Abstract
A cryo-macerated must of V. vinifera L. cabernet sauvignon was processed by ultra-high-pressure homogenisation (UHPH) sterilisation without the use of SO2. The UHPH treatment of the must was carried out continuously at a pressure of 300 MPa and reaching a maximum temperature of 77 °C for less than 0.2 s. The colloidal structure of the UHPH must was evaluated by atomic force microscopy (AFM) measuring an average particle size of 457 nm. The initial microbial load was 4-log CFU/mL (yeast), 3-log CFU/mL (bacteria). No yeast and non-sporulating bacteria were detected in 1 mL and 10 mL of the UHPH-treated must, respectively. Furthermore, no fermentative activity was detected in the non-inoculated UHPH-treated musts for more than 50 days. A strong inactivation of the oxidative enzymes was observed, with lower oxidation (≈ × 3) than controls. The antioxidant activity of the UHPH-treated must was much higher (106%) than that of the control must. UHPH had a protective effect in total anthocyanins, and especially in acylated anthocyanins (+ 9.3%); furthermore, the fermentation produces fewer higher alcohol (-44,3%) and more 2-phenylethyl acetate (+ 63%).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Elimination of SO2 is currently a topic of great interest in oenology, and the use of ultra-high-pressure homogenisation (UHPH) to eliminate oxidative enzymes and wild microorganisms is a powerful tool to reach this objective. UHPH is the continuous pressurisation at higher than 200 MPa of a fluid and the instantaneous release at atmospheric pressure (or low pressure) across a specially designed valve. UHPH has an ultrashort in-valve time (< 0.2 s) which helps to protect the nutritional and sensory quality, even when high temperatures can be reached in the valve. Several recent reviews have included the most important features and applications of UHPH processing (Comuzzo & Calligaris, 2019; Morata & Guamis, 2020; Patrignani & Lanciotti, 2016; Zamora & Guamis, 2015). UHPH is more efficient than high-pressure homogenisation (HPH) or microfluidisation concerning the elimination of microorganisms and enzyme inactivation, which normally needs multi-passes for efficient microbial control (Szczepańska et al., 2021). Additionally, HPH or microfluidisation are used to improve colloidal stability (Leite et al., 2016; Oliete et al., 2019); however, UHPH produces nanofragmentation, with lower than average size particles stabilising better colloidal structure (Morata & Guamis, 2020). This is because the higher pressure (300 MPa) used in UHPH increases collision effects in valves compared with HPH or microfluidisation (< 200 MPa).
The antimicrobial effect is produced by the intense impact and extreme shear efforts in colloidal structures and microorganisms during the in-valve depressurisation, which produces the nanofragmentation of cells and particles (Bañuelos et al., 2020; Morata & Guamis, 2020). Concerning the antimicrobial effect, it is highly effective, destroying yeast and bacteria in the grape musts (Loira et al., 2018) and, depending on the in-valve temperature, sporulated bacteria (Bañuelos et al., 2020). Compared with discontinuous high hydrostatic pressure (HHP), it shows a more intense antimicrobial effect in grape must; even in intense HHP treatments at 550 MPa-10 min, residual bacterial cells are detected (Morata et al., 2015). The intense microbial inactivation by HHP and UHPH technologies facilitates the use of new wine biotechnologies, such as non-Saccharomyces yeasts and yeast-bacteria co-inoculations, providing better implantation of fewer competitive starters (Bañuelos et al., 2016).
As with HHP, the continuous UHPH produces a gentle effect on fruit juices, with a low impact in colour (Patrignani et al., 2019), and delicate aroma compounds such as terpenes (Bañuelos et al., 2020), protecting, therefore, the sensory quality. A short processing time produces a low number or an absence of thermal markers. No HMF has been detected in musts processed by UHPH, and neither has 5-methyl furfural (Bañuelos et al., 2020; Suárez-Jacobo et al., 2012).
