Abstract
The food processing industry is currently facing challenges in delivering safe, healthy, and high-quality food. Constant monitoring at each step of the supply chain of food is vital to resolve the issue of food contamination. To achieve this aim and to meet consumer prospects, the technologies promoting the concept of clean label food have been widely cherished. Ozonation is one such advanced technology that assists in maintaining food product quality and safety. Its manifold approach and zero-by-product production make it a promising food disinfectant technique. Ozone due to its oxidative property has been widely used in sanitizing, washing, odor removal, water treatment, and in equipment, fruits, vegetable, and meat processing disinfection. Ozonation in foods is done in such a way that no nutritional, sensory, and physicochemical characteristics are altered. In this review, an attempt is made to give an overview of the impact and contribution of ozone as a disinfectant in food processing while comparing it with conventional disinfectants and its overall application in the food industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Food preservation is the process of extending of shelf-life of food products in prepared as well as raw form, beyond their natural decay period. The actual preservation of food products has been a tough task for mankind for ages. The food industries are usually focused on either killing or inactivating microbes and enzymes as a mode of preservation. several methods that involve heat (Søltoft-Jensen & Hansen, 2005). Advanced novel techniques, including flash pasteurization, steam pasteurization, and steam vacuuming, continue to depend on the heat for the reduction of harmful microbes which are responsible for food deterioration. However, the application of thermal-based technologies leads to the various transformation of food products triggering side reaction that causes undesired changes in nutritional, physicochemical, and sensory attributes. In a report, this has been suggested that thermal treatment leads to the reduction of total phenolic content and antioxidant activity in several fruits and vegetables (Al-Juhaimi et al., 2018). Conversely, non-thermal technologies such as high-pressure processing, ultrasonication, pulse electric field, and cold plasm technology involve the processing of food near room temperature. Hence, there is no damage caused by heat sensitivity (Jan et al., 2017). But the lack of cost-effectiveness is a key point for consideration when it comes to commercialization. On the other hand in chemical treatments, a cost-effective strategy to achieve contamination-free food products by affecting the cellular activity and composition of pathogens is also there (Kaavya et al., 2021). However, due to the hesitation for the use of artificial chemical preservatives in foods, the consumers demand safe and fresh-like products (Søltoft-Jensen & Hansen, 2005). Also, the advent of clean label Food has been widely valued as a global food trend. It embraces minimal processing and the use of natural ingredients in food processing to achieve a higher consumer preference for healthy foods (Aschemann-witzel et al., 2019). The pandemic phase of COVID-19 has also added to the evolution of this clean label trend. According to Food and Health survey by IFIC (International Food Information Council) in the year 2020, around 85 percent of consumers have changed their eating and food preparation habits due to COVID-19 (Ific, 2020).
Consequently, innovative technologies in the food industry are vital to meet consumers’ desires. The requirement has sped up the research and has led the studies toward the innovation of promising alternative technologies for food processing and disinfection (Leadley & Williams, 2006). Keeping in view all these concerns, ozone technology is one such technique that has become a focal point as an effective sanitizer that may fulfill the prospects of Food industries, regulating authorities, and consumers. Ozone, due to its sporicidal activity, is an efficient disinfectant and sanitizer which can be effective against harmful pathogens including their spores and can act as an excellent antimicrobial agent (Owais et al., 2018). Ozone treatment has been found effective in most food products during processing as well as storage and to maintain the quality, safety, and shelf-life of these products. Besides the inactivation of microbes, ozone also reduces the level of pesticides in fresh produce. Ozone treatment is very effective in decreasing the biological and chemical oxygen demand of water used in processing and washing. Ozone acts as a promising eco-friendly sanitizer but its efficacy in sanitization depends on dosage and type of products (Gonçalves, 2009). The main purpose of this review is to provide limelight on the ozonation as an advanced green technology along with its promising and multi-dimensional applicability in the food industries. Commercialization of ozone technology will ensure a safe, contamination-free food supply chain along with an eco-friendly approach.
Conventional Chemical Disinfectants
The purpose of cleaning and disinfection is to produce safe and contamination-free products along with better shelf life and quality. Food industries generally have the trend of longer production runs with short recesses for sanitization. Cleaning schedules should be of the shortest possible time, with low chemical usage and without producing residue (Moerman & Mager, 2016). The mechanical and chemical power, temperature, and contact time in the disinfection process should be wisely chosen to attain an adequate cleaning effect (Wirtanen, 2008). The usage of effective disinfecting agents minimizes the contamination caused due to microorganisms attached to the surface of food product, enhances shelf life, and ultimately reduces risks of foodborne illness (O'Donnell et al., 2012; Rice et al., 2002). Choice of disinfectant for use in food industries depends on several factors such as pH range, stability on dilution and reactivity, effectiveness at high temperatures, spectrum of action, and especially safety. Challenge for food industry involves excess use of water and wastewater discharge rates, constituting chemical residues. Even in some of the sectors, the use of water in the industry is more than that of agriculture. Among the industries, food industry ranks third in water consumption and wastewater discharge rates preceded by the chemical and refinery industries (Gil et al., 2015). Conventional chemical disinfectants like chlorine, chlorine dioxides, peracetic acid, and hydrogen peroxides are some of the most widely used chemical disinfectants in the industries. But in the recent years, outbreaks related to contaminating pathogens in food products raised apprehensions about the efficacy of conventional disinfectants in assuring safety of the products. Such as a multistate outbreak of salmonella Kottbus infection caused due to inadequate seed disinfection by heat and chlorine. Use of conventional chemical disinfectants is also linked with the production of high amounts of wastewater with extreme levels of chemical residue in the form of hazardous disinfection by-products (DBP) and biological oxygen demand (BOD). Production of DBP by chlorine and its dioxides, low antimicrobial efficacy of organic acids, peracetic acid, and hydrogen peroxide are some of the limitations associated with the conventional chemical disinfectants in food industries (Ölmez & Kretzschmar, 2009) (Table 1).
