Abstract
Gelatin (GEL) and whey protein isolate (WPI) are often taken into account as carriers of phytoantioxidants for developing active packaging. The materials obtained, however, have not yet been systematically compared to demonstrate their potential benefits and drawbacks. Fireweed extract (FE) is a rich source of polyphenols with high antioxidant activity. Therefore, in this study, the structural, physicochemical, and antiradical properties of GEL and WPI films incorporated with freeze-dried fireweed extract (FE; 0, 0.0125, 0.025, 0.05%) were simultaneously evaluated. As verified by X-ray diffraction, the GEL-based films were more crystalline and, consequently, mechanically stronger (~ 9–11 vs. ~ 6 MPa) and less permeable to water vapor than the WPI films (44.95–52.02 vs. 61.47–70.49 g mm m−2 day−1 kPa−1). Furthermore, GEL offered a bit more transparent, less yellow, and more stretchable films (~ 50–59% vs. ~ 26%). In turn, the WPI films had superior UV-protective potential. The higher FE concentration yielded more yellow films with improved UV-blocking ability. The FE (0.05%) made the GEL cryogel denser. Based on the half-time reduction of 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) radical cation (tABTS50%), the 0.025, and 0.05% FE-supplemented WPI films exhibited ~ 1.6 and ~ 1.9 times better antiradical potential than the GEL counterparts. This result implies that the WPI-based films, being more soluble (35.12–36.74 vs. 31.51–33.21%) and less swellable (192.61–205.88 vs. 1056.93–2282.47%), ensured faster release of FE into aqueous medium. The slower building up of the antiradical activity of the FE-supplemented GEL films suggests that GEL could be more useful in the development of slow/less migratory active packaging systems for high moisture food.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protein-based products have been studied and developed as structural, apparel, and biomedical materials for decades. Recently, the growing problem of synthetic plastic waste has stimulated research interests in proteins (especially derived from agro-food waste) as a renewable resource for eco-friendly products, including active food and non-food packaging (Ribeiro et al., 2021; Sabaghi et al., 2022; Vargas et al., 2022). Gelatin (GEL) and whey protein originate from by-products of the meat/leather and dairy industries, respectively. These proteins have proved to be highly versatile substances for various purposes. The outstanding potential of GEL in packaging systems (e.g., hard and soft capsules) is based on its high toughness, advantageous optical and oxygen barrier properties, sensory acceptability, and the rapid disintegration at body temperature (Kowalczyk & Baraniak, 2014; Kowalczyk et al., 2020c; Tyuftin & Kerry, 2021). Based on the source, the global GEL market is dominated by porcine GEL, which is regarded to be more stable in comparison with GEL from other sources (Market Research Report, 2019). Plasticized whey proteins form transparent and flexible films with good barrier properties against oxygen (Ramos et al., 2013) and aroma compounds (Miller et al., 1998). The films obtained from whey protein isolate (WPI) are rapidly dissolved after coming in contact with water. The water-insolubility, however, can be obtained by heating of the film-forming solution (FSS). Due to denaturation, previously buried hydrophobic and sulfhydryl groups of whey proteins become exposed which enables building structural integrity by hydrophobic aggregation and formation of covalent intermolecular disulfide bonds (Pérez-Gago et al., 1999). It has been demonstrated that water resistance of WPI films is proportionately dependent on temperature and heating time of FFSs (Perez-Gago & Krochta, 1999), while inhibition of intermolecular disulfide bonds increases protein solubility of WPI films (Fairley et al., 1996).
Active packaging (AP) is an emerging and existing area of food technology that extends the food shelf life better than traditional packaging. Since the exposure of chemicals in food to oxygen in the air destroys essential nutrients and produces off-flavor and toxic compounds, the development of AP materials with antioxidant function is one of the key issues. The principle of antioxidant AP consists in trapping pro-oxidant compounds from food or its environment and/or releasing antioxidants. Ingredients required to achieve the antioxidant effect may be incorporated into the packaging material itself or packed in a sachet or label inserted into the package. The advantage of AP is that a smaller quantity of active compounds can be used since antioxidants act directly on the food surface, i.e., where they are most needed. Current efforts in the development of AP involve both controlled-release formulations and non-migratory systems. The immobilization of additives is desirable if the active compound negatively alters the sensory profile of the product intended to be in direct contact with packaging (e.g., wrapping, sachets, casings, removable coatings).
In response to the increasing demand for chemical-free foods, the development of AP materials incorporated with natural compounds occurring in herbs and spices has received a lot of attention (Luciano et al., 2021; Maroufi et al., 2022; Pluta-Kubica et al., 2021; Zanela et al., 2021). Fireweed (Epilobium angustifolium L.) is a widely distributed plant with well-proven biological activities. A recent study has shown that a freeze-dried aqueous fireweed extract (FE) exhibits a set of numerous antioxidant activities (Szwajgier et al., 2021). Based on this finding, the FE has been used for producing active films based on methyl cellulose (MC) and corn starch (CS). It was found that a partially water-insoluble CS, in contrast to fast-dissolving MC, ensured incomplete release of FE into water (aqueous food simulant). It was concluded, therefore, that the CS film in direct contact with high moisture food would promote maintenance of a higher amount of the antioxidant on the food surface, where the oxidation reactions are most intense (Kowalczyk et al., 2021a, c).
Comparative studies can help in selection of the best material for a given application (Gökkaya et al., 2021). So far, a small number of studies have been focused on the effect of polyphenol-rich plant extracts on the properties of films made of different protein sources. GEL and WPI are often taken into account as carriers of phytoantioxidants for developing AP. The materials obtained, however, have not yet been systematically compared to demonstrate their potential benefits and drawbacks. Polyphenols can interact differently with different proteins through both weak reversible associations (such as hydrogen bonding, hydrophobic bonding, and van der Waals forces) and irreversible interactions (via the formation of covalent bonds as a result of oxidation and nucleophilic addition processes) (Bandyopadhyay et al., 2012). Consequently, the incorporation of polyphenols into protein-based packaging materials may improve or dramatically worsen their functionality (Choi et al., 2018). Therefore, the aim of this study was to assess the effect of increasing concentrations of freeze-dried FE on the functional properties of edible films based on GEL and WPI.
Materials and Methods
Materials
A pork GEL (type A, Bloom strength of 240, Kamis; McCormick Polska, Poland) and WPI (Pure Whey Isolate 97%, Bulk Powders, UK) were used as film formers. Glycerol (Sigma-Aldrich, USA) was used as a plasticizer. The freeze-dried FE was prepared as previously described (Kowalczyk et al., 2021c).
Preparation of Films
The films were prepared from aqueous solutions of the proteins (5% w/w), glycerol (2% w/w), and FE (0, 0.0125, 0.025, 0.05% w/w) according to the previous procedure (Kowalczyk et al., 2021c). Briefly, the protein/glycerol solutions were heated (90 °C, 30 min), cooled (~ 40 °C), mixed with FE, homogenized (30,000 rpm, 2 min), degassed, cast on trays, and dried (~ 25 °C, 50% relative humidity (RH), 24 h). A constant mass of solids (0.0125 g/cm2) was placed onto the trays in order to maintain a comparable film thickness.
