Abstract
To maximise tenderness, minimise cooking loss, and decrease the adverse effects on the colour of beef short ribs, third-order multiple regression models were used to optimise the processing parameters during the pulsed electric fields (PEF) followed by sous vide (SV) processing. Electric field strength (EFS) and specific energy (SE) of PEF ranged from 0 to 0.85 kV/cm and 0 to 110.96 kJ/kg, respectively, and SV processing for 12, 24, or 36 h at 60 °C was applied. The optimum PEF-SV processing conditions for short ribs were found at EFS of 0.85 kV/cm and SE of 110.96 kJ/kg with SV processing at 60 °C for 23.96 h. In the absence of PEF pre-treatment, the optimum SV time at 60 °C was 36 h. PEF pre-treatments (SE, 99–110.96 kJ/kg) followed by SV (24 h) at 60 °C resulted in a significantly lower cooking loss (%) and texture profile analysis hardness compared to PEF at all treatment intensities, followed by SV at 60 °C for 36 h. Furthermore, significant differences were not observed in the redness, hue angle, and chroma at different PEF-SV processing parameters. Therefore, for industrial applications, PEF-SV processing with EFS (0.85 kV/cm), pulse width (20 µS), pulse frequency (50 Hz) and SE (110.96 kJ/kg), and SV temperature–time combination of 60 °C and 23.96 h is highly recommended.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sous vide (SV) processing can improve the tenderness of meat with little impact on its colour and flavour (Alahakoon et al., 2018; Baldwin, 2012; Bhat et al., 2020). SV processing at temperatures in the range between 60 and 75 °C for 17 to 36 h has been reported to enhance the tenderness of beef (Bhat et al., 2020; Christensen et al., 2013; Rinaldi et al., 2014), pork (Christensen et al., 2011), lamb (Roldán et al., 2013, 2014), goat (Tangwatcharin et al., 2019), and chicken meat (Karpińska-Tymoszczyk et al., 2020; Kerdpiboon et al., 2019; Silva et al., 2017). However, the long cooking time required for SV processing implies that this processing is uneconomical and impractical for industrial applications. Previous efforts to shorten SV cooking time have involved injecting exogenous proteolytic enzymes into the meat prior to SV processing. However, as the enzymes have a high inactivation temperature, the residual enzymes found in meat can cause over-tenderisation, a mushy texture, off-flavours development, and quality loss (Alahakoon et al., 2019; Bekhit et al., 2016).
Subjecting the meat to pulsed electric fields (PEF) processing prior to SV demonstrated a potential as a means to reduce SV time (Alahakoon et al., 2018, 2019; Roldán et al., 2013). Alahakoon et al., (2018, 2019) reported that for tough beef brisket (deep and superficial pectoralis), PEF pre-treatments (EFS, 0.7–1.5 kV/cm; SE, 90–100 kJ/kg) before SV processing (60 °C/24–72 h) enhanced tenderness without adversely affecting colour, cooking loss, or lipid oxidation and reduced the effect of biological variability (Alahakoon et al., 2018). PEF treatment is postulated to cause electroporation in the muscle fibres, causing the release of Ca2+ and µ-calpain enzymes, thereby stimulating early proteolysis (Bekhit et al., 2014; Bhat et al., 2019b; Suwandy et al., 2015a). The onset of protein breakdown increases the fragmentation of myofibrils and the disintegration of myosin heavy chains (Faridnia et al., 2014). Additionally, PEF processing reduces the size of the muscle and increases the gaps between muscle cells (Gudmundsson & Hafsteinsson, 2001) leading to an increase in the porosity and thermal solubility of the connective tissue, which enhances tenderness (Alahakoon et al., 2017).
Adverse effects of PEF treatment on meat quality were also reported, which include PEF treatments with processing parameters of EFS in the range of 2–2.8 kV/cm and SE in the range of 149.8–226 kJ/kg, which reduced the water holding capacity (WHC) and an increase in the drip loss of meat, reducing tenderness (Bhat et al., 2019a; Khan et al., 2017; O'Dowd et al., 2013). Such changes have been speculated to be due to an increase in shrinkage and denaturation of myofibrillar proteins and enzymes (Khan et al., 2017; O'Dowd et al., 2013).
Before PEF-SV processing can be used commercially, it is crucial that its effect on meat quality is well understood and that the process is optimised. Optimisation of PEF processing parameters however is not a trivial exercise as animal age, breed, sex, muscle types, sample preparation (freezing, thawing, and method of deboning of the animals) before PEF treatment also affect meat quality (Bekhit et al., 2016; Faridnia et al., 2015). Furthermore, the distribution of electric field during PEF processing is affected by quantity and distribution of connective tissue and fat and muscle fibre orientation, which affect the outcome of PEF treatment (Alahakoon et al., 2017; Suwandy et al., 2015c).
To date, all published studies on the impact of PEF on meat quality have been on boneless meat cuts. This study is the first one that is investigating the optimisation of PEF treatment on bone-in meat cuts such as short ribs. Short ribs are heterogeneous bone-in tough cuts, which have a variable distribution of connective tissue, fat, bone, fibre orientation, and a wide range of electrical conductivity in meat as well as in the bone. Short ribs could potentially benefit from PEF-SV processes considering quality parameters like tenderness, cooking loss, colour, collagen solubility, and fragmentation of myofibrils. In addition, optimum PEF processing parameters and optimum SV time and temperature should be determined. Hence, this study was designed to find an optimum PEF pre-treatment and investigate the effect of PEF-SV processing parameters on the quality properties of beef short ribs.
Materials and Methods
Meat Samples
Beef short ribs (Transversus thoracis muscle) were obtained from six Angus steers (New Zealand). The weights of the left and right sides of the carcass, bone-in, were 138.6–160.2 kg and 134.8–161.6 kg, respectively. The post-rigour meat was vacuum-packed and frozen to − 18 °C in a blast freezer. Using a band saw, the frozen ribs were cut longitudinally into single short rib sections (~ 5 × 5 × 5 cm), which were stored at − 18 °C. Prior to use, the short ribs were thawed at 4 °C for 16 h. On average, the electrical conductivity of the muscle portion around the bone of short ribs was ranging between 5 and 6.5 mS/cm.
A 4 × 3 complete factorial design was used, taking PEF pre-treatments and SV time as the factors. High-intensity (SE, 84–111 kJ/kg), medium-intensity (SE, 57–71 kJ/kg), and low-intensity PEF (SE, 28–35 kJ/kg) and without PEF (SE, 0 kJ/kg) pre-treatments were followed by SV treatments for 12, 24, or 36 h at 60 °C. The SV at 60 °C was the optimum temperature elucidated from an earlier SV optimisation study by the authors (Karki et al., 2022). The PEF-SV treatment combinations were assigned using Latin square design, and the number of replicates for each treatment combination was 16.