Due to the environmental and health risks posed by the use of conventional chemical disinfectants, their use in the organic product is prohibited. The natural organic matter serves as a precursor in water for DBP production. This DBP has certain limits such as for total trihalomethanes, it is 0.080 mg/L; for haloacetic acids, it is 0.069 mg/L; and for bromate and chlorite, it is 0.010 and 1.0 mg/L respectively (Gee, 2016). The food processing sector is now seeking alternatives to conventional chemical disinfectants which may assure the safety of the products and, maintain the quality enabling shelf-life, besides minimizing the consumption rates of water in processing. Keeping in view these prepositions, the ozonation technique seems to be a potential prospect for the solution of these problems. Ozone is an evolving alternative chemical disinfectant that has already gained the generally recognized safe (GRAS) status and its usage in foods has also been approved. The Food and Drug Administration (FDA) has amended the food additive regulations to provide for the safe use of ozone in gaseous as well as aqueous phases as an antimicrobial agent in food, including meat and poultry, and also in raw agricultural commodities in the preparation, packing, or holding of these products for commercial purposes (Ölmez & Kretzschmar, 2009).
Ozonation: an Evolving Disinfectant Technology for the Food Industry
The name ozone was given by Christian Friedrich Schonbein, a German-Swiss chemist, derived from the Greek word “ozein” which stands for “smell” (Kaur, 2014). Ozone serves as a strong anti-microbial agent due to its powerful oxidizing capacity. In the year 1982, use of ozone in bottled water was granted GRAS by FDA. It was decreed by a panel of experts in 1997 that under good manufacturing practices (GMP), ozone can be used as both disinfectants and as a sanitizer for food (Guzel et al., 2004). Ozone is an endorsed sanitizer with little or no lethal residue. Ozone is naturally formed in the stratosphere due to the irradiation of Ultra-Violet rays (< 240 nm) on oxygen molecules. The stability of ozone in water depends on the purity of water as the requirement of ozone is least in pure water compared to other solutions (Pandiselvam et al., 2020).
Ozonation (ozone treatment) is a chemical food decontamination method in which the exposure of contaminated edibles (perishable/non-perishable) to ozone (O3) is done either in the aqueous or the gaseous phase. In the gaseous phase of ozone treatment, deactivation of microbes is attained at specified ozone concentration, constant pressure, flow rate, and the caused contamination level (Brodowska et al., 2015). In contrast to chlorine which is an extensively used traditional decontaminating agent, the use of ozone (2.07 V) has tremendously increased due to its stronger oxidative properties compared to Chlorine (1.36 V). Furthermore, the US FDA 2001 recognized and authorized ozone for its strong antimicrobial property and its use in food products. The effectiveness of ozone as an antibacterial agent is based on its action on a wide range of bacteria such as Gram-positive, Gram-negative, and even the spores of bacteria (Guzel-Seydim et al., 2004). Besides microbial decontamination, ozone is also used in reducing the pesticides level potentially, such as captan, ethylene thiourea, formetanate hydrochloride, and azinphosmethyl from the fresh produce (James et al., 2013).
Antimicrobial Activity Mechanism of Ozone
A wide-range spectrum of antimicrobial activities of ozone is due to its high oxidation potential (2.07 V) for free radicals. The high reactivity and instability of ozone tend to decay into the radicals of hydroperoxyl, superoxide, and hydroxyl (Pirani, 2010). However, the main inactivator of microorganisms is claimed to be the molecular ozone. Researchers had revealed that highly reactive by-products are formed during the disintegration of ozone (Pirani, 2010).
The inactivation of microbes by the ozone treatment involves the attack of ozone in the gas or liquid phase on the cell membrane, cell envelopes, cytoplasm, spore coat, and other constituents of microorganisms (Guzel-Seydim et al., 2004). As per the researchers, the inactivation of microorganisms can be done through ozone by two possible mechanisms (Fig. 1):
-
The first mechanism embraces the oxidation of amino acids and sulfhydryl groups of peptides, proteins, and enzymes to yield small peptides at the time of ozone exposure.
-
The second mechanism embraces the poly-unsaturated fatty acids oxidation and the formation of acid peroxides.
Deactivation of microorganisms is caused due to the damage to the cell envelope which will eventually result in the consequential leakage of cellular material or cell lysis (Pandiselvam et al., 2020). Particularly, double-bonded unsaturated lipids are more susceptible to ozone exposure (Lanni et al., 2019). Potent destruction or damage of nucleic acid and oxidation of cellular proteins extensively lead to rapid cellular death. Thymine is the most sensitive nucleic acid to the ozone (Pandiselvam et al., 2020). Alteration in polypeptide chains of a viral protein coat and RNA destruction of the virus is also caused by ozone (Guzel-Seydim et al., 2004).
Effectiveness Against Coronavirus (COVID-19)
The issue of shielding the individual against air-borne transmission has now become a great matter of concern due to the recent outbreak of the Coronavirus also known as COVID-19 (Rabail et al., 2021). The transmission of COVID-19 occurs when droplets that contain the virus enter the respiratory tract of humans. During this pandemic phase, fruits and vegetables are brought home directly by people from the vendors. This inevitable contact may increase the chance of association between the people and the contaminating body. Since a contamination-free food supply chain is the first and foremost step of food processing it is essential to disinfect the food produced just after it is harvested. Reports have suggested that ozone due to strong virucidal activity is capable of inactivating the virus as well as bacteria (Hudson et al., 2009; Yaneva et al., 2022; Alimohammadi & Naderi, 2021). Considering this challenge, the Central Institute of Post harvesting Engineering and Technology (CIPHET), India, has developed an ozone-based fruit and vegetable washer-cum-purifier known as Ozo-C, which is a portable and economical machine (ICAR-CIPHET, 2020). It is a venerable device working on the principle of the corona discharge method and removes the virus, bacteria, pesticides, and chemicals from food surfaces such as fruits, vegetables, meat, and seafood. The general mechanism of virucidal activity consists of the denaturation of virus lipid, and the formation of protein peroxide (Turkmen et al., 2015). The enveloped viruses possess cysteine, an amino acid with sulfhydryl as a functional group, also known as the thiol group, which is liable to oxidation by ozone (Yaneva et al., 2022; Alimohammadi & Maziar, 2021). Compared to non-enveloped viruses, COVID-19, an enveloped virus is sensitive to chemical disinfectants. Sensitivity is due to the intact lipid envelope of the virus infecting host cells, which can be affected by the use of strong disinfectors like ozone.