Analysis of Film-Forming Solutions (FFSs)
The frozen (in liquid nitrogen) FFS samples were fractured, sublimed (15 min, − 95 °C), and observed at − 145° using a scanning electron microscope (Carl Zeiss Ultra Plus, Oberkochen, Germany). A glass electrode connected to a pH-meter (Elmetron CPC 401, Poland) was used for pH measurement in the FFSs at 30 °C. Foam capacity (FC, %) and foam stability (FS, %) of the FFSs were determined according to a slightly modified method of He et al. (2016). The FFSs (20 mL placed in precisely graduated ISO 100-mL bottle) were preheated to 40 °C (the elevated temperature was necessary to prevent gelling of GEL-based FFSs) and stirred at 30,000 rpm for 30 s (time sufficient to reach a maximum foam capacity) using a MiuLab MT 30 K homogenizer (Hangzhou Miu Instruments Co., Ltd., China). The height of the obtained foams (mm) was measured intermediately with a digital caliper. The whipped FFSs were allowed to stand for 3 h at 40 °C in a water bath. Meanwhile, the height of the foams was remeasured. The height of the foam was converted into the volume of foam (mL). The FC and FS were calculated using the following formulas (Eq. (1) and (2)):
where V1 is the initial volume of the FFS, V2 is the initial volume of the foam, and V3 is the volume of the foam after the storage.
The analyses of the FFSs were performed in triplicate.
Film Thickness and Conditioning
The specimen thickness was determined using a Mitotuyo 547–401 digital thickness gauge (Mitotuyo, Tokyo, Japan). Before testing, the samples were maintained in an MLR-350 climatic test chamber (Sanyo Electric Biomedical Co. Ltd., Japan) for 48 h at 25 °C and 50% RH.
Scanning Electron Microscopy (SEM) of Films
The surface of the films was directly examined (i.e., without applying a metal coating) with a Carl Zeiss Ultra Plus scanning electron microscope (Oberkochen, Germany). The samples were imaged in high vacuum (5 × 10−3 Pa) using a secondary electron detector at 5 kV.
Protein Patterns of Films
The protein patterns of the films were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) according to the method proposed by Laemmli (1970) with slight modifications. The protein powders and film samples were heated in distilled water (5 mL) at 100 °C until completely dissolved. The final concentration of the protein in the solutions was ≈ 2.5 mg/mL. After cooling, the solutions (20 μL) were mixed with a non-reducing loading buffer (0.5 M Tris–HCl buffer (pH 6.8) containing glycerol (20% v/v), SDS (5% v/v), and bromophenol (0.01%)) or a reducing loading buffer (as above + β-mercaptoethanol (ME, 5% v/v)) at a ratio 1:1. The mixtures were incubated at 90 °C for 5 min. Subsequently, 20 μL of each solution and molecular weight markers were loaded onto the gel (4% stacking gel and 12% resolving gel). Electrophoresis was conducted by a Mini-PROTEAN Tetra Cell (Bio-Rad, USA) with a constant voltage of 130 V per gel until the bromophenol blue marker reached the bottom of the resolving gel. The protein bands were stained (methanol (45% v/v), acetic acid (10% v/v), Coomassie Brilliant Blue R-250 (0.1% w/v)) for 30 min, and then destained (methanol (10% v/v) and acetic acid (10% v/v)).
Fourier-Transform Infrared (FTIR) Spectroscopy
FTIR tests of control and 0.05% FE-supplemented films (in the form of sheets) were carried out at ~ 25 °C using an infrared spectrometer (PerkinElmer SP 100, Waltham, MA, USA) and the attenuated total reflectance (ATR) mode. One hundred scans were co-added with a resolution of 4 cm−1 in the range of 4000–650 cm−1. The obtained spectra were normalized, baseline corrected, and analyzed using PerkinElmer Spectrum 10 software. Each sample was scanned twice, observing good reproducibility.
Wide-Angle X-ray Diffraction (WAXD)
WAXD patterns of control and 0.05% FE-supplemented films were obtained on a URD 6 Seifert X-ray diffractometer (FPM-Seifert, Freiberg, Germany) with a Cu Kα radiation source. The operating parameters were as follows: 30 mA, 40 kV, 2θ scanning from 2° to 50° with a step size 0.1°, scanning speed of 0.1° per 15 s, ~ 25 °C. Each sample was scanned twice, observing good reproducibility.
Characterization of Physicochemical and Antioxidant Properties of Films
The analyses of the light transmission (T), opacity (Op), moisture content (MC), water vapor permeability (WVP), tensile strength (TS), elongation at break (E), and elastic modulus (EM) were performed as described previously (Kowalczyk et al., 2021c). Furthermore, the yellowness index (YI) was determined (Kowalczyk et al., 2020c). Additionally, the increase in the film mass (WV gain, %) during the WVP analysis was determined using an analytical balance. Also, the films were analyzed for solubility (So), swelling (Sw) and antiradical kinetics (at 25 °C) as reported previously (Kowalczyk et al., 2021a). The antioxidant activity (estimated using the 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical cation-based assay) was expressed as time needed to reduce the ABTS* + concentration by 50% (tABTS50%). The values were obtained with DDSolver: an add-in software for Microsoft Excel (Zhang et al., 2010) by applying the Weibull model. The values of the coefficient of determination (R2) were provided to present the accuracy of the tABTS50% calculations.
Statistical Analysis
The analysis of variance with p ≤ 0.05 and Fisher’s post hoc test was performed with the Statistica 13.3 software (StatSoft Inc., Tulsa, USA).
Results and Discussion
Analysis of FFSs
Cryo-SEM
Regardless of the protein type, the FE-free (control) FFSs formed honeycomb-like structures with comparable wall thickness and non-uniform pore distribution (Fig. 1A), which is an indicator of heterogeneous processes of ice nucleation. The loading of FE (0.05%) into the GEL FFS made the scaffolds denser (Fig. 1A). A possible explanation for this effect might be that the FE polyphenols, acting as cross-linkers, favored the gelation process in the GEL solution (Strauss & Gibson, 2004; Wu et al., 2001). As with the conventional hydrogel formation, the introduction of extra physical crosslinks among polymer chains generates smaller pores in the cryogel due to the relatively lower volume of free water in the solution that can be crystallized (Henderson et al., 2013). It was not possible to identify the influence of FE on the cryogelling behavior of the WPI solution (Fig. 1A).
pH, FA, and FS
GEL yielded FFSs with significantly lower pH values compared to WPI (~ 5.5 vs. ~ 7.0, Table 1). It is difficult to explain this result, but it might reflect the acid treatment and pH adjustment applied in GEL type A (GMIA, 2012) and WPI production (O’Regan et al., 2009), respectively. The incorporation of FE, regardless of its concentration, did not affect the pH of the FFSs (p > 0.05).
In film-forming solutions, foaming is an unwanted complication that results in microstructural defects (e.g., pinholes) and, consequently, loss of gloss, clarity, and protective function of the material. Since defoaming process slows down material production, the low-foaming components (i.e., with low FC and/or FS values) are highly desirable. Unfortunately, both GEL and WPI are excellent foaming agents comparable to egg white protein (Abu-Ghoush et al., 2010). As can be seen from Table 1, in most cases, the WPI-based FFSs were better foamed than the GEL-based counterparts (111.59–123.55 vs. 95.92–113.08%). Furthermore, the WPI foams tended to be more stable (20.79–34.70 vs. 10.09–29.89%, Table 2), which explains why in pilot testing the WPI films were more prone to pinhole defects (Fig. S1). Consequently, to obtain high-quality films, the WPI-based FFSs had to be carefully degassed to remove macro- and microfoam.
