a significant relationship was obtained between the descriptor “wrinkles” and the storage method in the 14- and 21-day samplings. At the end of storage, 75% of the control peppers showed wrinkles whereas only 12.5% of the MAP peppers presented them. Therefore, MAP showed beneficial effects in reducing the fraction of peppers with water loss. The use of silica gel was effective in reducing excess moisture inside packaging bags because the condensation of water vapor was notably reduced. Moreover, the tasters did not observe rottenness in MAP peppers throughout the storage time and the fruit always showed a good appearance and color. The use of silica gel and the modified atmosphere created inside pallets prevented microbial growth. In the previous assays, rottenness occurred during storage in both control and MAP fruit. Sahoo et al. (2014) assessed marketability of cold-stored MAP green bell peppers by looking at the level of visible mold growth, shriveling, decay, shine, and smoothness. Marketability was 61.30% using perforated LDPE and increased to 86.53% using perforated PP. A disadvantage of using MAP was the loss of external odor intensity which was detected in the sensory analysis performed by the tasters. Owoyemi et al. (2021) reported that the use of a micro-perforated film in red bell peppers created an atmosphere of 15–18% O2/2–5% CO2, similar to that obtained in our work, whereas an atmosphere of 20–21% O2/0.1–0.5% CO2 was created by a macro-perforated film. These gas compositions reduced weight loss, shriveling, and softening and retained flavor acceptance and visual appearance.
Conclusions
A simple and easy way to apply palletized MAP system was developed for ‘Padrón’ peppers. Packaging conditions were optimized through three assays. This packaging system prevented the development of anaerobic conditions and kept suitable CO2 levels throughout the entire storage period. The use of silica gel considerably reduced water vapor condensation. The shelf-life of peppers was extended to 21 days of storage due to the gaseous atmosphere created inside packaging bags and the use of silica gel. No rotting or other types of undesirable alterations were observed during the storage time. The percentage of MAP samples with wrinkles was minimal when compared to control samples indicating a reduced fraction of peppers with water loss. Overall, good pigment stability was obtained. In addition, chlorophyll a and β-carotene were not affected by MAP in any of the third assays carried out.
Based on the results of this study, we can conclude that the MAP system in pallets developed could be effective as a complement to conservation by refrigeration to extend the shelf-life of ‘Padrón’ peppers with good marketability. The use of other packaging film with a greater permeability to water vapor could also be evaluated and, if required, this could also be carried out in combination with silica gel.
Data Availability
Data available on request from the authors.
References
Akbudak, B. (2008). Effect of polypropylene and polyvinyl chloride plastic film packaging materials on the quality of ‘Yalova Charleston’ pepper (Capsicum annuum L.) during storage. Food Science and Technology Research, 14(1), 5–11.
Andrade Cuvi, M. J., Vicente, A. R., Concellón, A., & Chaves, A. R. (2011). Changes in red pepper antioxidants as affected by UV-C treatments and storage at chilling temperatures. LWT-Food Science and Technology, 44(7), 1666–1671. https://doi.org/10.1016/j.lwt.2011.01.027
Arkus, K. A. J., Cahoon, E. B., & Jez, J. M. (2005). Mechanistic analysis of wheat chlorophyllase. Archives of Biochemistry and Biophysics, 438(2), 146–155. https://doi.org/10.1016/j.abb.2005.04.019
Artés Calero, F. (2006). El envasado en átmósfera modificada mejora la calidad de consumo de los productos hortofrutícolas intactos y mínimamente procesados en fresco. Revista Iberoamericana De Tecnología Postcosecha, 7(2), 61–85.