Methods of Ozone Generation
The generation of ozone is done by the air present in the atmosphere, subjected to the high-energy electrical field or ultraviolet (UV) radiation (Khadre et al., 2001). However, due to the rapid degradation of ozone into oxygen, the production of ozone has to be continuous at the time of its utilization as it cannot be collected. To generate ozone, various methods such as Ultraviolet radiation, corona discharge, and electrolysis method are used (Fig. 2). Among these, ultraviolet radiation and corona discharge methods are most widely used in food processing industries due to their cost-efficiency. Along with it, many domestic types of ozone-generating facilities are also being invented in form of different equipment which can be procured in homes, small-scale shops, vendors, etc. More particularly the aim of making fresh food produces sanitized and disinfected as a habit of its processing (Okpala et al., 2015).
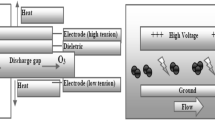
Corona Discharge Method
Corona discharge method has been widely used for the generation of ozone in large amounts. It comprises two electrodes of low and high tension. These electrodes are parted by a dielectric medium made of ceramic and a discharge gap (Goncalves, 2009). When in presence of oxygen, a high voltage alternate current flows through the discharge gap, the electrons of oxygen excite and therefore, initiate the splitting of molecules of oxygen. These cleaved oxygen atoms are associated with other oxygen molecules and lead to the formation of ozone molecules. The production of ozone is influenced by various factors such as voltage, current frequency, discharge gap, thickness, and property of the material used as dielectric (Khadre et al., 2001). Production of ozone is 1–4% when air is passed through the generator as a feed gas. The utilization of oxygen in the pure form for the production of ozone allows a rise in the yield and reaches 6–14%. It is observed that the ozone spontaneously degrades back to oxygen and, thus, cannot be stored (Guzel-Seydim et al., 2004).
Ultraviolet Radiation Method
The ultraviolet (UV) radiation method involves the transformation of oxygen into ozone with the help of a UV lamp of wavelength 188 nm. The generation of ozone is done by the ventilation of electric discharge of high voltage in fresh air (20% O2) or pure oxygen (Gonçalves, 2009; Guzel-Seydim et al., 2004). The atmospheric oxygen molecules are affected by the effect of radiation and produce splitting, then after the separation of molecules into atoms of oxygen. These oxygen atoms in free form collide with other oxygen molecules and result in the formation of ozone (Chawla et al., 2007). The reaction mechanism of ozone generation by UV radiation method involves the cleavage of diatomic oxygen molecules first and then the formation of free radicals of oxygen. This means the resultant radical of oxygen is free to react further with other oxygen molecules for the formation of ozone. Although higher energy is required to break the bond of diatomic oxygen molecules (Gonçalves, 2009).
Electrolysis Method
The electrolysis method has been first familiarized by Lynntech, Inc. (College Station, Tex.). It involves the splitting of water (H2O) molecules by the phenomenon of electrolysis, into the atoms of hydrogen and oxygen. The hydrogen atom is then parted from the molecule of water and the oxygen atom is combined to form ozone molecules. One of the major advantages stated by the producer of ozone via this method is that it produces 3–4 times higher ozone i.e., 10–18% than that of the corona discharge method (Pandiselvam et al., 2017a, b).
Application of Ozone in the Food Industry
Applicability in the Disinfection of Food Products
The food processing sector is the major platform to give momentum to the ozone treatment as an advancement. Technologies derived from ozone are coming in limelight as the chemical-free, cost-effective, and safe way to ensure food safety (Gonclave, 2009). The safety level of ozone on food and humans is limited up to a certain concentration and exposure time. Ozone is applied in the food industries either in gaseous or aqueous form. The preference for the form of ozone treatment must be chosen according to the type of food commodity and required action. Usually, aqueous ozone is found to be more effective on food-related microorganisms than gaseous one. But to achieve a significant effect, high concentration or long exposure times of gaseous ozone are required (Panebianco et al., 2022). Therefore, the utilization of ozone in various sectors of food processing industries along with the safety level of its usage is a matter of concern and requires more research.
Meat and Poultry Industry
Meat and poultry products’ safety continues with the global concern of the ailments related to several nasty pathogens such as Salmonella, E. coli, and Listeria Campylobacter. This fear has led the meat industry to continue its research and implement newer strategies to fight against pathogens. The applicability of ozone in storage is seen in warehouses and freezing chambers of the meat industry. The key goal is to reduce the bacteriological level in storage and attain the peak durability of foods in fresh storage, freezing, or refrigeration. This as a result will help in eliminating the growth of bacteria and molds in meat products (Chawla et al., 2007). In a comparative study, 12% trisodium phosphate, 5% hydrogen peroxide, 2% acetic acid, 0.5% ozonated water, and 0.3% commercial sanitizer were observed for their antimicrobial efficacy on beef brisket against E. coli. Results indicated that hydrogen peroxide and ozonated water were most effective in the reduction of bacterial counts (Hassenberg et al., 2007; Castillo et al., 2003) (Table 2). Ozone in aqueous form was effective in the decline of Salmonella count on the skin surface of chicken drumsticks below the detectable limit when treated with 8 ppm concentration along with the combination of lactic acid (Megahed et al., 2020) (Table 2). The application of ozone can also be perceived before cooking, as a pre-treatment step for meat to check for any activity of spoiling microorganisms. In a study, beef surfaces are treated with ozone and then cooked at 45 to 75 °C. A decline of Clostridium perfringens by 1–2 log CFU/g was observed while treating with aqueous ozone treatment and 45–75 °C of heat treatment. Additionally, a reduction in the spore count was also recorded but in a small quantity (Novak & Yuan, 2004). An observation of a study on turkey breast meat results stated an improved microbial quality with the reduction of the log by 2.9, 2.3, and 1.9 in the counts of yeast, mold, Enterobacteriaceae, and aerobic mesophilic bacteria respectively when treated with ozone for 8 h (Ayranci et al., 2020) (Table 2).