To produce the best foaming properties, a protein must rapidly migrate to the air–water interface resulting in the reduction of surface tension, unfold, and reorganize its structure (Hailing & Walstra, 1981). Therefore, the obtained result suggests that the whey proteins had a more flexible structure than GEL. This outcome is contrary to that reported by Aziz et al. (2014), who concluded that the weaker foamability of WPI in comparison to GEL (1% w/w solutions) was caused by the presence of cysteine residues which formed covalent S–S bridges restricting reorganization of protein molecules at the water–air interface. The discrepancy between our results and previous findings can be explained by the different protein concentrations, different processing temperatures, and the presence of glycerol, which is a modifier of foaming properties (Safouane et al., 2001; Lexis & Willenbacher, 2014).
It has been demonstrated that polyphenols can contribute to the stability of protein foam structures (Diaz et al., 2022). In the present study, the incorporation of FE into the GEL-based FFS significantly increased the FC (Table 1) but at the same time decreased the long-term FS (Fig. S2). As regards the WPI-based FFSs, the incorporation of higher levels of FE tended to have antifoaming action, i.e., decreased both FC and FS (Table 1, Fig. S2). FS is often related to the strength of the interfacial layer around the air bubble; therefore, it can be speculated that FE weakened the protein-stabilized interface. In agreement with our findings, previous studies have demonstrated that sinapic acid reduced FS of WPI (Yang et al., 2021). Nevertheless, opposite results of studies on FS of WPI/polyphenol blends can also be found in existing literature (Meng & Li, 2021). A note of caution is due here since both cited studies concerned native (unheated) proteins.
SDS-PAGE
The GEL-containing samples produced blurred migration patterns, which supports evidence from previous studies (Rahayu et al., 2015). The weak mobility of GEL may be a result of copolymerization with SDS–polyacrylamide gel (Heussen & Dowdle, 1980). Consistent with the literature, the GEL included high molecular weight polypeptides (≥ 100 kDa, Fig. 2). The smears with MW of ~ 116 kDa can be ascribed to α peptide chain (~ 100 kDa) of collagen. In turn, the strong band at the top of resolving gel reflects the presence of dimers (β-chain) and trimers (γ-chain) of covalently cross-linked α-chains (Derkach et al., 2019). The patterns of GEL (P line) and the films made of GEL were comparable.
Whey proteins are primarily composed of 50% β-lactoglobulin (β-Lg), 20% α-lactalbumin (α-La), 15% glycomacropeptide (GMP), and 8% bovine serum albumin (BSA). These proteins differ inter alia in the content of the free thiol group and disulfide bonds. Each β-Lg monomer has one free thiol group and two disulfide bonds, α-La contains four disulfide groups, but no thiol group, while BSA contains one thiol group and 17 disulfide bridges (Guo & Wang, 2016). In our study, β-Lg (a MW of ~ 18 kDa), α-La (a MW of ~ 14 kDa), and BSA (a MW of ~ 66 kDa) were detected as the main protein fractions of the WPI (Fig. 2, ME ( −)). Clearly visible differences were found between the protein-banding profiles of the WPI (P line) and the WPI films. Namely, the electropherograms of the non-reduced WPI-based samples revealed that the film-making procedure caused strong streaking in the gel and increased the intensity of the gel region between ~ 25 and 40 kDa. Concurrently, it weakened the BSA band and, most importantly, yielded high-molecular bands located on the top of the stacking and separating gels (Fig. 2). These changes can be ascribed to the heat-induced aggregation of whey proteins (Sava et al., 2005).
Marked differences were found between the WPI patterns obtained in the reducing and non-reducing conditions. As ME cleave disulfide bonds, it is obvious that weaker symptoms of whey protein polymerization were noticed in the reducing conditions (Fig. 2). These results clearly indicate that that S–S bonds played the major role in the protein cross-linking. Apart from the covalent bonds, non-covalent interchange reactions (hydrophobic, electrostatic, and steric) may also be involved in the whey protein aggregation process (Bu et al., 2009; Cavallieri et al., 2007). This may explain why some amounts of reduced high-molecular-mass polymers (too large to enter the stacking gel) were still detected (Fig. 2).
Interestingly, in the reducing conditions, the BSA band was not observed (both in the WPI and WPI film samples, Fig. 2). It may suggest formation of hydrophobically bonded aggregates (visible as bands located on top of the stacking and separating gels) between the reduced BSA and other whey proteins. Moreover, in the reduction conditions, the band corresponding to β-Lg dimers (MW of ~ 36 kDa, as reported by some authors (Fenelon et al., 2018)) was more clearly evident. Also, the intensities of the electrophoretic bands of α-La and β-Lg decreased after the film-making procedure and gradually decreased with the increase in the FE concentration (Fig. 2). It is possible that these proteins were cross-linked by the polyphenols of FE. Nevertheless, no equiponderant increase in the amount of the aggregates was observed.
SEM, FTIR, and WAXD
The SEM images did not reveal differences in the surface morphology of the GEL- and WPI-based films (Fig. 1B). The films were smooth, however, due to their better foam ability (Table 1), the WPI-based FFSs required very careful degassing in order to avoid film defects (Fig. S1). The FE did not affect the microstructure of the films (Fig. 1B).
All ATR-FTIR spectra of the films exhibited broadbands in the range of 3600–2800 cm−1 (Fig. 3A) attributed to vNH and vOH vibrations. The amide A band of the WPI films appeared at lower wavelengths and was more pointed than that of the GEL films. This finding was also reported by Jiang et al. (2010). The less sharp shape of the amide A peak of the GEL films may be explained in part by the absence of tryptophan, which exhibits a very strong and narrow N–H band (Sadhasivam & Muthusamy, 2016). Consistent with the literature (Pereira et al., 2020; Staroszczyk et al., 2014), the spectra of the GEL films showed characteristic bands at 1630, 1547, and 1237 cm−1 corresponding to vC=O and vNH vibrations in amide I, δNH and vCN vibrations in amide II, and vCN and δNH vibrations in the amide III band, respectively. In the case of the WPI films, these bands were located at lower wavelengths (i.e., 1628, 1537, and 1236 cm−1), which confirms previous observations (Guimarães et al., 2020; Gökkaya et al., 2021). The films showed a substantial peak around 1034 (GEL) or 1039 cm−1 (WPI). This peak is marginal in the spectra of GEL and WPI (Hossan et al., 2014; Popescu et al., 2021), so its presence is likely attributed to the C-O stretching in glycerol (Gorinstein & Deutsch, 1980; Guimarães et al., 2020).
In both types of films, the presence of FE did not provoke a significant shift of peaks in the amide I–III region, indicating no conformational changes in the proteinaceous matrices (Fig. 3A). In the case of the WPI film, the amide A band located at 3276 cm−1 was shifted towards 3272 cm−1 (Fig. 4). This red shift could indicate that the N–H group of a protein was involved in hydrogen bonding (Zhao et al., 2016) with the extract constituents (Mohammadian et al., 2021). The incorporation of FE into the GEL film resulted in a blue shift of A band (Fig. 3A), which suggests formation of new intermolecular associations, such as electrostatic interactions, which shortened the bond length of the -NH functional groups, thus increasing the wavenumber (Derkach et al., 2020).