PEF Processing Before Sous Vide Processing
Four PEF pre-treatments (high-, medium-, low-intensity PEF, and without PEF) were used in this study. The pilot-scale PEF system (Elcrack-HVP 5, DIL Quakenbruck, Germany) was used in batch mode configuration. Each short rib (~ 5 × 5 × 5 cm) was carefully placed in the centre of the PEF chamber (10 × 8 × 5 cm3 length × width × height) with bone perpendicular to the direction of the electric current (Fig. 1). The distance between the electrodes was 8 cm. Agar gel slab (1% agar, conductivity between 5 and 6.5 mS/cm) was tightly placed between the electrode and the short rib to ensure that the delivery of electric current could be applied consistently to each sample. PEF treatment with a constant input voltage of 10 kV, a constant pulse width of 20 µs, and a constant frequency of 50 Hz with variable pulse number (PN) resulting in variable specific energy (SE) was applied. A square wave bipolar pulse was applied in the treatment, and the pulse shape was measured continuously using an oscilloscope (Model UT2025C, Uni-Trend Group Ltd., Hong Kong, China). The electric field strength (kV/cm) and pulse energy along with other PEF processing parameters were recorded by the built-in sensors of PEF equipment during treatment, and the specific energy (kJ/kg) was calculated from Eq. (1). Electric field strength < 1 kV/cm was applied in this study based on the work of Alahakoon et al. (2019) using the same PEF Elcrack-HVP 5 system. Table 1 summarises the processing variables assigned to each treatment and the processing output recorded after treating short rib muscles from 6 different animals:
The reason EFS is changing even though voltage is constant is attributed by the product characteristics that pose electrical resistance during delivery of short pulses with fixed input voltage. In the context of short ribs, this is a heterogenous matrix with different meat fibre orientation, presence of bones (an insulator), variation of fat (an insulator) distribution, and the electrical conductivity value that can vary greatly within the same muscle tissue. The EFS value reported in Table 1 which varied from 0.5 to 0.85 kV/cm under constant input voltage clearly illustrates that EFS can be affected by the product/sample properties. In this study, the reported EFS value was based on short rib muscles from 6 different animals.
Sous Vide (SV) Treatment
Immediately after PEF pre-treatments, the temperature and electrical conductivity of the samples were measured. Each short rib was individually vacuum-packed (Audionvac VMS 153, Derby, UK) and sealed in a three-layer (polyethylene, ethylene vinyl alcohol (EVOH), and polyamide) and aluminium laminate (Contour Sales Packaging Systems, Tauranga, New Zealand). Each layer in the laminate was glued together using food-grade adhesives with a gauge of 79 µm, oxygen permeability of ≤ 0.1 cc/m2/day at 23 °C, 65% RH, and a WVTR of ≤ 0.1 g/m2/day.
The vacuum-packed short ribs were heated by submerging them in a water bath (PT 60; DIL, Quakenbruck, Germany) at 60 °C for 12, 24, or 36 h. The temperature–time profile of the water bath and inside the packaging was monitored using a temperature logger (i-button, Thermochron, Deventer, The Netherlands). After the SV treatment, the packed meat was immediately submerged in an ice water bath for 1 h. Then, the pack was opened, and short ribs were held at 4 °C for up to 24 h for analysis. The short rib sub-samples for further quality measurements were stored at − 80 °C.
Determination of Instrumental Meat Colour, Cooking Loss, Soluble Collagen, and Texture Profile Analysis (TPA) Parameters
Commission Internationale d’Eclairage (CIE) L* (white-black), a* (red-green), and b* (yellow blue) values were measured using a Hunter Lab Mini Scan XE Plus (Hunter Associates Laboratory Inc., Reston, VA, USA) following the procedure described by Karki et al. (2022). From these values, chroma (\({C}^{*}=\sqrt{{a}^{*2}+{b}^{*2}}\)), hue angle (\({\mathrm{h}=\mathrm{tan}}^{-1}\frac{{b}^{*}}{{a}^{*}}\)), and change or difference in L*, a*, b*, h, and C* after and before SV as \(\Delta {L}^{*}\),\(\Delta {a}^{*}\),\(\Delta {b}^{*}\), \(\Delta h\), and \(\Delta {C}^{*}\), respectively, were also determined.
Cooking loss was measured as a percentage of loss in weight before and after SV treatment, as described in Eq. (2):
TPA parameters were measured using a TA-XT 2i Texture Analyser (Stable Micro Systems Ltd., Surrey, UK) as per Karki et al. (2022). The amount of collagen solubilised after PEF-SV processing was determined using a modified procedure from Hill (1966) as per Alahakoon et al. (2018).
Determination of Myofibrillar Fragmentation Index (MFI)
A modified method by Aroeira et al. (2020) was used to determine the myofibrillar fragmentation index (MFI). Lean meat without fat or connective tissue (1 g) was mixed with 40-mL ice-cold (4 °C) buffer solution (a mixture of potassium chloride (100 mM), potassium phosphate (20 mM), magnesium chloride (1 mM), sodium azide (1 mM), and ethylenediaminetetraacetic acid disodium salt (1 mM)) and homogenised using an Ultra-Turrax (IKA® Works (Asia) Sdn Bhd, Selangor, Malaysia) at 15,000 rpm for 30 s. The homogenisation at 15,000 rpm for 30 s was repeated twice. After that, the homogenate was centrifuged at 2300 g for 30 min at 4 °C, and the supernatant was discarded. The pellet formed after the centrifugation was re-suspended using the same buffer as mentioned previously (40 mL) and re-centrifuged at 2300 g for 30 min at 4 °C. The supernatant was again discarded, and the remaining pellet was suspended in the same buffer (20 mL) and homogenised at 15,000 rpm for 30 s. Finally, the homogenate was sieved through an 18-mesh size metal sieve and collected for further analysis.
The protein concentration of the sieved suspension was determined using Lowry method (Wrolstad et al., 2005). The final protein concentration of the suspension was adjusted to 0.5 ± 0.05 mg/mL with the same buffer as above, and the absorbance was measured immediately at 540 nm using a spectrophotometer (Speccord 50 plus, Analytik Jena GmbH, Jena, Germany). Then, the mean of the triplicate absorbance readings was multiplied with a factor of 200 to obtain the MFI as described by Culler et al. ( 1978).
Data Analysis
The effect of SV time (12, 24, or 36 h) and SE of the PEF treatment on the cooking loss, TPA parameters, L*a*b* colour profile, soluble collagen, and MFI were analysed by a two-way analysis of variance (ANOVA) full factorial model. GraphPad Prism for Windows (GraphPad Software, California, USA, version 9.1.2 (226) was used for ANOVA. In addition, the post hoc Tukey test for multiple comparisons was performed at a 5% level of significance.
The optimisation of SV time, EFS, and SE of PEF treatment for response variable was carried out by fitting a third-order multiple regression model (Eq. 3) using Minitab version 18.1, Statistical Software (2017), State College, PA: Minitab, Inc., USA. (www.minitab.com):
where:
\(Y\) is response variable.
\({X}_{1},{X}_{2}, and{ X}_{3}\) are SV time, EFS, and SE, respectively.
\({\beta }_{0}\) is the coefficient or constant terms.
\({\beta }_{1}, {\beta }_{2}\), and \({\beta }_{3}\) are linear coefficients for \({X}_{1}, {X}_{2}, and {X}_{3}\), respectively.
\({\beta }_{4}, {\beta }_{5},\) and \({\beta }_{6}\) are quadratic coefficients.
\({\beta }_{7}, {\beta }_{8},\) and \({\beta }_{9}\) are linear interaction coefficients.
\({\beta }_{10}, {\beta }_{11},\) and \({\beta }_{12}\) are cubic coefficients.
\({\beta }_{13}, {\beta }_{14},{\beta }_{15} , {\beta }_{16}{, \beta }_{17}, {\beta }_{18},\) and \({\beta }_{19}\) are higher-order interaction coefficients for \({X}_{1},{X}_{2},\) and\({X}_{3}\), respectively.