Vegetables and Fruit Industry
Vegetables and fruits are perishables and are liable to water loss, physiological deterioration, and mechanical injury. The physiology and quality that affect the fruits and vegetables attributed to the exposure to ozone have been studied. Chlorine water has been commonly used as a vegetable and fruit washer in food processing industries as sanitization (Garcia & Heredia, 2017). Ozone is proven to be an excellent alternate sanitizer for fresh fruits and vegetables as chlorine produces trihalomethanes, a residual by-product that is carcinogenic (Han et al., 2002). Zhang and his team in a study observed that treating fresh-cut celery with ozone inhibits its respiration rate (Zhang et al., 2005). Also, in peach, respiration rate remains unchanged by exposure to ozone (0.3 ppm) for about three weeks (Palou et al., 2002). In the storage of fruits, it was observed that ozone can help in reducing the ethylene level in a cold storage room which ultimately leads to a longer storage time. Another issue for perishable commodities is the color change caused due to enzymatic browning, by the action polyphenol oxidase enzyme. It is observed that ozone-treated fresh-cut celery had an inhibiting effect on polyphenol oxidase activity (Zhang et al., 2005). In a study, it was reported that during storage, the browning and visual appearance of fresh-cut cabbage was maintained by ozonated water (Nie et al., 2020).
Grain Processing Industry
Ozone has an excellent application as a fumigant for grains and its storage by the inactivation of microbial pathogens, destruction of mycotoxin, and insect without affecting the quality of the grains. Studies have shown that ozone offers a unique benefit for the processing of grain by addressing the major concerns associated with the usage of harmful pesticides. Usually, organophosphate, methyl bromide, dichlorvos, phosphine, and malathion are used as insect controllers for stored grains. The feasibility of ozone in grain storage has been an emphasis in some studies due to its high reactivity, strong oxidizing property, and insecticidal activity. Several studies reported ozone as a capable fumigant against insects like Tribolium castaneum, Rhyzopertha Dominica, Ephestia elutella, Sitophilus oryzae, and Oryzaephilus surinamensis. Among insect species, Rhyzopertha Dominica, a lesser grain borer, was hard to kill as it closes spiracles for long period, which leads to lacking ozone to attain target tissue in an effective concentration (Pandiselvam et al., 2017a, b). A study has shown that Plodia interpunctella, an Indian meal moth, in wheat when exposed to a concentration of 50 ppm ozone for 3 days has achieved high mortality at its larval stage (Kells et al., 2001). However, eggs of Plodia interpunctella required an ozone concentration of 1800 ppm for 3 h (McDonough et al., 2011). It can be concluded that ozone efficacy as a fumigant varies with different species of insects and their respective life stage.
Fruit Juice Industry
Treatment of juices with ozone to regulate the juice quality is an active application of ozone technology. The rich nutrient content of fruit juices is supposed to support microbial activities and growth. Therefore, microbial inactivation during processing is necessary for maintaining the safety and nutritional quality of juices (Roobab et al., 2018). Ozone treatment in the juice processing industries is used in gaseous form (Miller et al., 2013). With the approval of ozone to be used as a direct additive by the FDA, the applicability of ozone has been explored widely in juice processing industries. Several studies showed that pathogenic species present in juices can be cut to 5 times when treated with ozone (Tiwari & Muthukumarappan, 2012). In other studies, clarified and unclarified watermelon juice was exposed to ozone for five successive durations (5–25 min). Yeast and mold count of clarified and unclarified ozonated watermelon juices showed a log reduction of 3.411 and 3.046 respectively with an exposure time of 25 min (Lee et al., 2021). In another study for sugarcane juice, ozone treatment of 10, 20, and 30% concentrations for 5, 12.5, and 20 min respectively result in the deactivation of the PPO (polyphenol oxidase) enzyme by 67.8% and a decline in Total Plate Count (TPC) by 3.72 logs (Panigrahi et al., 2020). The effect of ozonation has also been studied on the processing of many juices for the extension of shelf life including apple, tomato, and orange juice (Choi & Nielsen, 2005; Tiwari et al., 2009; Patil et al., 2010; Song et al., 2015).
Applicability in the Disinfection of Plant Equipment
Industrial wastewater contains harmful metals and natural pollutants in massive amounts as a result of industrial processes. The presence of these organic and inorganic impurities makes it difficult to reuse water (Tripathi et al., 2020). Cleaning in place (CIP) is an operation that involves the use of water, chemicals, and heat in blends for cleaning machinery, hose, and vessels without dismantling the plant. Ozone can be preferred as an alternative to other pre-existing disinfectants or sanitizers for CIP due to its environmental leads. Ozone efficiency in the CIP system of the wine industry was tested by Guillen and his team. The hosepipe in which wine is conveyed was exposed to various treatments of peracetic acid along with the solution of caustic soda, peracetic acid, and ozonated water (28 ± 1 °C). The outcomes reported that the treatment with ozonated water is the most effective of all (Guillen et al., 2010). It includes a quick breakdown of ozone into oxygen without any undesirable residue. Since it does not necessitate the final rinse to eliminate leftover residues and this makes it favorable for the food industry over other traditional disinfectants. As it does not leave any residues, disinfection of air is also a vital part of the food industry for hazard analysis and critical control point (HACCP). Ozone gas was shown to be effective against airborne microbes and can also be applied for the prevention of secondary impurities during food manufacturing (Naito & Takahara, 2006).