Both GEL and WPI films had semi-crystalline structure with two specific diffraction peaks (Fig. 3B). The GEL films showed a strong narrow peak located at 2θ = 6.7° (related to crystalline structure) and a broad peak located at 2θ ≈ 20° (related to an amorphous phase) (Kowalczyk et al., 2021b), which accords with earlier findings (Luciano et al., 2021). The WPI films exhibited peaks at 2θ = 8.7° and 2θ ≈ 20°, which is also in agreement with earlier observations (Zhang et al., 2020). As suggested by literature data (Luciano et al., 2021; Pereira et al., 2020), the renaturation process (partial triple-helix reversion) was mainly responsible for the partially crystalline nature of the GEL films. In turn, the narrow peak identified in the diffractograms of the WPI films may have been related to the unfolding of globular proteins during FFS heating and subsequent recrystallization of peptide molecules during cooling and solvent evaporation (Gomide et al., 2020). Notably, the GEL films had higher content of the crystalline fraction (the higher intensity of the first diffraction peak, Fig. 3B) compared to the WPI-based films. This result may be easily explained by the specific amino acid profile of GEL, i.e., the strict repetition of the triplets of glycine, proline, and hydroxyproline that ensured a high level of super-organization of protein chains (Terzi et al., 2018). Since WPI is a complex mixture of proteins (Fig. 2), the opportunity to generate self-organized crystalline domains was lower than in the GEL. The semi-crystalline structure of the films was not affected by the FE (Fig. 3B).
Optical Properties
Regardless of the FE concentration, the WPI FFS and films were more yellow and more opaque than the GEL counterparts (Fig. S3, Table 2). The YI increased with the increasing concentration of the FE. The WPI films offered better UV-blocking properties than the GEL films (Fig. 4A). Absorption of UV light by proteins predominantly arises from the presence of tryptophan, which has an absorption band in the range of 260–310 nm with a maximum at 280 nm (Voet & Voet, 2010). GEL contains almost no tryptophan, which is responsible for its weaker UV screening ability compared to WPI (Jiang et al., 2010).
The introduction of FE decreased the T of the films (predominately in the UV range) in a concentration-dependent manner (Fig. 4A). In agreement with the present results, previous studies also demonstrated that polyphenols (pure or present in plant extracts) enhanced the UV-blocking properties of films based on different biopolymers, including GEL (Li et al., 2014) and WPI films (Wang & Xiong, 2021).
Water Affinities
The WPI films exhibited higher MC than the GEL films (Table 2), which reflects the data obtained by Jiang et al. (2010). Additionally, the WPI offered a few percent more soluble films than the GEL (Fig. 4B). This result, however, does not support the previous research (Jiang et al., 2010), which can be easily explained in terms of the different approaches to the dissolution methodology. The cited authors dried (105 °C) the samples before determining total soluble matter (TSM). Since the dehydrothermal treatment can significantly strengthen film integrity throughout the dissolution procedure (Kowalczyk et al., 2015), the TSM values do not show the accurate dissolution behavior of hydrocolloid-based materials. Therefore, in our study, the film were just directly immersed in water.
The partial insolubility of the GEL films was predictable as GEL is soluble in hot water (≥ 30 °C). In turn, the partial water resistance of WPI films proves formation of cohesive matrix. As mentioned previously, it is a consequence of unfolding and aggregation of whey proteins provoked by heating of the FFSs (Perez-Gago et al., 1999). Since whey proteins are rich in cysteine, namely, β-Lg, α-La, and BSA contain 5, 8, and 35 cysteines, respectively (Guo & Wang, 2016), they could form intermolecular disulfide bridges, as evidenced by the comparison of results of SDS-PAGE analysis under reducing and non-reducing conditions (Fig. 2).
To sum up, it can be concluded that glycerol may represent most of the soluble matter in the GEL and WPI films.
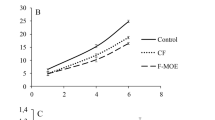
GEL yielded films that absorbed about 5–11 times more water than the WPI counterparts (Fig. 4C). The Sw ability of GEL increased with time, while a decreasing trend was observed for the more erodible WPI films. The incorporation of FE did not affect the MC and So of the GEL and WPI films (Table 2, Fig. 4B). In turn, the FE reduced the Sw of the GEL film in a dose-dependent manner. This result may be ascribed to the decreased porosity of the FE-supplemented GEL matrix (Fig. 1A). Previous studies also demonstrated that GEL cross-linked with polyphenols exhibited reduced water absorption abilities (Haroun & El Toumy, 2010; Zhao & Sun, 2018). In the case of the WPI film, the noticeably decreased Sw was observed only for a 1-h soaking period.
The GEL films exhibited lower WVP than the WPI films (Table 2). The measurement of the mass gain of the films after the WVP testing did not reveal differences (p > 0.05) in the moisture retention ability (Table 2), which excludes this hypothesis that GEL films could immobilize passing WV. The observed differences in the WVP can be attributed to differences in the compactness of the films. It is possible that the more complex fractional composition of WPI (Fig. 2), together with more diversified amino acid composition, resulted in weaker cohesion forces between its constituents, as partially evidenced by less ordered crystal structure of the WPI films (Fig. 3B). It is believed that amorphous regions of polymers allow water to penetrate more easily than densely packaged crystalline regions (Jenkins & Stamboulis, 2012). In contrast to the findings presented here (Table 2), Jiang et al. (2010) showed that GEL offered more permeable films than WPI. As in the case of So, this inconsistency may be related to the methodological differences (the wet cup method vs. the dry cup method). It is well known that, although the RH gradients (ΔRH) may be the same (e.g. 50–0% and 50–100%), the WVP results obtained at the lower and upper half of the RH spectrum may be different (Mali et al., 2019). In most cases, packaging materials are intended to come into contact with high moisture food vulnerable to dehydration. Therefore, knowledge of the WVP properties of edible films at 50–100% ΔRH is more useful, as these conditions are far more common to foods than are the gradients generated with the use of desiccants (Greener Donhowe & Fennema, 1992).
It was found that when the FE concentration was increased to 0.05%, the WVP of the GEL and WPI films decreased significantly by 13.6 and 12.8%, respectively, compared to the controls (p < 0.05, Table 2). This suggests that the interactions between the proteins and phenolic compounds of FE (Fig. 3A) may be favorable for the reduction of free space within the network formation. The results partially support previous findings reported by Choi et al. (2018), who observed that incorporation of different phenolic substances (tannic acid, caffeic acid, and green tea extract) into GEL films resulted in cross-links between protein chains, leading to improvement in the WV barrier properties. In turn, the study conducted by Wang and Xiong (2021) revealed that, although the incorporation of oxidized ferulic and tannic acids (2.5 and 5%) promoted protein cross-linking, this modification had no measurable effect on the WVP, likely due to the hydrophilic nature of the WPI. The results reported above suggest that, due to the differences in molecular properties of different proteinaceous sources, the effect of polyphenols (that differ in their chemical structures and forms) on the WV barrier behavior of protein films cannot be generalized and remains a complex issue.
Mechanical Properties
As shown in Table 2, the GEL films were ~ 2 times stronger, than the WPI counterparts. This result was predictable, since when a GEL-based FFS is left to evaporate below ~ 40 °C, part of the collagen will recover its strong triple-helical structure (Ghoshal et al., 2014). In turn, the WPI does not offer high organizational hierarchy (Fig. 3B) likely due to its more complex fractional (Fig. 2) and amino acid composition. This outcome is contrary to that reported by Jiang et al. (2010), who found that GEL and WPI films had very close mechanical strength (as indicated by a puncture test). The inconsistency is probably caused by the fact that the cited authors adjusted the pH of FFSs to 8.0. In contrast to GEL films, mechanical strength of WPI films increases after alkalization (Wang et al., 2008) as a result of unfolding of globular proteins, thus exposing -SH groups, which associate together upon drying to form covalent intermolecular S–S bonds.
The E value of the GEL films was two times greater than that of the WPI films (Table 2). It is possible that, due to the higher internal cohesiveness, the GEL films were able to withstand the load longer up to the point of breaking. Surprisingly, no significant differences were found between the GEL and WPI films in terms of the EM values. The high rigidity of WPI films may be attributed to the previously mentioned heat-induced polymerization of whey proteins through sulfhydryl-disulfide exchange. As porcine GEL does not contain cysteine, the rigidity of GEL films is believed to originate from proline and hydroxyproline that form hydrogen bonding stabilizing the triple-helix structure (Suderman et al., 2018). The FE did not affect most of the mechanical features of the films (Table 2).