Discontinuous HHP technologies used in batch processes in grapes can control yeasts and moulds, but can also increase the extraction of phenols (Corrales et al., 2009; Morata et al., 2015). Even when continuous UHPH must be applied in liquids, the intense nanofragmentation of colloidal particles from fruit cells has proven to have positive effects on the extraction of phenolic compounds, thereby increasing their availability and the antioxidant capacity of juices (Bañuelos et al., 2020; Loira et al., 2018; Patrignani et al., 2019; Suárez-Jacobo et al., 2012).
HHP has a limited effect on the inactivation of oxidative enzymes such as polyphenol oxidases (PPOs) and, frequently, its effectiveness depends on the use of simultaneous thermal treatments (Buckow et al., 2009; Sulaiman et al., 2015), even though higher browning effects can be observed when used with high pressure levels (Martínez-Hernández et al., 2019). Conversely, UHPH has proven to cause intense inactivation of PPOs, with the consequent improvement of the antioxidant capacity in fruit juices (Bañuelos et al., 2020; Loira et al., 2018; Patrignani et al., 2019; Velázquez-Estrada et al., 2013).
UHPH technology is available at an industrial scale reaching 50.000 L/h based on modular systems. UHPH pumps that can work at 300 MPa are available to process at 10.000 L/h (Ypsicon, 2018). The technology was approved by the International Organization of Vine and Wine (OIV) as an authorised practice in oenology in 2020 (OENO-MICRO 16–594B, OIV, 2020) and has been approved by the EU (European Commission, 2020).
This work aims to evaluate the effectiveness of UHPH in the production of red wine in controlling wild microorganisms and the oxidative processes, as well as analyse the repercussions in the colloidal structure, fermentation, colour, aroma, and sensory profile of the wine. The effect of UHPH on colour, stability of anthocyanin pigments, inhibition of PPO monitoring colour hue, and colloidal average size using atomic force microscopy (AFM) has been studied and assessed.
Materials and Methods
Must Preparation
The must used in this study was obtained from destemmed Vitis vinifera L. cabernet sauvignon grapes collected in the Costers del Segre region (Lérida, Spain), which were crushed and added to 3 g/HL of pectolytic enzymes (Rapidase™, Oenobrands, Montpellier, France) and then left to macerate at 0 °C for 15 days. This process is necessary to reach enough tannins and anthocyanins in order to produce red wines in the absence of skin contact. UHPH technology can only be applied to liquids with colloidal size below 0.5 mm. After this maceration of the grapes, the must was separated using a pneumatic press, then settled at 8 °C and kept in an inert CO2 atmosphere until the start of the experiment. Half of this clean must was used as a control treatment and the other half was treated by UHPH. The must processing by UHPH was carried out in a continuous operating mode using equipment including an improved tungsten carbide valve patented by the company Ypsicon Advanced Technologies (Barcelona, Spain; patent number: EP2409583B1). The working flow rate was 60 L/h at 300 ± 3 MPa, with an inlet temperature of 4 ºC, an in-valve temperature of 78 ± 2 °C for less than 0.2 s (measured by a sensor placed immediately downstream of the valve), and an outlet temperature of 15 °C (Fig. 1). At the outlet of the valve, the UHPH-treated must was cooled in a heat exchanger through which water was circulating at 3 °C. The total volume of must processed by UHPH was 100 L.
Optical and AFM
Samples of control and UHPH musts were observed and photographed using an optical microscope at 630 × (63 × objective) using a trinocular Leitz Diaplan microscope and a Jenoptik Gryphax digital camera. For AFM measurements, samples were prepared by freezing 10 μL drops of the control and UHPH-processed musts on a coverslip in a CO2 freezer at 80 °C.