Applicability in the Disinfection of Food Packaging
Sterilization of packaging materials is another range of widely studied applications of ozone. According to an observation, when plastic packaging films are treated with ozonated water, a five-log reduction in the bacterial count has been noticed (Natha et al., 2014). When ozone interacts with the packaging material (polymer) surface, it causes alteration in the properties of the packaging material surface in terms of surface tension and polarity. Treating polymers like polyethylene, polypropylene, and polyethylene terephthalate with ozone significantly increases the surface tension, and hydrophilicity and improves their adhesion properties (Ozen et al., 2002). According to study, ozonation on packaging material (molten LDPE and aluminium foil) leads to the establishment of a carbon–oxygen bond which makes the basic composition oxidized and ultimately improved adhesion (Nordin, 2010). The low surface tension of plastic films leads to poor adhesion properties which are caused due to the oxidation of polymer comprising functional group and degradation of polymer chains which varies with the chemical structure of the polymer. It was also observed that biofilms contaminated with Pseudomonas fluorescence on multi-laminated packaging material; when exposed to repeated ozone flow, contamination was reduced up to 188 colony forming units per area of 12.5 cm2 (Khadre et al., 2001). This proves the potential of ozonation in the sanitization of packaging materials.
Challenges of Ozonation
Instability of Ozone
Ozone can either oxidize or decompose the compound to oxygen in the form of free radicals. The mechanism of ozone decomposition is a complicated process. The decomposition rate of ozone is influenced by many factors like the type of radical formation in the solution and the type of organic matter present in the medium, pH, and temperature (Am Water Works Res et al., 1991). Organic matter in the medium helps in initiating, inhibiting, or promoting radical chain reactions. It is, therefore, not easy to specify a specific ozone concentration that will always be effective in the inhibition of microbes of definite concentration in a food product. In a study, each ozone and heat sterilized water was inoculated with water bacteria of a normal population. A prominent bacterial growth was noticed in the ozone sterilized water of the same origin (Kim et al., 1999). The observation showed that the products obtain after the breakdown of organic contaminants of water during ozonation are a good source of nutrients for water bacteria. In a report, this has been shown that the secondary half-life (time for reduction of concentration) of ozone is affected by pH. In an alkaline condition (pH 10), it has been observed to be about 25 s; in neutral (pH 7), it is 17 min; and in an acidic medium (pH 4), it took 7 h (Von Sonntag & Von Gunten, 2012).
Safety Limit of Ozone
Ozone shows high reactivity towards organic bodies such as the human body. Hence, monitoring the environment as well as people coming in contact with ozone is important. The human respiratory tract gets affected by ozone gas; therefore, working with ozone or being exposed to it requires specific care (Zhu, 2018). The toxicity of ozone, either acute or in chronic form, depends on the exposure time and concentration of ozone. Burning sensations in the eyes and throat, headache, cough, and dizziness are the common symptoms of ozone toxicity. Symptoms of chronic toxicity involve memory loss, bronchitis, and muscular excitability (Guzel-Seydim et al., 2004). Based on the ozone concentration in an environment, three different zones of health effects are categorized are acceptable zone, hazardous zone, and critical zone (White, 2007). Occupational Safety and Health Administration (OSHA) has recognized a 0.1 ppm concentration of ozone gas as an endorsed limit with a time of 8 h of exposure at the workplace in the food industry (Namdari et al., 2020). Fumigation of crops with ozone in the storage room is executed in the absence of workers or during the night. High pressure and warm water raise the ozone toxicity chances. Hence, it is advised to cover the area of application in such a way that air can be easily liberated (Smilanick, 2003). Researchers use potassium iodine solution in labs to absorb an excess ozone gas liberated while testing to prevent its release into the air (Khadre et al., 2001). Therefore, while working with aqueous ozone, ensuring all the safety precautions is highly endorsed.
Time–Temperature and Concentration Dependency of Ozone
Ozone exposure time, temperature, and concentration are the key points to analyze the efficiency of ozonolysis. High temperature (> 50 °C) leads to the rapid decomposition of ozone into the free radicals and elevates the effectiveness of the ozone treatment (Diao et al., 2013). A high rate of degradation of Aflatoxin B1 was stated for peanut flour and kernels at above 50 °C (Proctor et al., 2004). The solubility of ozone has an inverse relationship that is higher the temperature lower the solubility of ozone and vice versa (Brodowska et al., 2018). High temperatures are not ideal for the majority of agricultural products, as it shows undesirable outcomes on the quality attributes of the food products. Ozonation is, thus, preferred at room temperature, and the effectiveness increases along with its concentration and exposure time. However, adverse effects have been observed on the quality of food products due to extended exposure and higher concentration of ozone treatment. High pigmented food such as fruits and vegetables are more susceptible to ozone and lead to decoloration after treatment due to their strong oxidizing potential; at the same time, it is found to affect the stability of high fat-containing food such as dairy products (Panebianco et al., 2022). Hence, the application of ozone should not exceed the recommended threshold limit of products (Diao et al., 2013). Optimization of the ozonation is thus a significant issue that will result in utmost benefits without altering the quality and nutritional content of food products.
Future Perspectives of Ozonation
As discussed above, ozone is a likely solution for various operations in the food industry. Its potential to assurance of the microbial quality of the various products, potent sanitizer for plant equipment, disinfection of wash water, wastewater recycling, and treatment indicates that ozonation has a perspective and a bright future in food processing industries. However few conditions need to be resolved. Like as ozone effectiveness for the different community of produce need to be emphasized more by the researcher. Ozone has been shown to hinder the quality of some food products. On the other hand, process operation and system design challenges are faced by the operators working on ozone in a plant. Hence, their safety needs to be assured by studying the effect of ozone on humans as well. Also, consumers’ acceptability of ozone-treated food products is an area that needs to be focused on. Rigorous research work is requisite to determine the interface between ozone and the food constituents along with the effect of final compounds during the process of inactivation. The process parameters need to be optimized that follow more effective and specific process-related studies on its action mechanisms. Improper utilization of ozone may lead to lethal effects on the physiology and quality of the food products, so for the safe and effective utilization in food processing, contact time, the optimal concentration of ozone, and various other parameters of treatment must be well-defined for different types of food products.