Likely as a result of higher purity of WPI (97% vs. > 90%), the TS of the control WPI film obtained in our study was higher (6.10 MPa, Table 2) than reported by Gökkaya et al. (2021) (4.17 MPa). Likewise, the WPI film was 10 times stronger than that obtained from whey protein concentrate (Guimarães et al., 2020). Regarding the control GEL film, it exhibited smaller TS but higher E values, as compared to the previous results (Kowalczyk et al., 2021b; Xu et al., 2021). The reason was its higher (doubled) amount of glycerol that weakened the compactness of the polymeric network by decreasing the intermolecular interaction and increasing the molecular mobility. The previous studies have shown that GEL films with satisfactory mechanical properties can be obtained from 5% (w/w) GEL solutions containing 1% (w/w) of glycerol (Kowalczyk et al., 2021b; Łupina et al., 2019). The results of preliminary research showed, however, that, at this plasticization level, the WPI film was brittle and broke when peeled off. Therefore, the properties of films obtained from FFS containing 2% (w/w) glycerol had to be examined in the present study.
Antioxidant Activity
The FE-free GEL and WPI films exhibited much better inhibition of ABTS*+ (≥ 50%, Fig. S4) than the previously obtained MC and CS films (~ 15%, Kowalczyk et al., 2021a). It confirms that protein-containing materials have an advantage over polysaccharide-based ones in terms of antiradical efficiency (Kowalczyk & Biendl, 2016; Kowalczyk et al., 2021b). This may explain why the FE provoked weaker enhancement of the antioxidant activity of the proteinaceous films (Fig. S4) in comparison to the polysaccharide-based films (i.e., with initially lower antioxidant potential) (Kowalczyk et al., 2021a, c).
Interestingly, the control GEL film exhibited significantly stronger antiradical potential than the WPI carrier (tABTS50% = 19.63 vs. 28.41 min, Table 2). This result may be associated with differences in amino acid composition, structure, and hydrophobic properties of the proteins. According to some studies, the high glycine and proline contents in GEL are responsible for its good antioxidant effect (Abuine et al., 2019).
The increasing level of FE in the films resulted in a gradual increase in ABTS+* scavenging (Table 2). Since the WPI film had initially lower antiradical effectiveness, the presence of FE in this carrier contributed to the greater gain of antioxidant activity, compared to that observed for the GEL film. After incorporation of 0.0125% of FE, the GEL-based film still had higher antioxidant activity than the WPL films. Nevertheless, in the case of 0.025–0.05% FE-supplemented films, the WPI films exhibited higher antioxidant activity (Table 2). A possible explanation for this finding might be that, at the higher FE content, the initial antioxidant potential of the carrier material had a smaller contribution to the overall antiradical capacity of the systems. At the bulky amounts of FE, the major role in shaping the ABTS+* scavenging profiles was probably taken by the release abilities resulting from the water affinities of the films. It can be assumed that the better water solubility and less swellable character of the WPI films (Fig. 4B, C) favored the migration of FE from the polymeric matrix into the ABTS+* solution. In support of this claim, several previous reports have shown that more soluble films offer quicker release of active compounds and thus higher antioxidant potential than less erodible and/or more swellable materials (Kowalczyk et al., 2020a, b; Łupina et al., 2021). This is associated with the fact that swellable polymers form a viscous hydrogel barrier layer around the dosage form that entraps the active substance, thereby impeding water penetration into the carrier (Langer & Peppas, 2003). There is, however, an additional possible explanation for the above results. Namely, by increasing the crosslinking density of the GEL hydrogel (Fig. 1A), FE may favor a delay in the release of the film components (able to scavenge ABTS+*) or alternatively may hinder the penetration of free radicals into the film matrix.
Conclusion
This study showed that GEL produced more compact and ordered film structure than WPI (a more complex mixture of proteins with a richer amino acid profile). Consequently, the GEL films were mechanically stronger and less permeable to WV. The WPI films were more yellow and opaque, more prone to pinhole defects, and less stretchable than the GEL counterparts, which can be a disadvantage for some applications. On the other hand, the WPI films may be more efficient in preventing photo-oxidation of food during storage. The addition of FE did not affect the mechanical properties of the films but significantly increased yellowness, UV-blocking, and the antiradical potential. The FE added at the highest level (0.05%) improved the WV barrier properties of both films. GEL (without an added antioxidant) yielded a material with better free radical scavenging ability than WPI. The GEL seemed to exhibit a stronger FE-trapping ability, as indirectly evidenced by the weaker antiradical capacity of the GEL-based films containing medium (0.025%) and high (0.05%) levels of FE. This result can be mainly explained in terms of the less soluble and more swellable character of the GEL carrier. In conclusion, the weaker building up of the antiradical activity by the FE-supplemented GEL films suggests that, in comparison to WPI, GEL is more useful in development of slow/less migratory active packaging systems for high moisture food.
Data Availability
Data may be made available on request.
References
Abu-Ghoush, M., Herald, T. H. J., & Aramouni, F. M. (2010). Comparative study of egg white protein and egg alternatives used in an angel food cake system. Journal of Food Processing and Preservation, 34, 411–425. https://doi.org/10.1111/j.1745-4549.2008.00284.x
Abuine, R., Rathnayake, A. U., & Byun, H. G. (2019). Biological activity of peptides purified from fish skin hydrolysates. Fisheries and Aquatic Sciences, 22(1), 1–14. https://doi.org/10.1186/s41240-019-0125-4
Aziz, A., Hailes, H., Ward, J., & Evans, J. (2014). Long-term stabilization of reflective foams in sea water. RSC Advances, 4(95), 53028–53036. https://doi.org/10.1039/C4RA08714C
Bandyopadhyay, P., Ghosh, A. K., & Ghosh, C. (2012). Recent developments on polyphenol-protein interactions: Effects on tea and coffee taste, antioxidant properties and the digestive system. Food and Function, 3(6), 592–605. https://doi.org/10.1039/c2fo00006g
Bu, G., Luo, Y., Zheng, Z., & Zheng, H. (2009). Effect of heat treatment on the antigenicity of bovine α-lactalbumin and β-lactoglobulin in whey protein isolate. Food and Agricultural Immunology, 20(3), 195–206. https://doi.org/10.1080/09540100903026116
Cavallieri, A. L. F., Costa-Netto, A. P., Menossi, M., & Da Cunha, R. L. (2007). Whey protein interactions in acidic cold-set gels at different pH values. Dairy Science and Technology, 87(6), 535–554. https://doi.org/10.1051/lait:2007032
Choi, I., Lee, S. E., Chang, Y., Lacroix, M., & Han, J. (2018). Effect of oxidized phenolic compounds on cross-linking and properties of biodegradable active packaging film composed of turmeric and gelatin. LWT, 93, 427–433. https://doi.org/10.1016/j.lwt.2018.03.065
Derkach, S. R., Kuchina, Y. A., Baryshnikov, A. V., Kolotova, D. S., & Voron’ko, N. G. (2019). Tailoring cod gelatin structure and physical properties with acid and alkaline extraction. Polymers, 11(10), 1–17. https://doi.org/10.3390/polym11101724