\(e\) is the error term.
The model was simplified by backward elimination, with elimination criteria (α) significance set at 0.05 to remove insignificant terms in hierarchical order. The optimum values of EFS and SE and SV time were calculated by response optimiser using a simplified model for cooking loss, hardness, hue angle, and change in hue angle and chroma after and before SV. The feasible regions for PEF and SV process parameters were determined from an overlaid contour plot, obtained from the significant response variable terms with 95% confidence intervals of optimum values of response variables as upper and lower limits for the contours.
Results and Discussion
Change in Temperature and Electric Conductivity After PEF Treatments
In this study, there was no significant difference in final temperature of short rib samples after high- (SE, 84–111 kJ/kg), medium (SE, 57–71 kJ/kg), and low- (SE, 28–35 kJ/kg) intensity PEF treatments (Fig. 2A). The average final temperature of sample never exceeds 23.66 ± 2.28 °C, even after high-intensity PEF treatment. Therefore, the result indicates that the temperature increase in sample after high-intensity PEF treatment is less likely to cause thermal-induced denaturation of myofibrillar proteins and pose adverse effect on meat quality. It is important to note that increases in the temperature of short ribs after high-, medium-, and low-intensity PEF treatments (ΔT < 8 °C) (Fig. 2B) were lower than those ΔT values reported by O’Dowd et al. (2013) (ΔT = 5–30 °C after PEF treatment of V, 10 kV; PW, 20 μs; EFS, 0.58–0.73 kV/cm; and PF, 90 Hz) for beef semitendinosus (ST) muscle and by Faridnia et al. (2015) (ΔT = 10–12 °C after PEF treatment of EFS, 1.4 kV/cm; PW, 20 μs; PF, 50 Hz; PN, 1032; and SE, 250 kJ/kg) for beef ST muscle. But, direct comparison of ΔT among these studies can be difficult due to differences in PEF processing conditions, chamber size, and types of muscles. The plausible reason for the lower ΔT value observed in the present study in PEF-treated short ribs could be the fact that PEF treatment involves treating the sample using short pulses (20 μs) of high voltage with a time gap between pulses; it is expected that the sample temperature increase will be intermittent and short (20 μs) during the pulse delivery. After which, the temperature will decrease and equilibrate with the surroundings during the time gap between pulses due to energy dissipation and hence attributed to minimal changes in ΔT of sample after PEF treatment.
Change in temperature (°C) and electrical conductivity (mS/cm) of beef short ribs after PEF treatment. The number of replicates (n) = 16. The mean values are presented as a histogram with standard error bars. Different letters (a, b, and c) above the histograms indicate a statistically significant (p < 0.05) difference between the treatments
The final electrical conductivity of beef short ribs after PEF treatments was not significantly different (p > 0.05) (Fig. 2C). However, the increase in conductivity after high and medium intensities PEF are significantly (p < 0.05) higher than low-intensity PEF treatment (Fig. 2D). The increase in conductivity might be due to leaching of ions in the sarcoplasm due to the cellular damage (Faridnia et al., 2014), but there is no significant difference in water loss (%) after different intensity PEF treatment (Fig. 3B).
Weight loss after PEF-SV processing (A). Cooking loss (%) after PEF pre-treatments and sous vide at different times (B). Weight loss (%) after PEF pre-treatments. The number of replicates (n) = 16. The mean values are presented as a histogram with standard error bars. Different letters above the histograms indicate a statistically significant (p < 0.05) difference between the treatments
Weight and Cooking Loss (%) After PEF and SV Treatment
The cooking loss (%) of short ribs significantly (p < 0.001) increased as cooking time was prolonged, with PEF pretreated short ribs having a significantly (p = 0.001) lower cooking loss (%) than untreated short ribs (Supplementary Table S1). However, the interaction term between PEF treatments and cooking time was not significant (p = 0.672). SV processed short ribs for 24 and 36 h had significantly (p < 0.05) higher cooking loss (%) (17.83 ± 2.62 and 20.39 ± 2.71, respectively) than for 12 h (16.57 ± 2.53). Figure 2A shows the difference in cooking loss (%) among PEF-SV combinations. However, there was no significant difference (p > 0.05) in weight loss (%) of short ribS after PEF among the three (i.e. low, medium, and high intensity) PEF treatments (Fig. 3B).
An increase in cooking time significantly increased the cooking loss (%). The cooking loss was significantly lower after PEF treatment than without PEF treatment. The current results are similar to those reported in earlier studies. PEF treatment (EFS between 0.36 and 1.5 kV/cm and SE between 18.63 and 250 kJ/kg with a pulse width (PW) of 20 µs and frequency (PF) of 20–200 Hz) did not significantly increase cooking loss for beef deep pectoralis (DP) muscle (Alahakoon et al., 2019), semimembranosus (SM) muscle (Bhat et al., 2019a), longissimus lumborum (LL) muscle (Khan et al., 2017), and semitendinosus (ST) muscle (Faridnia et al., 2015). Lower cooking loss enhances the juiciness, succulence, and tenderness of meat (Arroyo et al., 2015a, 2015b). However, Suwandy et al. (2015b) observed a significant increase in cooking loss (%) of PEF-treated (EFS 0.28–0.56 kV/cm, PW 20 µs, PF 20–90 Hz, SE 3.2–70.1 kJ/kg) hot-boned beef LL and SM muscles compared to untreated samples. That result may be because the muscle was removed from the carcass in a pre-rigour state, making it more susceptible to contraction and shortening than cold boned muscle owing to the lack of a skeleton framework, which resulted in a higher water expulsion from the muscle fibres (Bekhit et al., 2016).
Texture Profile Analysis (TPA) Parameters After PEF-SV Treatment
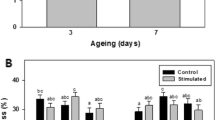
The difference in TPA parameters at different PEF-SV treatment combinations was shown in Fig. 4. There was a significant effect of SV time (p < 0.001) and PEF treatments (p < 0.001) on the TPA hardness of short ribs (Supplementary Table S2). The interaction between SV time and PEF treatment was also significant (p = 0.001), which supported the idea that the effect of SV time on TPA hardness was affected by the intensity of PEF pre-treatment used. Short ribs SV processed for 12 or 24 h with high-intensity PEF pre-treatment (SE, 84–111 kJ/kg) had significantly (p < 0.05) lower TPA hardness (N) than short ribs SV processed for 36 h either with or without PEF (Fig. 4). Thus, an increase in PEF treatment intensity increased meat tenderness.
Texture profile analysis (TPA) parameters of short ribs after PEF pre-treatments and sous vide (SV) processing A TPA hardness (N), B TPA adhesiveness (Ns), C TPA cohesiveness, D TPA springiness, E TPA gumminess, F TPA chewiness, and G TPA resilience. The number of replicates (n) = 48 (16 meat pieces with 3 reading each). The mean values are presented as a histogram with standard error bars. Different letters above histograms indicate a statistically significant (p < 0.05) difference between the treatments
There was no significant difference (p > 0.05) in TPA adhesiveness of short ribs after different PEF pre-treatments and SV for 12, 24, or 36 h; the interaction term between PEF pre-treatment and SV time was also insignificant (p > 0.05) (Supplementary Table S2). However, there was a significant effect of SV time (p < 0.001) and PEF pre-treatment (p < 0.01) on TPA cohesiveness, springiness, gumminess, chewiness, and resilience of short ribs. The interaction between PEF pre-treatment and SV time parameters for TPA cohesiveness, springiness, gumminess, chewiness, and resilience was also significant (p = 0.015, p = 0.002, p < 0.001, p = 0.002, p < 0.001, respectively). Short ribs SV processed for 24 h with high-intensity (SE, 84–111 kJ/kg) PEF had significantly higher (p < 0.05) cohesiveness than short ribs SV processed for 36 h either with medium intensity (SE, 57–71 kJ/kg) or without PEF treatment (Fig. 4).