Conclusion
Ozonation is an advanced technology that assists in maintaining a contamination-free food supply chain without harming the environment. Ozone has been extensively used in sanitizing, washing, water treatment, odor removal, in-plant equipment, fruits, vegetable, and meat processing disinfection. The regulatory approval of ozone utilization in food by the FDA and by FSIS/USDA clears all the regulatory hurdles in its industrial application. Due to the diverse effective factors, the applicability of ozone has surely accelerated. It has acted as an effective sanitizer/disinfectant in food processing industries for vegetables, fruits, cereal, meat, and its products, fish, and processing plants. No special handling, generation of absolutely no hazardous residue, eco-friendly, and its hurdle applicability make it more valuable for sanitation purposes in the food industry than other chemical sensitization methods. The most vital point that needs to be considered for the ozone process is the concentration of ozone varies with the type of treated materials, environmental conditions, microorganisms, and contaminants present in the product. Research needs to be more focused on the product-based optimization of ozone treatment which can lead to headway of ozonation as the absolute disinfection technique in the food processing industries.
References
Alimohammadi, M., & Naderi, M. (2021). Effectiveness of ozone gas on airborne virus inactivation in enclosed spaces: A review study. Ozone: Science & Engineering, 43(1), pp.21–31.
Al-Juhaimi, F., Ghafoor, K., Özcan, M. M., Jahurul, M. H. A., Babiker, E. E., Jinap, S., Sahena, F., Sharifudin, M. S., & Zaidul, I. S. M. (2018). Effect of various food processing and handling methods on preservation of natural antioxidants in fruits and vegetables. Journal of Food Science and Technology, 55(10), 3872–3880.
Am Water Works Res, F., Langlais, B., Reckhow, D. A. & Brink, D. R. (1991). Ozone in water treatment: Application and engineering. CRC press. https://krishi.icar.gov.in/jspui/handle/123456789/36470
Aschemann-Witzel, J., Varela, P., & Peschel, A. O. (2019). Consumers’ categorization of food ingredients: Do consumers perceive them as ‘clean label’producers expect? An exploration with projective mapping. Food Quality and Preference, 71, 117–128.
Aslam, R., Alam, M. S., & Kaur, P. (2022). Comparative study on efficacy of sanitizing potential of aqueous ozone and chlorine on keeping quality and shelf-life of minimally processed onion (Allium Cepa L.). Ozone: Science & Engineering, 44(2), pp.196–207.
Ayranci, U. G., Ozunlu, O., Ergezer, H., & Karaca, H. (2020). Effects of ozone treatment on microbiological quality and physicochemical properties of turkey breast meat. Ozone: Science & Engineering, 42(1), pp.95–103.
Brodowska, A. J., Nowak, A., & Śmigielski, K. (2018). Ozone in the food industry: Principles of ozone treatment, mechanisms of action, and applications: An overview. Critical Reviews in Food Science and Nutrition, 58(13), 2176–2201.
Brodowska, A. J., Śmigielski, K., Nowak, A., Czyżowska, A., & Otlewska, A. (2015). The impact of ozone treatment in dynamic bed parameters on changes in biologically active substances of juniper berries. PLoS ONE, 10(12), e0144855.
Castillo, A., McKenzie, K. S., Lucia, L. M., & Acuff, G. R. (2003). Ozone treatment for reduction of Escherichia coli O157: H7 and Salmonella serotype Typhimurium on beef carcass surfaces. Journal of Food Protection, 66(5), 775–779.
Chamnan, S., Varith, J., Jaturonglumlert, S., Phimphimol, J., & Sujinda, N. (2022). Effects of high concentration ozone gas fumigation on the quality and shelf-life of longan fruit. Ozone: Science & Engineering, 44(1), pp.105–116.
Chawla, A., Bell, J. W., & Janes, M. E. (2007). Optimization of ozonated water treatment of wild-caught and mechanically peeled shrimp meat. Journal of Aquatic Food Product Technology, 16(2), 41–56.
Choi, L. H., & Nielsen, S. S. (2005). The effects of thermal and nonthermal processing methods on apple cider quality and consumer acceptability. Journal of Food Quality, 28(1), 13–29.
Diao, E., Hou, H., & Dong, H. (2013). Ozonolysis mechanism and influencing factors of aflatoxin B1: A review. Trends in Food Science & Technology, 33(1), 21–26.
García, S., & Heredia, N. (2017). Microbiological safety of fruit and vegetables in the field, during harvest, and packaging: A global issue. In Global food security and wellness (pp. 27–48). Springer, New York, NY.
Gee, I. M. (2016). Removal of inorganic contaminants and natural organic matter by enhanced alum coagulation: Defluoridation at the pilot scale and application to arsenic (Doctoral dissertation).
Gil, M. I., Gómez-López, V. M., Hung, Y. C., & Allende, A. (2015). Potential of electrolyzed water as an alternative disinfectant agent in the fresh-cut industry. Food and Bioprocess Technology, 8(6), 1336–1348.
Gonçalves, A. A. (2009). Ozone: An emerging technology for the seafood industry. Brazilian Archives of Biology and Technology, 52(6), 1527–1539.
Guillen, A. C., Kechinski, C. P., & Manfroi, V. (2010). The use of ozone in a CIP system in the wine industry. Ozone: science & engineering, 32(5), 355–360.
Guzel-Seydim, Z. B., Greene, A. K., & Seydim, A. C. (2004). Use of ozone in the food industry. LWT-Food Science and Technology, 37(4), 453–460.
Han, Y., Floros, J. D., Linton, R. H., Nielsen, S. S., & Nelson, P. E. (2002). Response surface modeling for the inactivation of Escherichia coli O157: H7 on green peppers (Capsicum annuum) by ozone gas treatment. Journal of Food Science, 67(3), 1188–1193.
Hassenberg, K., Idler, C., Molloy, E., Geyer, M., Plöchl, M., & Barnes, J. (2007). Use of ozone in a lettuce-washing process: An industrial trial. Journal of the Science of Food and Agriculture, 87(5), 914–919.