Derkach, S. R., & Voron’ko, N. G., Sokolan, N. I., Kolotova, D. S., & Kuchina, Y. A. (2020). Interactions between gelatin and sodium alginate: UV and FTIR studies. Journal of Dispersion Science and Technology, 41(5), 690–698. https://doi.org/10.1080/01932691.2019.1611437
de Vargas, V. H., Marczak, L. D. F., Flôres, S. H., & Mercali, G. D. (2022). Advanced Technologies applied to enhance properties and structure of films and coatings: A review. Food and Bioprocess Technology, 15, 1224–1247. https://doi.org/10.1007/s11947-022-02768-6
Diaz, J. T., Foegeding, E. A., Stapleton, L., Kay, C., Iorizzo, M., Ferruzzi, M. G., & Lila, M. A. (2022). Foaming and sensory characteristics of protein-polyphenol particles in a food matrix. Food Hydrocolloids, 123, 107148. https://doi.org/10.1016/j.foodhyd.2021.107148
Fairley, P., Monahan, F. J., German, J. B., & Krochta, J. M. (1996). Mechanical properties and water vapor permeability of edible films from whey protein isolate and sodium dodecyl sulfate. Journal of Agricultural and Food Chemistry, 44(2), 438–443. https://doi.org/10.1021/jf9505234
Fenelon, M. A., Hickey, R. M., Buggy, A., McCarthy, N., & Murphy, E. G. (2018). Whey proteins in infant formula. In H. C. Deeth & N. Bansal (Eds.), Whey proteins: From milk to Medicine (1st ed., pp. 439–494). Elsevier Inc. https://doi.org/10.1016/B978-0-12-812124-5.00013-8
Ghoshal, S., Stapf, S., & Mattea, C. (2014). Protein renaturation in the gelatin film formation process. Applied Magnetic Resonance, 45, 145–154. https://doi.org/10.1007/s00723-014-0514-x
GMIA (2012). Gelatin handbook. Gelatin Manufacturers Institute of America. https://nitta-gelatin.com/wp-content/uploads/2018/02/GMIA_Gelatin-Handbook.pdf
Gomide, R. A. C., de Oliveira, A. C. S., Luvizaro, L. B., Yoshida, M. I., de Oliveira, C. R., & Borges, S. V. (2020). Biopolymeric films based on whey protein isolate/lignin microparticles for waste recovery. Journal of Food Process Engineering, 44(1), 1–12. https://doi.org/10.1111/jfpe.13596
Gorinstein, B. S., & Deutsch, J. (1980). Spectroscopic determination of glycerol, polyphenols, and nitrogenous compounds in beer and wine. MBAA Technical Ouarterly, 17(3), 6–9.
Greener Donhowe, I., & Fennema, O. (1992). The effect of relative humidity gradient on water vapor permeance of lipid and lipid-hydrocolloid bilayer films. Journal of the American Oil Chemists’ Society, 69(11), 1081–1087. https://doi.org/10.1007/BF02541041
Guimarães, A., Ramos, Ó., Cerqueira, M., Venâncio, A., & Abrunhosa, L. (2020). Active whey protein edible films and coatings incorporating Lactobacillus buchneri for Penicillium nordicum control in cheese. Food and Bioprocess Technology, 13, 1074–1086. https://doi.org/10.1007/s11947-020-02465-2
Guo, M., & Wang, G. (2016). Whey protein polymerisation and its applications in environmentally safe adhesives. International Journal of Dairy Technology, 69(4), 481–488. https://doi.org/10.1111/1471-0307.12303
Gökkaya Erdem, B., Dıblan, S., & Kaya, S. (2021). A Comprehensive study on sorption, water barrier, and physicochemical properties of some protein- and carbohydrate-based edible films. Food and Bioprocess Technology, 14, 2161–2179. https://doi.org/10.1007/s11947-021-02712-0
Hailing, P. J., & Walstra, P. (1981). Protein - stabilized foams and emulsions. C R C Critical Reviews in Food Science and Nutrition, 15(2), 155–203.
Haroun, A. A., & El Toumy, S. A. (2010). Effect of natural polyphenols on physicochemical properties of crosslinked gelatin-based polymeric biocomposite. Journal of Applied Polymer Science, 116(5), 2825–2832. https://doi.org/10.1002/app.31736
He, X., Mao, L., Gao, Y., & Yuan, F. (2016). Effects of high pressure processing on the structural and functional properties of bovine lactoferrin. Innovative Food Science and Emerging Technologies, 38, 221–230. https://doi.org/10.1016/j.ifset.2016.10.014
Henderson, T., Ladewig, K., Haylock, D., McLean, K., & O’Connor, A. (2013). Cryogels for biomedical applications. Journal of Materials Chemistry B, 1, 2682–2695. https://doi.org/10.1039/C3TB20280A
Heussen, C., & Dowdle, E. B. (1980). Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Analytical Biochemistry, 102(1), 196–202. https://doi.org/10.1016/0003-2697(80)90338-3
Hossan, J., Gafur, M. A., Kadir, M. R., & Karim, M. M. (2014). Preparation and characterization of gelatin- hydroxyapatite composite for bone tissue engineering. International Journal of Engineering & Technology, 57(1), 113–122.
Jenkins, M., & Stamboulis, A. (2012). Durability and reliability of medical polymers (1st ed.). Woodhead Publishing.
Jiang, Y., Li, Y., Chai, Z., & Leng, X. (2010). Study of the physical properties of whey protein isolate and gelatin composite films. Journal of Agricultural and Food Chemistry, 58(8), 5100–5108. https://doi.org/10.1021/jf9040904
Kowalczyk, D., & Baraniak, B. (2014). Effect of candelilla wax on functional properties of biopolymer emulsion films - A comparative study. Food Hydrocolloids, 41, 195–209. https://doi.org/10.1016/j.foodhyd.2014.04.004
Kowalczyk, D., & Biendl, M. (2016). Physicochemical and antioxidant properties of biopolymer/candelilla wax emulsion films containing hop extract - A comparative study. Food Hydrocolloids, 60, 384–392. https://doi.org/10.1016/j.foodhyd.2016.04.010
Kowalczyk, D., Kordowska-Wiater, M., Karaś, M., Zięba, E., Mężyńska, M., & Wiącek, A. E. (2020a). Release kinetics and antimicrobial properties of the potassium sorbate-loaded edible films made from pullulan, gelatin and their blends. Food Hydrocolloids, 101, 105539. https://doi.org/10.1016/j.foodhyd.2019.105539
Kowalczyk, D., Kordowska-Wiater, M., Nowak, J., & Baraniak, B. (2015). Characterization of films based on chitosan lactate and its blends with oxidized starch and gelatin. International Journal of Biological Macromolecules, 77, 350–359. https://doi.org/10.1016/j.ijbiomac.2015.03.032
Kowalczyk, D., Pytka, M., Szymanowska, U., Skrzypek, T., Łupina, K., & Biendl, M. (2020b). Release kinetics and antibacterial activity of potassium salts of iso-α-acids loaded into the films based on gelatin, carboxymethyl cellulose and their blends. Food Hydrocolloids, 109, 106104. https://doi.org/10.1016/j.foodhyd.2020.106104
Kowalczyk, D., Skrzypek, T., Basiura-Cembala, M., Łupina, K., & Mężyńska, M. (2020c). The effect of potassium sorbate on the physicochemical properties of edible films based on pullulan, gelatin and their blends. Food Hydrocolloids, 105, 105837. https://doi.org/10.1016/j.foodhyd.2020.105837
Kowalczyk, D., Szymanowska, U., Skrzypek, T., Bartkowiak, A., Materska, M., & Łupina, K. (2021a). Release of fireweed extract (Epilobium angustifolium L.) from corn starch- and methylcellulose-based films - A comparative study. Food Hydrocolloids, 120, https://doi.org/10.1016/j.foodhyd.2021.106887
Kowalczyk, D., Szymanowska, U., Skrzypek, T., Basiura-Cembala, M., Łupina, K., & Biendl, M. (2021b). Edible films based on gelatin, carboxymethyl cellulose, and their blends as carriers of potassium salts of iso-α-acids: Structural, physicochemical and antioxidant properties. Food Hydrocolloids, 115, 106574. https://doi.org/10.1016/j.foodhyd.2020.106574
Kowalczyk, D., Szymanowska, U., Skrzypek, T., Basiura-Cembala, M., Materska, M., & Łupina, K. (2021c). Corn starch and methylcellulose edible films incorporated with fireweed (Chamaenerion angustifolium L.) extract: Comparison of physicochemical and antioxidant properties. Carbohydrate Polymers, 118159. https://doi.org/10.1016/j.ijbiomac.2021.09.079
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature Publishing Group, 227, 726–734.