The samples were then lyophilised. Topographic measurements of the cells were carried out using a Nano-Observer AFM (Concept Scientific Instruments, Les ULIS, France) operating in resonant mode. A 1 N/m rectangular silicon cantilever (model Fort, AppNano, Mountain View, CA, USA) with an 8 nm nominal tip radius was selected. Typical setpoint amplitudes of 4–5 V were used during the measurements, with high values of feedback of proportional and integral gains (P and I) to compensate for the high topographic variations (1–4 microns).
Microbial Counts
Microbiological counts were performed on both control and UHPH-treated must to verify the antimicrobial effectiveness of the non-thermal processing technique and assess the degree of implantation of the inoculated starters. Vegetative forms were assessed by growth on plates with selective media. In the case of total aerobic bacteria and lactic acid bacteria, 1 mL of serial decimal dilutions in saline peptone (0.85% NaCl and 0.1% peptone) were pour-plated into PCA supplemented with nystatin (50 mg/L) and into MRS agar (Pronadisa, Barcelona, Spain) and MLO agar (Pronadisa, Barcelona, Spain) supplemented with nystatin (50 mg/L), respectively. The inoculated plates were incubated for 6 days at 30 °C until colony growth, maintaining anaerobic conditions for lactic acid bacteria by placing the plates in a jar with CO2 atmosphere. For yeast counts, 100 μL or 1 mL were spread-plated into glucose chloramphenicol agar (GCA; Pronadisa, Barcelona, Spain) for total yeast count and into synthetic lysine agar (Oxoid, Hampshire, UK) for non-Saccharomyces counts. These plates were incubated aerobically at 25 °C for 4 and 6 days, respectively. Aerobic bacterial endospores were analysed from 10 mL of must pasteurised at 80 °C for 30 min (to remove vegetative forms), which was cooled down and filtered through 0.45 μm membrane filters (Millipore) and surface incubated on PCA plates for 6 days at 30 °C.
Fermentations
Fermentations were carried out at 20 °C and assayed in triplicate in 1-L flasks filled with must at 90% capacity, because the UHPH system produces two 2-L bags in conditions of full sterility. The fermentation temperature was set at 20 °C because it is suitable for young red wines and avoids excessive aroma losses during fermentation. All fermentation flasks were inoculated with 20 mL of Saccharomyces cerevisiae 7VA starter (enotecUPM, Madrid, Spain) pre-cultured for 24 h in YPD broth (Conda, Madrid, Spain), containing 5 × 107 CFU/mL (checked by plating).
A parallel assay was performed in triplicate in 100-mL vials to further confirm the antimicrobial power of the UHPH technique. These vials were filled with 60 mL of must, sealed with Müller valves and left to ferment at 20 °C with the native population (without microbial inoculation). The weight loss in the vials was recorded daily to monitor fermentation.
Oenological Parameters by Infrared Spectroscopy
Oenological parameters were evaluated by Fourier transform infrared spectroscopy (FTIR) with OenoFoss™ equipment (FOSS, Barcelona, Spain).
Determination of PPO Enzymatic Activity
PPO activity was evaluated spectrophotometrically (Agilent Technologies™ 8453, Palo Alto, CA, USA) by measuring the ratio of absorbances 420/520 nm at a path length of 1 mm and with a high aeration surface of 1 cm2/mL at room temperature. The ratio between absorbance at 420 nm (yellow) and 520 nm (red) is an estimation of the browning processes, which, over a short period of time and exposure to air, is due to enzymatic oxidation.
Antioxidant Capacity (ABTS Method)
The transformation in the colourless form of the cationic ABTS•+ [2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonate) radical cation] by phenols and other compounds was used to evaluate the antioxidant activity of musts (Re et al., 1999). Samples of 50 µL of diluted wines were analysed at four concentrations in duplicate.
Colour Parameters and Total Phenol Content
The colour intensity and hue were analysed according to the Glories method (1984), spectrophotometrically with a device 8453 Agilent Technologies™ (Palo Alto, CA, USA). Total phenols were also analysed in the same spectrophotometer at 280 nm in a 1 mm quartz cuvette (Ribéreau-Gayon et al., 1980).