Soluble Collagen (%) After PEF-SV Treatment
There was a significant effect of SV time (p < 0.001) and PEF pre-treatment (p = 0.010) on the percentage of soluble collagen (%) of SV treated short ribs (Table S3). The interaction effect between SV time and PEF pre-treatment was not significant (p = 0.736). The SV processed short ribs with high-intensity PEF had significantly higher (p < 0.05) soluble collagen (%) (16.99 ± 3.97) than low-intensity PEF-treated ones (14.15 ± 4.56). Short ribs SV processed for 36 and 24 h had significantly higher soluble collagen (%) (18.85 ± 3.02 and 16.30 ± 3.86, respectively) than short ribs SV processed for 12 h (11.25 ± 3.50). Short ribs treated with medium- and low-intensity PEF or without PEF that were subsequently SV processed for 12 h had significantly lower (p < 0.05) soluble collagen (%) than SV processed for 24 or 36 h with high- and medium-intensity PEF pre-treatment (Fig. 5).
Soluble collagen (%) after PEF pre-treatments and sous vide (SV) processing. The number of replicates (n) = 11. The mean values are presented as a histogram with standard error bars. Different letters above the histograms indicate a statistically significant (p < 0.05) difference between the treatments
Myofibrillar Fragmentation Index (MFI) After PEF-SV Treatment
PEF treatments had a significant effect (p = 0.001) on myofibrillar fragmentation index (MFI) of SV processed short ribs. The impact of SV time (p = 0.992) and the interaction between SV time and PEF pre-treatment (p = 0.277) on MFI of short ribs were not significant (Supplementary Table S3). Short ribs that are SV processed without PEF pre-treatments had significantly lower (p < 0.05) MFI (184.99 ± 11.39) than that with high-intensity PEF (197.01 ± 16.17) and then SV processed. SV processed for 36 h after high-intensity PEF pre-treatments had significantly (p < 0.05) higher MFI values (202.56 ± 13.33) than 36 h SV processed without PEF pre-treatment (182.55 ± 11.19). Similarly, short ribs SV processed for 12 h after medium-intensity PEF had significantly (p < 0.05) higher MFI values (201.42 ± 14.49) than short ribs SV processed for 12 h at 60 0C without PEF (181.89 ± 13.07) (Fig. 6).
Myofibrillar fragmentation index (MFI) after PEF pre-treatments and sous vide (SV) processing. The number of replicates (n) = 10. The mean values are presented as a histogram with standard error bars. Different letters above the histograms indicate a statistically significant (p < 0.05) difference between the treatments
In the current study, short ribs treated with high-intensity PEF treatment and subjected to SV processing for 24 h had significantly lower TPA hardness than short ribs SV processed for 36 h with or without PEF treatment. TPA hardness is the perceived physical quantity, which is related to the peak force in the first compression to attain a crack or a fracture in the ingested food (Nishinari & Fang, 2018). Roldán et al. (2013) correlated lower hardness values of meat to the solubilisation of connective tissue and partial denaturation of sarcoplasmic protein. A higher percentage of collagen solubilisation with an increase in PEF treatment intensity was also observed in the current study. Alahakoon et al. (2019) also observed the lowest value of TPA hardness (< 20.2 N) with PEF pre-treatment (EFS, 0.7–1.4 kV/cm; PW, 20 µs; PF, 50 Hz; SE, 90–100 kJ/kg) followed by SV processing for 20.2–23.7 h. However, Alahakoon et al. (2018) observed that the hardness value was lowest for both PEF-treated (EFS, 1.5 kV/cm; PW, 20 µs; PF, 50 Hz; SE, 90–100 kJ/kg) DP muscles or untreated one followed by SV for 72 h at 60 or 65 °C.
The current study found significantly higher hardness after SV for 36 h compared to SV for 24 h and higher cooking loss (%) after SV for 36 h than after 24 h, which contributed to the meat becoming dry and tough as the water holding capacity (WHC) of meat affects its texture, tenderness, and flavour (Arroyo et al., 2015a, b) and higher cooking loss increases the toughening of meat (Roldán et al., 2013). Furthermore, there was a significant increase in soluble collagen (%) after high-intensity PEF than low-intensity PEF when cooked for 24 h, which enhanced the tenderness of short ribs compared to SV for 12 h with low-intensity PEF or without PEF. This may be due to increased solubilisation of connective tissue and gelation of sarcoplasmic protein, which enhances the tenderness of meat (Roldán et al., 2013). Prolonged cooking in the moist SV environment causes an uncoupling of the triple helix structure of collagen, increasing its solubility, leading to gelation and an increase in the tenderness of the meat (Alahakoon et al., 2018; Purslow, 2018). The argument is further supported by Alahakoon et al. (2018). They reported a more porous surface and breakdown in the collagen of isolated connective tissue from PEF-treated DP muscle than non-PEF-treated samples. Furthermore, Zielbauer et al. (2016) reported that SV for > 4 h lowered the denaturation temperature and enthalpy of meat proteins below the peak temperatures, which reiterated the fact that prolonging cooking time at lower temperature and in a moist environment increases the solubilisation and gelation of collagen and sarcoplasmic protein and enhances the tenderness of the meat.
Apart from the amount and distribution of connective tissue, the fragmentation and overlapping of myofibrils also affect meat tenderness (Aroeira et al., 2020; Sawdy et al., 2004). Culler et al. (1978) stated that half of the variation in the tenderness of loin muscles is due to myofibrillar fragmentation and only 10% by collagen solubility. Significant (p < 0.01) correlation had been observed between MFI and sensory tenderness, and Warner–Bratzler shear force in tough DP and rhomboideus (RB) beef muscles (Paterson & Parrish Jr, 1986). Rajagopal and Oommen (2015) also observed a strong correlation between MFI and meat tenderness in buffalo LD muscles. Thus, the MFI can be regarded as an indicator of the degree of meat fragmentation and tenderisation (Aroeira et al., 2020). The results discussed in the current study also agree with the argument above. Significantly, lower TPA hardness and significantly higher MFI were observed after a high-intensity PEF-SV than without PEF-SV. There was no significant difference in MFI values of short ribs after SV for 24 or 36 h, but short ribs SV cooked for 24 h had lower cooking loss than short ribs for 36 h resulting in lower TPA hardness values of short ribs after SV for 24 h than 36 h. Therefore, myofibrillar fragmentation and cooking loss might be the major contributors to the variation in meat tenderness observed in the current study. Thus, lower cooking loss and a similar degree of collagen solubility and myofibrillar fragmentation of short ribs after high-intensity PEF and SV for 24 h and 36 h might be the reason for enhanced tenderness after high-intensity PEF pre-treatments and SV for 24 h than SV for 36 h either with or without PEF.