Baardseth, P., Bjerke, F., Martinsen, B. K., & Skrede, G. (2010). Vitamin C, total phenolics and antioxidative activity in tip-cut green beans (Phaseolus vulgaris) and swede rods (Brassica napus var. napobrassica) processed by methods used in catering. Journal of the Science of Food and Agriculture, 90(7), 1245–1255. https://doi.org/10.1002/jsfa.3967
Bae, H., Jayaprakasha, G. K., Crosby, K., Yoo, K. S., Leskovar, D. I., Jifon, J., & Patil, B. S. (2014). Ascorbic acid, capsaicinoid, and flavonoid aglycone concentrations as a function of fruit maturity stage in greenhouse-grown peppers. Journal of Food Composition and Analysis, 33(2), 195–202. https://doi.org/10.1016/j.jfca.2013.11.009
Barbosa, C., Machado, T. B., Alves, M. R., & Oliveira, M. B. P. P. (2020). Fresh-cut bell peppers in modified atmosphere packaging: improving shelf life to answer food security concerns. Molecules, 25, Article 2323. https://doi.org/10.3390/molecules25102323
Belay, Z. A., Caleb, O. J., Mahajan, P. V., & Opara, U. L. (2018). Design of active modified atmosphere and humidity packaging (MAHP) for ‘wonderful’ pomegranate arils. Food and Bioprocess Technology, 11(8), 1478–1494. https://doi.org/10.1007/s11947-018-2119-0
Bonaccorsi, I., Cacciola, F., Utczas, M., Inferrera, V., Giuffrida, D., Donato, P., Dugo, P., & Mondello, L. (2016). Characterization of the pigment fraction in sweet bell peppers (Capsicum annuum L.) harvested at green and overripe yellow and red stages by offline multidimensional convergence chromatography/liquid chromatography–mass spectrometry. Journal of Separation Science, 39, 3281–3291. https://doi.org/10.1002/jssc.201600220
Caleb, O. J., Mahajan, P. V., Al-Said, F. A. J., & Opara, U. L. (2013). Modified atmosphere packaging technology of fresh and fresh-cut produce and the microbial consequences-A review. Food and Bioprocess Technology, 6(2), 303–329. https://doi.org/10.1007/s11947-012-0932-4
Cano, M. P., Monreal, M., de Ancos, B., & Alique, R. (1998). Effects of oxygen levels on pigment concentrations in cold-stored green beans (Phaseolus vulgaris L. cv. Perona). Journal of Agricultural and Food Chemistry, 46, 4164–4170. https://doi.org/10.1021/jf980158r
Castro, S. M., Saraiva, J. A., Da Silva Lopes, J. A., Delgadillo, I., Loey, A. V., Smout, C., & Hendrickx, M. (2008). Effect of thermal blanching and of high pressure treatments on sweet green and red bell pepper fruits (Capsicum annuum L.). Food Chemistry, 107(4), 1436–1449. https://doi.org/10.1016/j.foodchem.2007.09.074
Cerit, I., & Demirkol, O. (2020). Effects of modified atmosphere packaging conditions and ethylene absorber on the quality of red bell pepper. Journal of Food and Nutrition Research, 59(1), 35–43.
Chen, L. B., & Fan, K. (2021). Influence of ultrasound treatment in combination with modified atmosphere on microorganisms and quality attributes of fresh-cut lettuce. International Journal of Food Science and Technology, 56(10), 5242–5249. https://doi.org/10.1111/ijfs.15256
Chitravathi, K., Chauhan, O. P., & Kizhakkedath, J. (2020). Shelf life extension of green chillies (Capsicum annuum L.) using passive modified atmosphere packaging and gamma irradiation. Journal of Food Processing and Preservation, 44(8), Article e14622. https://doi.org/10.1111/jfpp.14622
Chitravathi, K., Chauhan, O. P., & Raju, P. S. (2015a). Influence of modified atmosphere packaging on shelf-life of green chillies (Capsicum annuum L.). Food Packaging and Shelf Life, 4, 1–9. https://doi.org/10.1016/j.fpsl.2015.02.001
Chitravathi, K., Chauhan, O. P., Raju, P. S., & Madhukar, N. (2015b). Efficacy of aqueous ozone and chlorine in combination with passive modified atmosphere packaging on the postharvest shelf-life extension of green chillies (Capsicum annuum L.). Food and Bioprocess Technology, 8(6), 1386–1392. https://doi.org/10.1007/s11947-015-1511-2