Hudson, J. B., Sharma, M., & Vimalanathan, S. (2009). Development of a practical method for using ozone gas as a virus decontaminating agent. Ozone: science & engineering, 31(3), 216–223.
Ianni, A., Grotta, L., & Martino, G. (2019). Feeding influences the oxidative stability of poultry meat treated with ozone. Asian-Australasian Journal of Animal Sciences, 32(6), 874.
International Food Information Council. (2020). Food and Health Survey 2020, www.ifci.org
James, R., Ellis, J., & Duehl, A. (2013). The potential for using ozone to decrease pesticide residues in honey bee comb. Agricultural Science, 1(1), 1–16.
Jan, A., Sood, M., Sofi, S. A., & Norzom, T. (2017). Non-thermal processing in food applications: A review. International Journal of Food Science and Nutrition, 2(6), 171–180.
Kaavya, R., Pandiselvam, R., Abdullah, S., Sruthi, N. U., Jayanath, Y., Ashokkumar, C., Khanashyam, A. C., Kothakota, A., & Ramesh, S. V. (2021). Emerging non-thermal technologies for decontamination of Salmonella in food. Trends in Food Science & Technology.
Kaur, R. K. (2014). Ozone therapy: A new paradigm in periodontics. Journal of Advanced Medical and Dental Sciences Research, 2(4).
Kells, S. A., Mason, L. J., Maier, D. E., & Woloshuk, C. P. (2001). Efficacy and fumigation characteristics of ozone in stored maize. Journal of Stored Products Research, 37(4), 371–382.
Khadre, M. A., Yousef, A. E., & Kim, J. G. (2001). Microbiological aspects of ozone applications in food: A review. Journal of Food Science, 66(9), 1242–1252.
Kim, J. G., Yousef, A. E., & Dave, S. (1999). Application of ozone for enhancing the microbiological safety and quality of foods: A review. Journal of Food Protection, 62(9), 1071–1087.
Leadley, C. E., & Williams, A. (2006). Pulsed electric field processing, power ultrasound and other emerging technologies. Food processing handbook, p.201.
Lee, B. J., Ting, A. S. Y., & Thoo, Y. Y. (2021). Impact of ozone treatment on the physico-chemical properties, bioactive compounds, pectin methylesterase activity and microbiological properties of watermelon juice. Journal of Food Science and Technology, pp.1–11.
McDonough, M. X., Mason, L. J., & Woloshuk, C. P. (2011). Susceptibility of stored product insects to high concentrations of ozone at different exposure intervals. Journal of Stored Products Research, 47(4), 306–310.
Megahed, A., Aldridge, B., & Lowe, J. (2020). Antimicrobial efficacy of aqueous ozone and ozone–lactic acid blend on Salmonella-contaminated chicken drumsticks using multiple sequential soaking and spraying approaches. Frontiers in Microbiology, 11, 3121.
Miller, F. A., Silva, C. L., & Brandão, T. R. (2013). A review on ozone-based treatments for fruit and vegetables preservation. Food Engineering Reviews, 5(2), 77–106.
Moerman, F., & Mager, K. (2016). Cleaning and disinfection in dry food processing facilities. In Handbook of Hygiene Control in the Food Industry (pp. 521–554). Woodhead Publishing.
Naito, S., & Takahara, H. (2006). Ozone contribution in food industry in Japan. Ozone: science and Engineering, 28(6), 425–429.
Namdari, M., Lee, C. S., & Haghighat, F. (2020). Active ozone removal technologies for a safe indoor environment: A comprehensive review. Building and Environment, p.107370.
Natha, A., Mukhimb, K., Swerb, T., Duttaa, D., Vermaa, N., Dekab, B. C., & Gangwara, B. (2014). A review on application of ozone in the food processing and packaging.
Nie, M., Wu, C., Xiao, Y., Song, J., Zhang, Z., Li, D., & Liu, C. (2020). Efficacy of aqueous ozone combined with sodium metasilicate on microbial load reduction of fresh-cut cabbage. International Journal of Food Properties, 23(1), 2065–2076.
Nordin, A. (2010). Effect of ozone treatment on molten LDPE on to aluminum foil.
Novak, J. S., & Yuan, J. T. (2004). Increased inactivation of ozone-treated Clostridium perfringens vegetative cells and spores on fabricated beef surfaces using mild heat. Journal of Food Protection, 67(2), 342–346.
O'Donnell, C., Tiwari, B. K., Cullen, P. J., & Rice, R. G. eds. (2012). Ozone in food processing. John Wiley & Sons.
Okpala, C. O. R., Bono, G., Abdulkadir, A., & Madumelu, C. U. (2015). Ozone (O3) process technology (OPT): An exploratory brief of minimal ozone discharge applied to shrimp product. Energy Procedia, 75, 2427–2435.
Ölmez, H., & Kretzschmar, U. (2009). Potential alternative disinfection methods for organic fresh-cut industry for minimizing water consumption and environmental impact. LWT-Food Science and Technology, 42(3), 686–693.
Owais, Y., Sushree, T., & Anupama, S. (2018). Technological aspects for assuring food safety in food production chain. Environment and Ecology, 36(4), 953–958.
Ozen, B. F., Mauer, L. J., & Floros, J. D. (2002). Effects of ozone exposure on the structural, mechanical and barrier properties of select plastic packaging films. Packaging Technology and Science: An International Journal, 15(6), 301–311.
Palou, L., Crisosto, C. H., Smilanick, J. L., Adaskaveg, J. E., & Zoffoli, J. P. (2002). Effects of continuous 0.3 ppm ozone exposure on decay development and physiological responses of peaches and table grapes in cold storage. Postharvest Biology and Technology, 24(1), pp.39–48.
Pandiselvam, R., Kothakota, A., Thirupathi, V., Anandakumar, S., & Krishnakumar, P. (2017a). Numerical simulation and validation of ozone concentration profile in green gram (Vigna radiate) bulks. Ozone: Science & Engineering, 39(1), pp.54–60.