Langer, R., & Peppas, N. A. (2003). Advances in biomaterials, drug delivery, and bionanotechnology. AIChE Journal, 49(12), 2990–3006. https://doi.org/10.1002/aic.690491202
Lexis, M., & Willenbacher, N. (2014). Yield stress and elasticity of aqueous foams from protein and surfactant solutions - The role of continuous phase viscosity and interfacial properties. Colloids and Surfaces a: Physicochemical and Engineering Aspects, 459, 177–185. https://doi.org/10.1016/j.colsurfa.2014.06.030
Li, J. H., Miao, J., Wu, J. L., Chen, S. F., & Zhang, Q. Q. (2014). Preparation and characterization of active gelatin-based films incorporated with natural antioxidants. Food Hydrocolloids, 37, 166–173. https://doi.org/10.1016/j.foodhyd.2013.10.015
Luciano, C. G., Rodrigues, M. M., Lourenço, R. V., Bittante, A. M. Q. B., Fernandes, A. M., Sobral, P. J., & do A. (2021). Bi-layer gelatin film: Activating film by incorporation of “Pitanga” leaf hydroethanolic extract and/or nisin in the second layer. Food and Bioprocess Technology, 14, 106–119. https://doi.org/10.1007/s11947-020-02568-w
Łupina, K., Kowalczyk, D., Lis, M., Raszkowska-Kaczor, A., & Drozłowska, E. (2021). Controlled release of water-soluble astaxanthin from carboxymethyl cellulose/gelatin and octenyl succinic anhydride starch/gelatin blend films. Food Hydrocolloids, 123, 107179. https://doi.org/10.1016/j.foodhyd.2021.107179
Łupina, K., Kowalczyk, D., Zięba, E., Kazimierczak, W., Mężyńska, M., Basiura-Cembala, M., & Wiącek, A. E. (2019). Edible films made from blends of gelatin and polysaccharide-based emulsifiers - A comparative study. Food Hydrocolloids, 96, 555–567. https://doi.org/10.1016/j.foodhyd.2019.05.053
Mali, S., Carvalho, F. A., Bilck, A. P., & Yamashita, F. (2019). Polyvinyl alcohol films with different degrees of hydrolysis and polymerization. Semina: Ciências Exatas e Tecnológicas, 40(2), 169. https://doi.org/10.5433/1679-0375.2019v40n2p169
Market Resarch Report, (2019). Gelatin Market by Source (Porcine, Bovine Skin, Bovine Bone, Fish & Poultry), Application (Food, Pharmaceuticals & Healthcare), Type (Type A, Type B), Function (Stabilizing, Thickening, Gelling), and Region - Global Forecast to 2023.
Maroufi, L. Y., Shahabi, N., Ghanbarzadeh, M., & Ghorbani, M. (2022). Development of antimicrobial active food packaging film based on gelatin/dialdehyde quince seed gum incorporated with apple peel polyphenols. Food and Bioprocess Technology, 15, 693–705. https://doi.org/10.1007/s11947-022-02774-8
Meng, Y., & Li, C. (2021). Conformational changes and functional properties of whey protein isolate polyphenol complexes formed by non-covalent interaction. Food Chemistry, 364, 129622. https://doi.org/10.1016/j.foodchem.2021.129622
Miller, K. S., Upadhyaya, S. K., & Krochta, J. M. (1998). Permeability of d-Limonene in whey protein films. Journal of Food Science, 63, 244–247. https://doi.org/10.1111/j.1365-2621.1998.tb15718.x
Mohammadian, M., Moghaddam, A. D., Sharifan, A., Dabaghi, P., & Hadi, S. (2021). Structural, physico-mechanical, and bio-functional properties of whey protein isolate-based edible films as affected by enriching with nettle (Urtica dioica L.) leaf extract. Journal of Food Measurement and Characterization, 15(5), 4051–4060. https://doi.org/10.1007/s11694-021-00988-6
O’Regan, J., Ennis, M.P., & Mulvihill, D.M. (2009). 13 - Milk proteins. In G.O. Phillips, P.A. Williams (Eds), Handbook of hydrocolloids (2nd ed. pp. 298–358). Woodhead Publishing. https://doi.org/10.1533/9781845695873.298
Pereira, P. F. M., Picciani, P. H. S., Calado, V. M. A., & Tonon, R. V. (2020). Gelatin-based nanobiocomposite films as sensitive layers for monitoring relative humidity in food packaging. Food and Bioprocess Technology, 13, 1063–1073. https://doi.org/10.1007/s11947-020-02462-5
Pérez-Gago, M., Nadaud, P., & Krochta, J. (1999). Water vapor permeability, solubility, and tensile properties of heat-denatured versus native whey protein films. Journal of Food Science, 64, 1034–1037. https://doi.org/10.1111/j.1365-2621.1999.tb12276.x
Pluta-Kubica, A., Jamróz, E., Juszczak, L., Krzyściak, P., & Zimowska, M. (2021). Characterization of furcellaran-whey protein isolate films with green tea or pu-erh extracts and their application as packaging of an acid-curd cheese. Food and Bioprocess Technology, 14, 78–92. https://doi.org/10.1007/s11947-020-02570-2
Popescu, V., Molea, A., Moldovan, M., Lopes, P. M., Mazilu Moldovan, A., & Popescu, G. L. (2021). The influence of enzymatic hydrolysis of whey proteins on the properties of gelatin-whey composite hydrogels. Materials, 14(13), 1–15. https://doi.org/10.3390/ma14133507
Rahayu, P. P., Purwadi, Radiati, L. E., & Manab, A. (2015). Physico chemical properties of whey protein and gelatine biopolymer using tea leaf extract as crosslink materials. Current Research in Nutrition and Food Science, 3(3), 224–236. https://doi.org/10.12944/CRNFSJ.3.3.06
Ramos Ó. L., Reinas I., Silva S. I., Fernandes, J.C., Cerqueira, M. A., Pereira R. N., Vicente, A.A., Poças M. F., Pintado M. E., Malcata F. X., (2013). Effect of whey protein purity and glycerol content upon physical properties of edible films manufactured therefrom. Food Hydrocolloids, 30 (1,) 110–122. https://doi.org/10.1016/j.foodhyd.2012.05.001
Ribeiro, A. M., Estevinho, B. N., & Rocha, F. (2021). Preparation and incorporation of functional ingredients in edible films and coatings. Food and Bioprocess Technology, 14, 209–231. https://doi.org/10.1007/s11947-020-02528-4
Sabaghi, M., Tavasoli, S., Jamali, S. N., Katouzian, I., & Esfanjani, A. F. (2022). The pros and cons of incorporating bioactive compounds within food networks and food contact materials: A review. Food and Bioprocess Technology. https://doi.org/10.1007/s11947-022-02837-w
Sadhasivam, B., & Muthusamy, S. (2016). Thermal and dielectric properties of newly developed L-Tryptophan-based optically active polyimide and its POSS nanocomposites. Designed Monomers and Polymers, 19(3), 236–247. https://doi.org/10.1080/15685551.2015.1136530
Safouane, M., Durand, M., Saint Jalmes, A., Langevin, D., & Bergero, V. (2001). Aqueous foam drainage. Role of the rheology of the foaming fluid. Journal de Physique IV, 11(11), 275–280.