Chuah, A. M., Lee, Y.-C., Yamaguchi, T., Takamura, H., Yin, L., & Matoba, T. (2008). Effect of cooking on the antioxidant properties of coloured peppers. Food Chemistry, 111(1), 20–28. https://doi.org/10.1016/j.foodchem.2008.03.022
Devgan, K., Kaur, P., Kumar, N., & Kaur, A. (2019). Active modified atmosphere packaging of yellow bell pepper for retention of physico-chemical quality attributes. Journal of Food Science and Technology, 56(2), 878–888. https://doi.org/10.1007/s13197-018-3548-5
Dwi Anggono, A., Rebezov, M., Mironov, S., Thangavelu, L., Aravindhan, S., Aljeboree, A. M., Al-Janabi, S., Abd Alrazzak, N., Alkaim, A.F., Kamal Abdelbasset, W. (2022). Fruit preservation packaging technology based on air adjustment packaging method. Food Science and Technology, 42, Article e29221. https://doi.org/10.1590/fst.29221
El-Mogy, M. M., & Kitinoja, L. (2019). Review of best postharvest practices for fresh market green beans. PEF White Paper No. 19-01. Ed. The Postharvest Education Foundation, La Pine, Oregon, USA. https://www.postharvest.org/PEF_White_Paper_19-01_El-Mogy_Kitinoja_GreenBeans.pdf
Escalona, V., Correa, J., & González, A. (2019). Manejo postcosecha de tomates y pimientos frescos y de IV gama. Serie Ciencias Agronómicas. https://repositorio.uchile.cl/bitstream/handle/2250/175675/Manejo-postcosecha-de-tomates-y-pimientos-fresco.pdf?sequence=1&isAllowed=y
Esturk, O., Ayhan, Z., & Ustunel, M. A. (2012). Modified atmosphere packaging of “Napoleon” cherry: Effect of packaging material and storage time on physical, chemical, and sensory quality. Food and Bioprocess Technology, 5(4), 1295–1304. https://doi.org/10.1007/s11947-011-0561-3
Fan, K., Zhang, M., Guo, C., Dan, W., & Devahastin, S. (2021). Laser-induced microporous modified atmosphere packaging and chitosan carbon-dot coating as a novel combined preservation method for fresh-cut cucumber. Food and Bioprocess Technology, 14(5), 968–983. https://doi.org/10.1007/s11947-021-02617-y
Feng, L., Zhang, M., Adhikari, B., & Guo, Z. (2018). Effect of ultrasound combined with controlled atmosphere on postharvest storage quality of cucumbers (Cucumis sativus L.). Food and Bioprocess Technology, 11(7), 1328–1338. https://doi.org/10.1007/s11947-018-2102-9
Figeroa Cares, I. E., Martínez Damián, M. T., Rodríguez Pérez, J. E., Cruz Álvarez, O., Colinas Leóns, M. T. B., Valle Guadarrama, S., & Ramírez Ramírez, S. P. (2015). Capacidad antioxidante en variedades de pimiento morrón (Capsicum annum L .). Interciencia, 40(10), 696–704.
Fornaris, G. J. (2005). Cosecha y manejo postcosecha. In Conjunto Tecnológico para la Producción de Pimiento: tipos ‘cubanelle’ y ‘campana’. Estación experimental agrícola. Universidad de Puerto Rico. https://www.uprm.edu/
Franczuk, J., Rosa, R., Zaniewicz-Bajkowska, A., & Ginter, A. (2021). The effect of treating string bean pods with modified atmosphere packaging and UV-C irradiation on their storage life. Agronomy, 11, Article 1747. https://doi.org/10.3390/agronomy11091747
Frans, M., Aerts, R., Ceusters, N., Luca, S., & Ceusters, J. (2021). Possibilities of modified atmosphere packaging to prevent the occurrence of internal fruit rot in bell pepper fruit (Capsicum annuum) caused by Fusarium spp. Postharvest Biology and Technology, 178, Article 111545. https://doi.org/10.1016/j.postharvbio.2021.111545
Gökmen, V., Kahraman, N., Demir, N., & Acar, J. (2000). Enzymatically validated liquid chromatographic method for the determination of ascorbic and dehydroascorbic acids in fruit and vegetables. Journal of Chromatography A, 881(1–2), 309–316.