Instrumental CIE Colour Parameters After PEF-SV Treatment
The change in CIE lightness (ΔL*), redness (Δa*), yellowness (Δb*), hue angle (Δh), and chroma (ΔC*) after and before SV was significantly affected by cooking time (p < 0.001, p < 0.001, p = 0.001, p < 0.001, and p = 0.003, respectively). PEF treatments also significantly affected the ΔL*, Δa*, Δh, and ΔC* of short ribs (p = 0.029, p = 0.001, p = 0.046, and p = 0.021, respectively). Δb* was not significantly affected by PEF pre-treatments (p = 0.549). The interaction terms between the SV time and PEF treatments for ΔL*, Δa*, Δb*, and ΔC* were significant (p = 0.002, p = 0.037, p = 0.002, p = 0.013, and p = 0.006, respectively), but Δh of short ribs did not have a significant interaction term (p = 0.845). The Δa* and ΔC* values of short ribs SV processed for 24 h without PEF were significantly lower (p < 0.05) than short ribs SV processed for 24 h after high-intensity PEF pre-treatments (Fig. 7).
CIE colour parameters of short ribs after PEF pre-treatments and sous vide (SV) processing A change in lightness (ΔL*), B change in redness (Δa*), C change in yellowness (Δb*) after and before SV, D hue angle (h) after SV, E chroma (C*) after SV, F change in the angle (Δh), and G change in chroma (ΔC*) after and before SV. The number of replicates (n) = 16. The mean values are presented as a histogram with standard error bars. Different letters above the histograms indicate a statistically significant (p < 0.05) difference between the treatments
From the current study, it was seen that there was less effect on the colour by PEF pre-treatments as shown by significantly higher Δa* values and lower ΔC* values after PEF and SV for 24 h than without PEF treatment. Alahakoon et al. (2018) also observed that PEF treatment did not have a significant effect on a* value of meat, even after SV for 48 h at 60 °C. Thus, PEF-SV treatment did not affect the colour of meat compared to conventional cooking as meat colour (before and after cooking) influences consumer decision to purchase and accept the cooked meat (Arroyo et al., 2015a, b). The meat colour depends on the myoglobin, such as purple due to reduced myoglobin, red due to oxymyoglobin, and brown by metmyoglobin (Abril et al., 2001). The redness of meat is highly dependent on the meat structure, the concentration, and the oxidation state of myoglobin present in the muscle (de Huidobro et al., 2005). The decrease in a* values is due to an increase in the oxidation of myoglobin to metmyoglobin (Faridnia et al., 2016; Khan et al., 2017; O'Dowd et al., 2013; Suwandy et al., 2015d). The preservation of redness due to PEF-SV may be due to the lower oxygen permeability of the SV packaging and lower level of denaturation of myoglobin (Dominguez-Hernandez et al., 2018; Roldán et al., 2013). C* and h are good indicators of metmyoglobin formation and increased brownness in meat (Khan et al., 2017; Suwandy et al., 2015d). C* explains the colour intensity, and the h value explains the relative position of colour between redness and yellowness (Bekhit et al., 2001). The h value generally increases because of oxidation of myoglobin and oxymyoglobin (Bekhit & Faustman, 2005), which depends on temperature, oxygen pressure, and lipid oxidation (Faustman et al., 2010). There were significantly low \(\Delta h\) values after high-intensity PEF than without PEF. Thus, lower oxidation permeability of laminate and possible lower oxidation and denaturation of myoglobin during PEF-SV processing produce pinkish coloured cooked meat, as seen by visual inspection.
Optimisation of PEF and SV Processing Parameters
The third-order multiple regression was computed with cooking loss (%), TPA parameters (hardness, cohesiveness, springiness, gumminess, chewiness, and resilience), hue angle after SV (h), change in hue angle after and before SV (Δh), change in chroma after and before SV (ΔC*), and soluble collagen (%) as the response variable with SV time (12, 24, and 36 h), EFS (0–0.85 kV/cm), and SE (0–110.96 kJ/kg) of PEF pre-treatments as factors. The multiple regression analysis is significant (p < 0.05) for all parameters (Table 2). The F test for lack of fit was not significant (p ≥ 0.05) for all dependent variables, except springiness, which explained that the predicted regression model for these variables depicted the actual values. The variance inflation factor was < 5 after the standardisation of the elements by subtracting the factors’ value from the mean, resulting in no multicollinearity among the factors.
There was a significant effect (p < 0.001) of EFS on cooking loss (%). The negative linear coefficient clarified that the cooking loss (%) decreased with increasing EFS keeping SV time and SE constant. SE had no significant coefficient (p value > 0.05). However, there was a significant effect (p < 0.001) of SV time on cooking loss (%) (Table 2). The positive coefficient for a quadratic term of SV time explains the curvature of a plot of cooking loss (%), which undergoes the minima at SV time between 19 and 25 h, EFS > 0.7 kV/cm, and SE > 80 kJ/kg (Fig. 8).
Contour plots and surface plots of response variables with processing parameters. A Contour plots of cooking loss (%) with specific energy (SE) and electric field strength (EFS) as a variable with sous vide time held at 19.4554 h; B contour plot of cooking loss (%) with SE and SV time as a variable with EFS held at 0.85 kV/cm; C contour plot of cooking loss (%) with SV time and EFS as a variable with SE held at 110.96 kJ/kg; D surface plot of cooking loss (%) with SV time (h) and SE (9 kJ/kg) as variables with EFS held at 085 kV/cm; E contour plot of hardness with SE and SV time as variables; F surface plot of TPA hardness with SV time and SE as variables
The EFS had no significant effect on TPA hardness (p value > 0.05). However, the linear terms for SV time and SE were significant (p < 0.05) (Table 2). The positive coefficient for quadratic terms of SV time significantly (p < 0.001) reached the minima near 20–25 h and SE > 90 kJ/kg (Fig. 8). Similarly, the negative linear coefficient for SE stated that increasing the SE with all other term constants decreased the hardness. For adhesiveness, the linear term of SE and higher-order interaction between SV time and SE were significant (p < 0.05) (Table 2). A surface plot showed that the adhesiveness reached the lowest value at SV time of 20–25 h and SE > 80 kJ/kg. Thus, SE had a significant (p < 0.001) linear coefficient for cohesiveness, and the negative coefficient iterated that cohesiveness would decrease with increasing SE of PEF pre-treatment (Table 2). The linear and quadratic terms for SV time were significant (p < 0.05); the negative quadratic term for SV time showed that cohesiveness reached maxima at SV time 20–25 h and SE 20–30 kJ/kg.
For springiness, EFS did not have a significant effect (p > 0.05). The linear coefficients for SE were significant (p < 0.001); for SE and SV time, the linear coefficients were positive (Table 2). However, the significant (p < 0.01) quadratic coefficient for SV time was negative with significant higher-order interaction between SV time and EFS of PEF pre-treatment. This stated that for low SE, the springiness reached maxima around SV time of 20–25 h and 0.25 kV/cm. In the case of gumminess, the linear term of SV time and SE were significant. The quadratic term of SV time and SE and higher-order interaction between SV time and EFS were substantial (p < 0.05) (Table 2). Higher EFS and SE resulted in lower gumminess values. For chewiness, the linear terms of SE and SV were significant (p < 0.001), and the negative coefficient explained that chewiness would decrease with an increase in SV time, EFS, and SE. The linear terms of SV time and SE were significant (p < 0.001) for resilience. The quadratic term of SV time was negative and significant (p < 0.05) (Table 2), explaining the curvature and the value of resilience, reaching a maximum of around 20–25 h. The negative coefficient for SE explained that stability would decrease with increased PEF pre-treatments keeping other factors constant.