Sava, N., Van Der Plancken, I., Claeys, W., & Hendrickx, M. (2005). The kinetics of heat-induced structural changes of β-lactoglobulin. Journal of Dairy Science, 88(5), 1646–1653. https://doi.org/10.3168/jds.S0022-0302(05)72836-8
Staroszczyk, H., Sztuka, K., Wolska, J., & Wojtasz-Paja̧k, A., & Kołodziejska, I. (2014). Interactions of fish gelatin and chitosan in uncrosslinked and crosslinked with EDC films: FT-IR study. Spectrochimica Acta - Part a: Molecular and Biomolecular Spectroscopy, 117, 707–712. https://doi.org/10.1016/j.saa.2013.09.044
Strauss, G., & Gibson, S. M. (2004). Plant phenolics as cross-linkers of gelatin gels and gelatin-based coacervates for use as food ingredients. Food Hydrocolloids, 18(1), 81–89. https://doi.org/10.1016/S0268-005X(03)00045-6
Suderman, N., Isa, M. I. N., & Sarbon, N. M. (2018). Characterization on the mechanical and physical properties of chicken skin gelatin films in comparison to mammalian gelatin films. IOP Conference Series: Materials Science and Engineering, 440(1). https://doi.org/10.1088/1757-899X/440/1/012033
Szwajgier, D., Baranowska-Wójcik, E., Kukula-Koch, W., Kowalik, K., Polak-Berecka, M., & Waśko, A. (2021). Evolution of the anticholinesterase, antioxidant, and anti-inflammatory activity of Epilobium angustifolium L. infusion during in vitro digestion. Journal of Functional Foods, 85, 104645. https://doi.org/10.1016/j.jff.2021.104645
Terzi, A., Storelli, E., Bettini, S., Sibillano, T., Altamura, D., Salvatore, L., Madaghiele, M., Romano, A., Siliqi, D., Ladisa, M., De Caro, L., Quattrini, A., Valli, L., Sannino, A., & Giannini, C. (2018). Effects of processing on structural, mechanical and biological properties of collagen-based substrates for regenerative medicine. Scientific Reports, 8(1), 1–13. https://doi.org/10.1038/s41598-018-19786-0
Tyuftin, A. A., & Kerry, J. P. (2021). Gelatin films: Study review of barrier properties and implications for future studies employing biopolymer films. Food Packaging and Shelf Life, 29, 100688. https://doi.org/10.1016/j.fpsl.2021.100688
Voet, D., & Voet, J. G. (2010). Protein folding, dynamics, and structural evolution. In D. Voet & J. G. Voet (Eds.), Biochemistry (4th ed., pp. 278–322). John Wiley & Sons, Inc.
Wang, Y., & Xiong, Y. L. (2021). Physicochemical and microstructural characterization of whey protein films formed with oxidized ferulic/tannic acids. Foods, 10(7), 1–13. https://doi.org/10.3390/foods10071599
Wang, L., Liu, L., Holmes, J., Huang, J., Kerry, J. F., & Kerry, J. P. (2008). Effect of pH and addition of corn oil on the properties of whey protein isolate-based films using response surface methodology. International Journal of Food Science and Technology, 43(5), 787–796. https://doi.org/10.1111/j.1365-2621.2007.01517.x
Wu, J., Chiu, S., Pearce, E., & Kwei, T. (2001). Effects of phenolic compounds on gelation behavior of gelatin gels. Journal of Polymer Science Part a: Polymer Chemistry, 39, 224–231. https://doi.org/10.1002/1099-0518(20010101)39
Xu, J., Li, X., Xu, Y., Wang, A., Xu, Z., Wu, X., Li, D., Mu, C., & Ge, L. (2021). Dihydromyricetin-loaded pickering emulsions stabilized by dialdehyde cellulose nanocrystals for preparation of antioxidant gelatin–based edible films. Food and Bioprocess Technology, 14, 1648–1661. https://doi.org/10.1007/s11947-021-02664-5
Yang, J., Lamochi Roozalipour, S. P., Berton-Carabin, C. C., Nikiforidis, C. V., van der Linden, E., & Sagis. L. M. C. (2021). Air-water interfacial and foaming properties of whey protein - sinapic acid mixtures. Food Hydrocolloids, 112(2020), 106467. https://doi.org/10.1016/j.foodhyd.2020.106467
Zanela, J., Casagrande, M., Radaelli, J. C., Dias, A. P., Júnior, A. W., Malfatti, C. R. M., & Yamashita, F. (2021). Active biodegradable packaging for foods containing baccharis dracunculifolia leaf as natural antioxidant. Food and Bioprocess Technology, 14, 1301–1310. https://doi.org/10.1007/s11947-021-02641-y
Zhang, Y., Huo, M., Zhou, J., Zou, A., Li, W., Yao, C., & Xie, S. (2010). DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS Journal, 12(3), 263–271. https://doi.org/10.1208/s12248-010-9185-1
Zhang, X., Zhao, Y., Li, Y., Zhu, L., Fang, Z., & Shi, Q. (2020). Physicochemical, mechanical and structural properties of composite edible films based on whey protein isolate/psyllium seed gum. International Journal of Biological Macromolecules, 153, 892–901. https://doi.org/10.1016/j.ijbiomac.2020.03.018
Zhao, Y., & Sun, Z. (2018). Effects of gelatin-polyphenol and gelatin–genipin cross-linking on the structure of gelatin hydrogels. International Journal of Food Properties, 20(3), 2822–2832. https://doi.org/10.1080/10942912.2017.1381111
Zhao, Y., Li, Z., Yang, W., Xue, C., Wang, Y., Dong, J., & Xue, Y. (2016). Modification of gelatine with galla chinensis extract, a natural crosslinker. International Journal of Food Properties, 19(4), 731–744. https://doi.org/10.1080/10942912.2015.1013633
Funding
This work was financed by the program of the Minister of Science and Higher Education “Regional Initiative of Excellence” in 2019–2022; project number 029/RID/2018/19; funding amount 11 927 330.00 PLN.
Author information
Authors and Affiliations
Contributions
Dariusz Kowalczyk: conceptualization, methodology, investigation, formal analysis, visualization, data curation, supervision, validation, writing—original draft preparation; project administration, funding acquisition; Urszula Szymanowska: investigation, formal analysis; Tomasz Skrzypek: investigation, visualization; Monika Basiura-Cembala: investigation, visualization; Artur Bartkowiak: investigation, visualization, writing—original draft preparation; Katarzyna Łupina: visualization; writing—review and editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kowalczyk, D., Szymanowska, U., Skrzypek, T. et al. A Comprehensive Study on Gelatin- and Whey Protein Isolate-Based Edible Films as Carriers of Fireweed (Epilobium angustifolium L.) Extract. Food Bioprocess Technol 15, 2547–2561 (2022). https://doi.org/10.1007/s11947-022-02898-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-022-02898-x