Gurnani, N., Gupta, M., Mehta, D., & Metha, B. K. (2016). Chemical composition, total phenolic and flavonoid contents, and in vitro antimicrobial and antioxidant activities of crude extracts from red chilli seeds (Capsicum frutescens L.). Journal of Taibah University of Science, 10(4), 462–470. https://doi.org/10.1016/j.jtusci.2015.06.011
Hernández-Pérez, T., Del Gómez-García, M. R., Valverde, M. E., & Paredes-López, O. (2020). Capsicum annuum (hot pepper): An ancient Latin-American crop with outstanding bioactive compounds and nutraceutical potential. A review. Comprehensive Reviews in Food Science and Food Safety, 19(6), 2972–2993. https://doi.org/10.1111/1541-4337.12634
Hernández-Ortega, M., Ortiz-Moreno, A., Hernández-Navarro, M. D., Chamorro-Cevallos, G., Dorantes-Álvarez, L., & Necoechea-Mondragón, H. (2012). Antioxidant, antinociceptive, and anti-inflammatory effects of carotenoids extracted from dried pepper (Capsicum annuum L.). Journal of Biomedicine and Biotechnology, 2012, Article ID 524019. https://doi.org/10.1155/2012/524019
Horvitz, S., & Cantalejo, M. J. (2015). Effects of gaseous O3 and modified atmosphere packaging on the quality and shelf-life of partially dehydrated ready-to-eat pepper strips. Food and Bioprocess Technology, 8(8), 1800–1810. https://doi.org/10.1007/s11947-015-1537-5
Howard, L. R., Talcott, S. T., Brenes, C. H., & Villalon, B. (2000). Changes in phytochemical and antioxidant activity of selected pepper cultivars (Capsicum species) as influenced by maturity. Journal of Agricultural and Food Chemistry, 48(5), 1713–1720. https://doi.org/10.1021/jf990916t
Imahori, Y., Kota, M., Ueda, Y., Ishimaru, M., & Cachin, K. (2002). Regulation of ethanolic fermentation in bell pepper fruit under low oxygen stress. Postharvest Biology and Technology, 25(2), 159–167. https://doi.org/10.1016/S0925-5214(01)00174-0
ISO 11035:1994 (1994). Sensory analysis. Identification and selection of descriptors for establishing a sensory profile by a multidimensional approach. Geneva. Switzerland: International Organization for Standardization.
ISO 8586:2012 (2012). Sensory Analysis. General guidelines for the selection, training and monitoring of selected assessors and expert sensory assessors. Geneve. Switzerland: International Organization of Standarization.
ISO 13299:2016 (2016). Sensory analysis. Methodology. General guidance for establishing a sensory profile. Geneva. Switzerland: International Organization for Standardization.
Koide, S., & Shi, J. (2007). Microbial and quality evaluation of green peppers stored in biodegradable film packaging. Food Control, 18(9), 1121–1125. https://doi.org/10.1016/j.foodcont.2006.07.013
Krongyut, W., & Duangsi, R. (2021). Organic chilli pepper (Capsicum annuum cv. superhot) quality and biochemical responses to sequential treatment with hot water, surface coating, equilibrium modified atmosphere packaging and cold storage. Agrivita Journal of Agricultural Science, 43(2), 325–337. https://doi.org/10.17503/agrivita.v43i2.2890
Lim, C. S., Kang, S. M., Cho, J. L., Gross, K. C., & Woolf, A. B. (2007). Bell pepper (Capsicum annuum L.) fruits are susceptible to chilling injury at the breaker stage of ripenes. HortScience, 42(7), 1659–1664. https://doi.org/10.21273/hortsci.42.7.1659
Lucera, A., Conte, A., & Del Nobile, M. A. (2011). Shelf life of fresh-cut green beans as affected by packaging systems. International Journal of Food Science and Technology, 46(11), 2351–2357. https://doi.org/10.1111/j.1365-2621.2011.02756.x
Lwin, H. P., Lee, J., & Lee, J. (2022). Perforated modified atmosphere packaging differentially affects the fruit quality attributes and targeted major metabolites in bell pepper cultivars stored at ambient temperature. Scientia Horticulturae, 301, Article 111131. https://doi.org/10.1016/j.scienta.2022.111131