In the case of CIE hue angle (h), only the linear term of SV time was negative and significant (p < 0.001), which clarified that the hue angle would decrease with an increase in SV time (Table 2). For chroma after SV, only the quadratic coefficient for SV time was significant (p < 0.01), which explained that C* would decrease with increasing SV time until a minimum value was reached and then would increase again. For a change in hue angle after and before SV (Δh), only the linear term for SV time and SE was significant (p < 0.05) (Table 2). The negative linear coefficient for SV time suggested that Δh would decrease with increasing SV time and SE. For a change in chroma after and before SV (ΔC*), the linear term for EFS was significant (p < 0.01) (Table 2). For ΔC*, the quadratic term for SE and SV time was significant (p < 0.05), and the coefficient was positive, but for EFS, the quadratic term is negative, which explained the maxima reached at about EFS of 0.7 kV/cm and the minima reached at SE of 40 kJ/kg. There were significant (p < 0.05) linear terms for SV time and SE of PEF pre-treatments for soluble collagen (%). The linear coefficients were positive, which explained that the soluble collagen (%) would increase with an increase in SV time and SE of PEF pre-treatment (Table 2).
The optimum values obtained by Minitab for the SV time at 60 °C, EFS, and SE of PEF were 23.96 h, 0.85 kV/cm, and 110.96 kJ/kg, respectively, with composite desirability of 0.6387 (Fig. 8). The achievable minimum value of cooking loss (%), TPA hardness, hue angle, C*, Δh, and ΔC* was 13.64, 9.43, 51.66, 23.51, 14.20, and 0.41, respectively. The predicted range (95% confidence interval) of cooking loss (%), hardness (N), h, C*, Δh, and ΔC* values at that optimum condition was in the range of 11.29–16.00, 8.401–10.46, 57–74–55.59, 9.18–9.23, and − 3.86 to 2.92, respectively. The feasible processing regions of PEF-SV at 60 °C (Fig. 9) were EFS (0.65–0.85 kV/cm), SE (100–110.96 kJ/kg), and SV time (18–29 h). The response optimisation of SV time with minimum cooking loss, TPA hardness, h, C*, Δh, and ΔC* for SV processing without PEF pre-treatment at 60 °C was 36 h. The optimum values of the above-mentioned dependent variables (cooking loss, TPA hardness, h, C*, Δh, and ΔC*) were 21.07%, 13.65 N, 50.63, 24.24, 14.02, and 3.27, respectively, with composite desirability of 0.493. Thus, PEF pre-treatment before SV decreased the optimum SV time approximately 12 h.
The overlaid contour plot showing the effect of PEF pre-treatment and sous vide time on the 95% confidence interval of optimum values of cooking loss (%), TPA hardness, change in hue angle (Δh), and chroma (ΔC*) after and before SV. The white area at PEF processing parameters of EFS, 80 kV/cm; SE, > 100 kJ/kg; and SV time of 18–30 h is the feasible area of sous vide processing
A third-order multiple regression model was used to optimise the PEF processing parameters and SV time. The lack-of-fit for cooking loss (%), TPA parameters, and soluble collagen (%) were not significant, which described that model depicted the actual model. There was a significant linear and quadratic term of SV time for cooking loss (%). TPA hardness had significant quadratic terms of SV time. The significant linear terms of SV time for h and \(\Delta h\) were determined. Significant quadratic terms of SV time were determined for C* and \(\Delta {C}^{*}\). In terms of PEF processing parameters, significant linear terms of EFS were observed for cooking loss (%) and \(\Delta {C}^{*}\) with a significant interaction term between EFS and SE for cooking loss and SV time and EFS for \(\Delta {C}^{*}\). For TPA hardness and \(\Delta h\), the significant linear term for SE was observed. From the model, the optimum PEF-SV processing conditions were found to be EFS of 0.85 kV/cm, SE of 110.96 kJ/kg, and SV time of 23.96 h at 60 °C. However, for SV processing without PEF, the optimum cooking time was found to be 36 h. Thus, PEF pre-treatment decreased the SV time considerably, which will provide benefits to the industry with increased productivity and reduced cost. Furthermore, the current study also developed the model for PEF-SV processing, which can be used to determine the effect of different PEF-SV processing conditions on the quality parameters of short ribs. Therefore, for industrial PEF-SV processing of short ribs to enhance their quality, PEF (EFS 0.85 kV/cm, PW 20 µS, PF 50 Hz, and SE 110.96 kJ/kg) and SV (60 °C/23.96 h) are recommended.
The study confirmed that PEF treatment enhanced the tenderness of beef short ribs without increasing cooking loss and affecting colour of beef short ribs. Furthermore, it shortened the sous vide cooking time significantly, which can benefit meat processing industries economically by valorising low value meat cuts, reducing losses, and saving process time and energy, which will also contribute to sustainability of meat processing industry. However, this research did not carried out analysis of processing cost and cost benefit study and recommends these analysis for future studies.
Conclusions
High-intensity (SE, 84–111 kJ/kg) PEF-SV resulted in a significantly lower cooking loss (%), TPA hardness (N), and significantly higher soluble collagen (%) and MFI in short ribs compared to the SV that was only subjected to SV. On the other hand, there was no significant difference in the change in redness, hue angle, and C* among short ribs treated with high intensity or without PEF after SV processing. The optimum PEF-SV processing condition for short ribs was found to be EFS (0.85 kV/cm) and SE (110.96 kJ/kg) for PEF and SV (23.96 h/60 °C) to maximise tenderness and minimise cooking loss and detrimental effect on colour. However, for SV processing without PEF, the optimum SV time at 60 °C was 36 h. Thus, PEF pre-treatment decreased the optimum SV time considerably. So, for industrial application of PEF-SV processing to enhance the quality of short ribs, the PEF treatments of EFS, 0.85 kV/cm; PW, 20 µS; PF, 50 Hz; and SE, 110.96 kJ/kg followed by SV temperature–time combination of 60 °C for 23.96 h are recommended.
Data Availability
The data presented in this study are available on request from the corresponding author. The data are not publicly available because of the results are from the ongoing PhD thesis examination.