Pandiselvam, R., Mayookha, V. P., Kothakota, A., Sharmila, L., Ramesh, S. V., Bharathi, C. P., Gomathy, K., & Srikanth, V. (2020). Impact of ozone treatment on seed germination–A systematic review. Ozone: Science & Engineering, 42(4), 331–346.
Pandiselvam, R., Sunoj, S., Manikantan, M. R., Kothakota, A., & Hebbar, K. B. (2017b). Application and kinetics of ozone in food preservation. Ozone: Science & Engineering, 39(2), 115–126.
Panebianco, F., Rubiola, S., & Di Ciccio, P. A. (2022). The use of ozone as an eco-friendly strategy against microbial biofilm in dairy manufacturing plants: A review. Microorganisms, 10(1), 162.
Panigrahi, C., Mishra, H. N., & De, S. (2020). Effect of ozonation parameters on nutritional and microbiological quality of sugarcane juice. Journal of Food Process Engineering, 43(11), e13542.
Patil, S., Valdramidis, V. P., Cullen, P. J., Frias, J., & Bourke, P. (2010). Inactivation of Escherichia coli by ozone treatment of apple juice at different pH levels. Food Microbiology, 27(6), 835–840.
Perna, A., Gambacorta, E., Simonetti, A., Grassi, G., & Scopa, A. (2022). Effect of ozone treatment exposure time on oxidative stability of cream milk. European Journal of Lipid Science and Technology, p.2100238.
Pirani, S. (2010). Application ofozone infood industries. Doctoral Program in Animal Nutrition and Food Safety. Doctoral Program in Animal Nutrition and Food Safety. Università degli Studi di Milano, 127 p.
Proctor, A. D., Ahmedna*, M., Kumar, J. V., & Goktepe, I. (2004). Degradation of aflatoxins in peanut kernels/flour by gaseous ozonation and mild heat treatment. Food additives and contaminants, 21(8), 786-793.
Rabail, R., Saleem, J., Tanveer, Z., Patching, S. G., Khalid, A. R., Sultan, M. T., Manzoor, M. F., Karrar, E., Inam‐Ur‐Raheem, M., Shabbir, M. A., & Aadil, R.M. (2021). Nutritional and lifestyle changes required for minimizing the recovery period in home quarantined COVID‐19 patients of Punjab, Pakistan. Food Science & Nutrition.
Rice, R. G., Graham, D. M., & Lowe, M. T. (2002). Recent ozone applications in food processing and sanitation. Food Safety Magazine, 8(5), 10–17.
Roobab, U., Aadil, R. M., Madni, G. M., & Bekhit, A. E. D. (2018). The impact of nonthermal technologies on the microbiological quality of juices: A review. Comprehensive Reviews in Food Science and Food Safety, 17(2), 437–457.
Sert, D., & Mercan, E. (2022). The impact of ozone treatment on whey concentrate on the flow behaviour, functional and microbiological characteristics of whey powder. International Dairy Journal, p.105447.
Smilanick, J. L. (2003). December. Use of ozone in storage and packing facilities. In Washington Tree Fruit Postharvest Conference (pp. 1–10).
Søltoft-Jensen, J., & Hansen, F. (2005). New chemical and biochemical hurdles. In Emerging Technologies for Food Processing (pp. 387–416). Academic Press.
Song, W. J., Shin, J. Y., Ryu, S., & Kang, D. H. (2015). Inactivation of E scherichia coli O 157: H 7, S almonella T yphimurium and L isteria monocytogenes in apple juice at different pH levels by gaseous ozone treatment. Journal of Applied Microbiology, 119(2), 465–474.
Tiwari, B. K., & Muthukumarappan, K. (2012). Ozone in fruit and vegetable processing. Ozone in food processing, pp. 55–80.
Tiwari, B. K., O’Donnell, C. P., Brunton, N. P., & Cullen, P. J. (2009). Degradation kinetics of tomato juice quality parameters by ozonation. International Journal of Food Science & Technology, 44(6), 1199–1205.
Tripathi, G., Yadav, V. K., Singh, J. & Mishra, V. (2020). Analytical methods of water pollutants detection. In Sensors in Water Pollutants Monitoring: Role of Material (pp. 63–78). Springer, Singapore.
Turkmen, A., Kesici, S., Elmali, N., Cangir, C. C., & Cakirguz, M. (2015). Chronic hepatitis B and ozone therapy. Journal of Medical Case Reports, 3(2), 38–39.
Von Sonntag, C., & Von Gunten, U. (2012). Chemistry of Ozone in Water and Wastewater Treatment. IWA publishing.
White, S. D. (2007). Using ozone to control fungi in high moisture corn. Iowa State University.
Wirtanen, G. (2008). Efficacy of cleaning agents and disinfectants used in decontamination procedures in food industry. Risk Assessment of Microbial Problems and Preventive Actions in Food Industry, p.26.
Yaneva, G., Dimitrova, T., Ivanov, D., Ivanova, D., Ingilizova, G., & Slavov, S. (2022). Modern applications of ozone for COVID-19 disinfection and treatment. Journal of IMAB–Annual Proceeding Scientific Papers, 28(1), 4284–4288.
Zhang, L., Lu, Z., Yu, Z., & Gao, X. (2005). Preservation of fresh-cut celery by treatment of ozonated water. Food Control, 16(3), 279–283.
Zhu, F. (2018). Effect of ozone treatment on the quality of grain products. Food Chemistry, 264, 358–366.
Acknowledgements
We are thankful to the Integral University, Lucknow, India, for providing the basic facilities.
Author information
Authors and Affiliations
Contributions
Priyanka Dubey: investigation, methodology, writing—original draft. Anupama Singh: writing—review and editing. Owais Yousuf: conceptualization, methodology, supervision.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dubey, P., Singh, A. & Yousuf, O. Ozonation: an Evolving Disinfectant Technology for the Food Industry. Food Bioprocess Technol 15, 2102–2113 (2022). https://doi.org/10.1007/s11947-022-02876-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-022-02876-3