Majidi, H., Minaei, S., Almassi, M., & Mostofi, Y. (2014). Tomato quality in controlled atmosphere storage, modified atmosphere packaging and cold storage. Journal of Food Science and Technology, 51(9), 2155–2161. https://doi.org/10.1007/s13197-012-0721-0
Mangaraj, S., Goswami, T. K., Giri, S. K., & Joshy, C. G. (2014). Design and development of modified atmosphere packaging system for guava (cv. Baruipur). Journal of Food Science and Technology, 51(11), 2925–2946. https://doi.org/10.1007/s13197-012-0860-3
Manolopoulou, H., Xanthopoulos, G., Douros, N., & Lambrinos, G. (2010). Modified atmosphere packaging storage of green bell peppers: Quality criteria. Biosystems Engineering, 106(4), 535–543. https://doi.org/10.1016/j.biosystemseng.2010.06.003
Muftuoǧlu, F., Ayhan, Z., & Esturk, O. (2012). Modified atmosphere packaging of Kabaaşi apricot (Prunus armeniaca L. ’Kabaaşi’): effect of atmosphere, packaging material type and coating on the physicochemical properties and sensory quality. Food and Bioprocess Technology, 5(5), 1601–1611. https://doi.org/10.1007/s11947-010-0482-6
Nishiyama, I., Fukuda, T., & Oota, T. (2005). Genotypic differences in chlorophyll, lutein, and β-carotene contents in the fruits of Actinidia species. Journal of Agricultural and Food Chemistry, 53(16), 6403–6407. https://doi.org/10.1021/jf050785y
Oboh, G. (2005). Effect of blanching on the antioxidant properties of some tropical green leafy vegetables. LWT-Food Science and Technology, 38(5), 513–517. https://doi.org/10.1016/j.lwt.2004.07.007
Olveira-Bouzas, V., Pita-Calvo, C., Vázquez-Odériz, M. L., & Romero-Rodríguez, M. A. (2021). Evaluation of a modified atmosphere packaging system in pallets to extend the shelf-life of the stored tomato at cooling temperature. Food Chemistry, 364, Article 130309. https://doi.org/10.1016/j.foodchem.2021.130309
Ornelas-Paz, J. J., Castañeda-Jiménez, A. C., Estrada-Alvarado, M. I., Ramos-Aguilar, O. P., Ibarra-Junquera, V., Pérez-Martínez, J. D., Escalante-Minakata, P., Guevara-Arauza, J. C., & Ruiz-Cruz, S. (2015). Effect of the perforation level of recycled-LDPE bags on the modification of the atmosphere development, bioactive compounds content, and other qualities of Jalapeño peppers during storage. Journal of Food Science and Technology, 52(10), 6415–6424. https://doi.org/10.1007/s13197-015-1749-8
Ospina Meneses, S. M., & Cartagena Valenzuela, J. R. (2008). La atmósfera modificada: Una alternativa para la conservación de los alimentos. Revista Lasallista De Investigación, 5(2), 112–123.
Owoyemi, A., Rodov, V., & Porat, R. (2021). Retaining red bell pepper quality by perforated compostable packaging. Food Science & Nutrition, 9, 3683–3692. https://doi.org/10.1002/fsn3.2329
Ozgur, M., Ozcan, T., Akpinar-Bayizit, A., & Yilmaz-Ersan, L. (2011). Functional compounds and antioxidant properties of dried green and red peppers. African Journal of Agricultural Research, 6(25), 5638–5644. https://doi.org/10.5897/AJAR11.709
Ozturk, A., Yildiz, K., Ozturk, B., Karakaya, O., Gun, S., Uzun, S., & Gundogdu, M. (2019). Maintaining postharvest quality of medlar (Mespilus germanica) fruit using modified atmosphere packaging and methyl jasmonate. LWT - Food Science and Technology, 111, 117–124. https://doi.org/10.1016/j.lwt.2019.05.033
Peano, C., Giuggioli, N. R., Girgenti, V., Palma, A., D’Aquino, S., & Sottile, F. (2017). Effect of palletized MAP storage on the quality and nutritional compounds of the Japanese plum cv. Angeleno (Prunus salicina Lindl.). Journal of Food Processing and Preservation, 41(2), Article e12786. https://doi.org/10.1111/jfpp.12786