References
Abril, M., Campo, M. M., Onenc, A., Sanudo, C., Alberti, P., & Negueruela, A. I. (2001). Beef colour evolution as a function of ultimate pH. Meat Science, 58(1), 69–78. https://doi.org/10.1016/s0309-1740(00)00133-9
Alahakoon, A. U., Faridnia, F., Bremer, P., Silcock, P., & Oey, I. (2017). Pulsed electric fields effects on meat tissue quality and functionality. In Handbook of Electroporation, 2455–2475. https://doi.org/10.1007/978-3-319-32886-7_179
Alahakoon, A. U., Oey, I., Bremer, P., & Silcock, P. (2018). Optimisation of sous vide processing parameters for pulsed electric fields treated beef briskets. Food and Bioprocess Technology, 11(11), 2055–2066. https://doi.org/10.1007/s11947-018-2155-9
Alahakoon, A. U., Oey, I., Bremer, P., & Silcock, P. (2019). Process optimisation of pulsed electric fields pre-treatment to reduce the sous vide processing time of beef briskets. International Journal of Food Science and Technology, 54(3), 823–834. https://doi.org/10.1111/ijfs.14002
Aroeira, C. N., Torres Filho, R. A., Fontes, P. R., Ramos, A. L. S., Contreras Castillo, C. J., Hopkins, D. L., & Ramos, E. M. (2020). Comparison of different methods for determining the extent of myofibrillar fragmentation of chilled and frozen/thawed beef across postmortem aging periods. Meat Science, 160, 107955. https://doi.org/10.1016/j.meatsci.2019.107955
Arroyo, C., Eslami, S., Brunton, N. P., Arimi, J. M., Noci, F., & Lyng, J. G. (2015a). An assessment of the impact of pulsed electric fields processing factors on oxidation, color, texture, and sensory attributes of turkey breast meat. Poultry Science, 94(5), 1088–1095. https://doi.org/10.3382/ps/pev097
Arroyo, C., Lascorz, D., O’Dowd, L., Noci, F., Arimi, J., & Lyng, J. G. (2015b). Effect of pulsed electric field treatments at various stages during conditioning on quality attributes of beef longissimus thoracis et lumborum muscle. Meat Science, 99, 52–59. https://doi.org/10.1016/j.meatsci.2014.08.004
Baldwin, D. E. (2012). Sous vide cooking: A review. International Journal of Gastronomy and Food Science, 1(1), 15–30. https://doi.org/10.1016/j.ijgfs.2011.11.002
Bekhit, A. E. -D. A., & Faustman, C. (2005). Metmyoglobin reducing activity [Review]. Meat Science, 71(3), 407–439. https://doi.org/10.1016/j.meatsci.2005.04.032
Bekhit, A. E. D., Geesink, G. H., Morton, J. D., & Bickerstaffe, R. (2001). Metmyoglobin reducing activity and colour stability of ovine longissimus muscle. Meat Science, 57(4), 427-435. https://doi.org/10.1016/S0309-1740(00)00121-2
Bekhit, A. E. -D. A., Suwandy, V., Carne, A., van de Ven, R., & Hopkins, D. L. (2016). Effect of repeated pulsed electric field treatment on the quality of hot-boned beef loins and topsides. Meat Science, 111, 139–146. https://doi.org/10.1016/j.meatsci.2015.09.001
Bekhit, A. E. -D. A., van de Ven, R., Suwandy, V., Fahri, F., & Hopkins, D. L. (2014). Effect of pulsed electric field treatment on cold-boned muscles of different potential tenderness. Food and Bioprocess Technology, 7(11), 3136–3146. https://doi.org/10.1007/s11947-014-1324-8
Bhat, Z. F., Morton, J. D., Mason, S. L., & Bekhit, A. E. -D. A. (2019a). Does pulsed electric field have a potential to improve the quality of beef from older animals and how? Innovative Food Science & Emerging Technologies, 56, 102194. https://doi.org/10.1016/j.ifset.2019a.102194
Bhat, Z. F., Morton, J. D., Mason, S. L., & Bekhit, A. E. -D. A. (2019b). Pulsed electric field operates enzymatically by causing early activation of calpains in beef during ageing. Meat Science, 153, 144–151. https://doi.org/10.1016/j.meatsci.2019.03.018
Bhat, Z. F., Morton, J. D., Zhang, X., Mason, S. L., & Bekhit, A. E.-D. A. (2020). Sous-vide cooking improves the quality and in-vitro digestibility of semitendinosus from culled dairy cows. Food Research International, 127, 108708. https://doi.org/10.1016/j.foodres.2019.108708
Christensen, L., Ertbjerg, P., Aaslyng, M. D., & Christensen, M. (2011). Effect of prolonged heat treatment from 48 °C to 63 °C on toughness, cooking loss and color of pork. Meat Science, 88(2), 280–285. https://doi.org/10.1016/j.meatsci.2010.12.035
Christensen, L., Ertbjerg, P., Løje, H., Risbo, J., van den Berg, F. W. J., & Christensen, M. (2013). Relationship between meat toughness and properties of connective tissue from cows and young bulls heat treated at low temperatures for prolonged times. Meat Science, 93(4), 787–795. https://doi.org/10.1016/j.meatsci.2012.12.001
Culler, R., Parrish, F., Smith, G., & Cross, H. R. (1978). Relationship of myofibril fragmentation Index to certain chemical, physical and sensory characteristics of bovine longissimus muscle. Journal of Food Science, 43, 1177.
de Huidobro, F. R., Miguel, E., Blazquez, B., & Onega, E. (2005). A comparison between two methods (Warner-Bratzler and texture profile analysis) for testing either raw meat or cooked meat. Meat Science, 69(3), 527–536. https://doi.org/10.1016/j.meatsci.2004.09.008
Dominguez-Hernandez, E., Salaseviciene, A., & Ertbjerg, P. (2018). Low-temperature long-time cooking of meat: Eating quality and underlying mechanisms. Meat Science, 143, 104–113. https://doi.org/10.1016/j.meatsci.2018.04.032
Faridnia, F., Bekhit, A. E. -D. A., Niven, B., & Oey, I. (2014). Impact of pulsed electric fields and post-mortem vacuum ageing on beef longissimus thoracic muscles. International Journal of Food Science & Technology, 49(11), 2339–2347. https://doi.org/10.1111/ijfs.12532
Faridnia, F., Bremer, P., Burritt, D. J., & Oey, I. (2016). Effects of pulsed electric fields on selected quality attributes of beef outside flat (Biceps femoris). In 1st World Congress on Electroporation and Pulsed Electric Fields in Biology, Medicine and Food & Environmental Technologies (pp. 51-54). Springer, Singapore.
Faridnia, F., Ma, Q. L., Bremer, P. J., Burritt, D. J., Hamid, N., & Oey, I. (2015). Effect of freezing as pre-treatment prior to pulsed electric field processing on quality traits of beef muscles. Innovative Food Science & Emerging Technologies, 29, 31–40. https://doi.org/10.1016/j.ifset.2014.09.007
Faustman, C., Sun, Q., Mancini, R., & Suman, S. P. (2010). Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Science, 86(1), 86–94. https://doi.org/10.1016/j.meatsci.2010.04.025
Gudmundsson, M., & Hafsteinsson, H. (2001). Effect of electric field pulses on microstructure of muscle foods and roes. Trends in Food Science & Technology, 12(3–4), 122–128. https://doi.org/10.1016/S0924-2244(01)00068-1
Hill, F. (1966). The solubility of intramuscular collagen in meat animals of various ages. Journal of Food Science, 31(2), 161–170. https://doi.org/10.1111/j.1365-2621.1966.tb00472.x
Karki, R., Bremer, P., Silcock, P., & Oey, I. (2022). Effect of sous vide processing on quality parameters of beef short ribs and optimisation of sous vide time and temperature using third-order multiple regression. Food and Bioprocess Technology, 15, 1629–1646. https://doi.org/10.1007/s11947-022-02849-6
Karpińska-Tymoszczyk, M., Draszanowska, A., Danowska-Oziewicz, M., & Kurp, L. (2020). The effect of low-temperature thermal processing on the quality of chicken breast fillets. Food Science and Technology International. https://doi.org/10.1177/1082013220912592
Kerdpiboon, S., Suraphantapisit, N., Pongpaew, P., & Srikalong, P. (2019). Properties changes of chicken breast during sous-vide cooking and acceptance for elderly. Chiang Mai University Journal of Natural Sciences, 18(2), 156–166. https://doi.org/10.12982/CMUJNS.2019.0012
Khan, A. A., Randhawa, M. A., Carne, A., Mohamed Ahmed, I. A., Barr, D., Reid, M., & Bekhit, A. E. -D. A. (2017). Effect of low and high pulsed electric field on the quality and nutritional minerals in cold boned beef M. longissimus et lumborum. Innovative Food Science & Emerging Technologies, 41, 135–143. https://doi.org/10.1016/j.ifset.2017.03.002
Minitab 17 Statistical Software (2017). [Minitab version 18.1]. State College, PA: Minitab, Inc. (www.minitab.com)
Nishinari, K., & Fang, Y. (2018). Perception and measurement of food texture: Solid foods. Journal of Texture Studies, 49(2), 160–201. https://doi.org/10.1111/jtxs.12327
O’Dowd, L. P., Arimi, J. M., Noci, F., Cronin, D. A., & Lyng, J. G. (2013). An assessment of the effect of pulsed electrical fields on tenderness and selected quality attributes of post rigour beef muscle. Meat Science, 93(2), 303–309. https://doi.org/10.1016/j.meatsci.2012.09.010
Paterson, B., & Parrish, F., Jr. (1986). A sensory panel and chemical analysis of certain beef chuck muscles. Journal of Food Science, 51(4), 876–879.