Pinto, L., Palma, A., Cefola, M., Pace, B., D’Aquino, S., Carboni, C., & Baruzzi, F. (2020). Effect of modified atmosphere packaging (MAP) and gaseous ozone pre-packaging treatment on the physico-chemical, microbiological and sensory quality of small berry fruit. Food Packaging and Shelf Life, 26, Article 100573. https://doi.org/10.1016/j.fpsl.2020.100573
Plaza, L., Sánchez-Moreno, C., Elez-Martínez, P., De Ancos, B., Martín-Belloso, O., & Cano, M. P. (2006). Effect of refrigerated storage on vitamin C and antioxidant activity of orange juice processed by high-pressure or pulsed electric fields with regard to low pasteurization. European Food Research and Technology, 223(4), 487–493. https://doi.org/10.1007/s00217-005-0228-2
Queiroz, C., Da Silva, A. J. R., Lopes, M. L. M., Fialho, E., & Valente-Mesquita, V. L. (2011). Polyphenol oxidase activity, phenolic acid composition and browning in cashew apple (Anacardium occidentale, L.) after processing. Food Chemistry, 125(1), 128–132. https://doi.org/10.1016/j.foodchem.2010.08.048
Ranjitha, K., Sudhakar Rao, D. V., Shivashankara, K. S., & Roy, T. K. (2015). Effect of pretreatments and modified atmosphere packaging on the shelf life and quality of fresh-cut green bell pepper. Journal of Food Science and Technology, 52(12), 7872–7882. https://doi.org/10.1007/s13197-015-1928-7
Rojas-Grau, M. A., Oms-Oliu, G., Soliva-Fortuny, R., & Martín-Belloso, O. (2009). The use of packaging techniques to maintain freshness in fresh-cut fruits and vegetables: A review. International Journal of Food Science and Technology, 44, 875–889. https://doi.org/10.1111/j.1365-2621.2009.01911.x
Sahoo, N. R., Bal, L. M., Pal, U. S., & Sahoo, D. (2014). A comparative study on the effect of packaging material and storage environment on shelf life of fresh bell-pepper. Journal of Food Measurement and Characterization, 8(3), 164–170. https://doi.org/10.1007/s11694-014-9177-4
Sakaldaş, M., & Kaynaş, K. (2010). Biochemical and quality parameters changes of green sweet bell peppers as affected by different postharvest treatments. African Journal of Biotechnology, 9(48), 8174–8181. https://doi.org/10.5897/AJB10.1021
Sánchez-Mata, M. C., Cámara, M., & Díez-Marqués, C. (2003). Extending shelf-life and nutritive value of green beans (Phaseolus vulgaris L.), by controlled atmosphere storage: Micronutrients. Food Chemistry, 80, 317–322.
Saxena, A., Saxena, T. M., Raju, P. S., & Bawa, A. S. (2013). Effect of controlled atmosphere storage and chitosan coating on quality of fresh-cut jackfruit bulbs. Food and Bioprocess Technology, 6(8), 2182–2189. https://doi.org/10.1007/s11947-011-0761-x
Severino, R., Ferrari, G., Vu, K. D., Donsì, F., Salmieri, S., & Lacroix, M. (2015). Antimicrobial effects of modified chitosan based coating containing nanoemulsion of essential oils, modified atmosphere packaging and gamma irradiation against Escherichia coli O157:H7 and Salmonella Typhimurium on green beans. Food Control, 50, 215–222. https://doi.org/10.1016/j.foodcont.2014.08.029
Sharma, K., Cardona, J., Sibomana, M., Nampeera, E., & Fallik, E. (2018). Quality attributes of modified atmosphere packaged bell pepper (Capsicum annuum L.) during storage. Journal of Nutrition, Food Research and Technology, 1(2), 56–62. https://doi.org/10.30881/jnfrt.00012
Singh, R., Giri, S. K., & Kotwaliwale, N. (2014). Shelf-life enhancement of green bell pepper (Capsicum annuum L.) under active modified atmosphere storage. Food Packaging and Shelf Life, 1(2), 101–112. https://doi.org/10.1016/j.fpsl.2014.03.001
Singleton, V. L., Orthofer, R., & Lamuela-Raventós, R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymology, 299, 152–178. https://doi.org/10.1016/S0021-9673(00)00080-7
Taheri, A., Behnamian, M., Dezhsetan, S., & Karimirad, R. (2020). Shelf life extension of bell pepper by application of chitosan nanoparticles containing Heracleum persicum fruit essential oil. Postharvest Biology and Technology, 170, Article 111313. https://doi.org/10.1016/j.postharvbio.2020.111313
Toledo-Martín, E. M., García-García, M. C., Font, R., Moreno-Rojas, J. M., Gómez, P., Salinas-Navarro, M., & Río-Celestino, M. D. (2016). Application of visible / near-infrared reflectance spectroscopy for predicting internal and external quality in pepper. Journal of the Science of Food and Agriculture, 96(9), 3114–3125. https://doi.org/10.1002/jsfa.7488
Vunnam, R., Hussain, A., Nair, G., Bandla, R., Gariepy, Y., Donnelly, D. J., Kubow, S., & Raghavan, G. S. V. (2014). Physico-chemical changes in tomato with modified atmosphere storage and UV treatment. Journal of Food Science and Technology, 51(9), 2106–2112. https://doi.org/10.1007/s13197-012-0690-3
Wahba, N. M., Ahmed, A. S., & Ebraheim, Z. Z. (2010). Antimicrobial effects of pepper, parsley, and dill and their roles in the microbiological quality enhancement of traditional Egyptian Kareish cheese. Foodborne Pathogens and Disease, 7(4), 411–418. https://doi.org/10.1089/fpd.2009.0412
Yahia, E. M., & Ornelas-Paz, J. J. (2010). Chemistry, stability, and biological actions of carotenoids. In L. A. De la Rosa, E. Álvarez-Parrilla, & G. A. González-Aguilar (Eds.), Fruit and Vegetable Phytochemicals: Chemistry, Nutritional Value, and Stability (pp. 177–222). Blackwell Publishing.
Zhang, X.-J., Zhang, M., Chitrakar, B., Devahastin, S., & Guo, Z. (2022). Novel combined use of red-white LED illumination and modified atmosphere packaging for maintaining storage quality of postharvest pakchoi. Food and Bioprocess Technology, 15(3), 590–605. https://doi.org/10.1007/s11947-022-02771-x
Zhang, X. T., Zhang, M., Devahastin, S., & Guo, Z. (2019). Effect of combined ultrasonication and modified atmosphere packaging on storage quality of pakchoi (Brassica chinensis L.). Food and Bioprocess Technology, 12(9), 1573–1. https://doi.org/10.1007/s11947-019-02316-9
Zhong, Z., Zhou, L., Yu, K., Jiang, F., Xu, J., Zou, L., Du, L., & Liu, W. (2022). Effects of microporous packaging combined with chitosan coating on the quality and physiological metabolism of passion fruit after harvest. Food and Bioprocess Technology. https://doi.org/10.1007/s11947-022-02845-w
Zhuang, Y., Chen, L., Sun, L., & Cao, J. (2012). Bioactive characteristics and antioxidant activities of nine peppers. Journal of Functional Foods, 4(1), 331–338. https://doi.org/10.1016/j.jff.2012.01.001
Acknowledgements
Our thanks to AGACA (Asociación Gallega de Cooperativas Agroalimentarias) for the supply and storage of samples, to Carburos Metálicos Company for packaging samples in modified atmospheres, and to JoDee Anderson for the linguistic support she provided.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research was supported by the Autonomous Government of Galicia (Spain) project PGIDIT09TAL003E.
Author information
Authors and Affiliations
Contributions
V.O-B. performed the processing and analyses; C.P–C. wrote the initial manuscript draft and interpreted statistical analysis; M.L. Vázquez-Odériz and M.A. Romero-Rodríguez designed the study and the selection of samples, interpreted the results and coordinated the study. All authors took part in the editing and approval of the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Olveira-Bouzas, V., Pita-Calvo, C., Romero-Rodríguez, M.Á. et al. Evaluation of a Packaging System in Pallets Under Modified Atmosphere to Extend the Shelf-life of ‘Padrón’ Peppers Stored at Refrigeration Temperature. Food Bioprocess Technol 16, 785–803 (2023). https://doi.org/10.1007/s11947-022-02966-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-022-02966-2