Purslow, P. P. (2018). Contribution of collagen and connective tissue to cooked meat toughness; some paradigms reviewed. Meat Science, 144, 127–134. https://doi.org/10.1016/j.meatsci.2018.03.026
Rajagopal, K., & Oommen, G. T. (2015). Myofibril fragmentation index as an immediate postmortem predictor of buffalo meat tenderness. Journal of Food Processing and Preservation, 39(6), 1166–1171. https://doi.org/10.1111/jfpp.12331
Rinaldi, M., Dall’Asta, C., Paciulli, M., Cirlini, M., Manzi, C., & Chiavaro, E. (2014). A novel time/temperature approach to sous vide cooking of beef muscle. Food and Bioprocess Technology, 7(10), 2969–2977. https://doi.org/10.1007/s11947-014-1268-z
Roldán, M., Antequera, T., Hernández, A., & Ruiz, J. (2014). Physicochemical and microbiological changes during the refrigerated storage of lamb loins sous-vide cooked at different combinations of time and temperature. Food Science and Technology International, 21(7), 512–522. https://doi.org/10.1177/1082013214552861
Roldán, M., Antequera, T., Martín, A., Mayoral, A. I., & Ruiz, J. (2013). Effect of different temperature–time combinations on physicochemical, microbiological, textural and structural features of sous-vide cooked lamb loins. Meat Science, 93(3), 572–578. https://doi.org/10.1016/j.meatsci.2012.11.014
Sawdy, J. C., Kaiser, S. A., St-Pierre, N. R., & Wick, M. P. (2004). Myofibrillar 1-D fingerprints and myosin heavy chain MS analyses of beef loin at 36 h postmortem correlate with tenderness at 7 days. Meat Science, 67(3), 421–426. https://doi.org/10.1016/j.meatsci.2003.11.014
Silva, F. A. P., Ferreira, V. C. S., Madruga, M. S., & Estevez, M. (2017). Aroma profile and consumer liking of salted and dried chicken meat: Effects of desalting and cooking methods. International Journal of Food Properties, 20(12), 2954–2965. https://doi.org/10.1080/10942912.2016.1263653
Suwandy, V., Carne, A., van de Ven, R., Bekhit, A. E. -D. A., & Hopkins, D. L. (2015a). Effect of pulsed electric field on the proteolysis of cold boned beef M. longissimus lumborum and M. semimembranosus. Meat Science, 100, 222–226. https://doi.org/10.1016/j.meatsci.2014.10.011
Suwandy, V., Carne, A., van de Ven, R., Bekhit, A. E. -D. A., & Hopkins, D. L. (2015b). Effect of pulsed electric field treatment on hot-boned muscles of different potential tenderness. Meat Science, 105, 25–31. https://doi.org/10.1016/j.meatsci.2015.02.009
Suwandy, V., Carne, A., van de Ven, R., Bekhit, A. E. -D. A., & Hopkins, D. L. (2015c). Effect of pulsed electric field treatment on the eating and keeping qualities of cold-boned beef loins: Impact of Initial pH and fibre orientation. Food and Bioprocess Technology, 8(6), 1355–1365. https://doi.org/10.1007/s11947-015-1498-8
Suwandy, V., Carne, A., van de Ven, R., Bekhit, A. E. A., & Hopkins, D. L. (2015d). Effect of repeated pulsed electric field treatment on the quality of cold-boned beef loins and topsides. Food and Bioprocess Technology, 8(6), 1218–1228. https://doi.org/10.1007/s11947-015-1485-0
Tangwatcharin, P., Sorapukdee, S., & Kongsrirat, K. (2019). Sous-vided restructured goat steaks: Process optimized by thermal inactivation of Listeria monocytogenes and their quality characteristics. Food Science of Animal Resources, 39(6), 863–876. https://doi.org/10.5851/kosfa.2019.e64
Wrolstad, R. E., Acree, T. E., Decker, E. A., Penner, M. H., Reid, D. S., Schwartz, S. J., Shoemaker, C. F., Smith, D. M., & Sporns, P. (2005). Handbook of food analytical chemistry, volume 1: Water, proteins, enzymes, lipids, and carbohydrates. John Wiley & Sons.
Zielbauer, B. I., Franz, J., Viezens, B., & Vilgis, T. A. (2016). Physical aspects of meat cooking: Time dependent thermal protein denaturation and water loss. Food Biophysics, 11(1), 34–42. https://doi.org/10.1007/s11483-015-9410-7
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This work was supported by the Food Industry Enabling Technologies (FIET) program funded by the New Zealand Ministry of Business, Innovation and Employment (contract MAUX1402). Oey is affiliated with the Riddet Institute, a New Zealand Centre of Research Excellence, funded by the Tertiary Education Commission. The authors are not engaged in any research project and involved in present or anticipate employment by any organisation that may gain or lose financially through publication of this manuscript.
Author information
Authors and Affiliations
Contributions
Roman Karki, data curation, methodology, software, formal analysis, investigation, validation, visualisation, roles/writing — original draft, conceptualisation, and roles/writing — original draft and editing. Phil Bremer, conceptualisation, funding acquisition, investigation, methodology, project administration, and writing — review and editing. Sze Ying Leong, investigation, methodology, project administration, writing — review and editing. Patrick Silcock, conceptualisation, funding acquisition, investigation, methodology, project administration, and writing — review and editing. Indrawati Oey, conceptualisation, funding acquisition, investigation, methodology, project administration, resources, software, supervision, and writing — review and editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karki, R., Oey, I., Bremer, P. et al. Effect of Pulsed Electric Fields (PEF) Pre-Treatment on the Quality of Sous Vide (SV) Processed Beef Short Ribs and Optimisation of PEF and SV Process Parameters Using Multiple Polynomial Regression Model. Food Bioprocess Technol 16, 216–231 (2023). https://doi.org/10.1007/s11947-022-02932-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-022-02932-y