Anthocyanins by LC-DAD
Must and wine anthocyanins and pyranoanthocyanins were analysed using an LC-DAD chromatograph system (Agilent Technologies 1260, Palo Alto, CA) according to Bañuelos et al. (2016). Concentrations were calculated with a calibration curve of malvidin-3-O-glucoside (r2 = 0.9999, LOD = 0.1 mg/L) and an injection volume of 50 µL.
Analysis of Volatile Fermentative Compounds by GC-FID
Fermentative aroma compounds were analysed by gas chromatography with flame ionisation detection (GC-FID) using an Agilent Technologies 6850 GC (Network GC System) in a DB-624 column, according to Bañuelos et al. (2020). One microliter with 10% internal standard (4-methyl-2-pentanol at 500 mg/L) and prefiltered at 0.45 µm was injected. External standards calibration (r2 > 0.999) was performed using compounds from Fluka (Buchs SG, Switzerland, GC quality > 98%).
Sensory Evaluation
The sensory quality of the wines was evaluated by means of a blind attribute difference test, following a procedure similar to that described by Loira et al. (2018). The tasting panel consisted of nine expert tasters from the Department of Food Chemistry and Technology of the Universidad Politécnica de Madrid (age range: 30 to 60 years old, four women and five men). The wines were served randomly at a temperature of 20 ± 2 °C in two standard tasting glasses. After reaching a general consensus on the description of the attributes to be evaluated, the panellists used a scale from 0 to 5 to rate the intensity of the attributes, the lowest rating being considered as a “not perceived attribute” and the highest rating as a “strongly perceived attribute.” Finally, the global perception of the wines was also evaluated, taking into account visual, olfactory, and taste characteristics. Significant differences between samples were determined by ANOVA analysis (p < 0.05).
Statistical Analysis
Statistical analyses were performed with PC Statgraphics v.5 software (Graphics Software Systems, Rockville, MD, USA). The significance level was 5%.
Results and Discussion
Colloidal and Molecular Structures Produced by UHPH
Must processed by UHPH is affected by ultra-high impact forces, and shear efforts strongly affect the nature of the molecular structure of the colloidal particles. The effect is a nanofragmentation of the suspended particles in the must, producing higher colloidal stability. It was observed that, after centrifugation, the colloidal particles appeared intensively dyed in the control (Fig. 2a, left) but only slightly in the must processed by UHPH (Fig. 2a, right), probably because the smaller average size reduces the adsorbent capacity of the anthocyanins. This could have an impact on colour stability. The optical microscopy of the control must without UHPH treatment showed a colloidal structure with large fragments (several micrometres) dyed by anthocyanins (Fig. 2b). Fibres of vegetal structures could be observed, as could other cell wall fragments from the vegetal cells of the grapes. The optical image of the UHPH must showed smaller particles in the submicron range and, again, less colour (Fig. 2c). Additionally, more tartrate crystals could be observed, probably because of their formation in the intense depressurisation after the UHPH valve. Using high-resolution AFM, we characterised and measured the size of the fragments of a dry drop of must by topographic scanning in resonant mode. A flat surface could be observed in the control, with many polyhedric fragments spread across the entire surface (Fig. 2d). A thinner mass with fewer and smaller polyhedric structures could be observed in the UHPH-processed must (Fig. 2d).
External appearance of the control and UHPH-processed must after centrifugation A, optical microscopy (600 ×) of a centrifuged drop of cabernet sauvignon must B, optical microscopy (600 ×) of the UHPH centrifuged must C, AFM scanning in resonant mode of a drop of dried control must D, and the UHPH dried must E. Topographic renders of the 3D external topography in the control F and UHPH dried drops G
In a previous study, the size of the colloids in the UHPH must was estimated by laser diffraction (Bañuelos et al., 2020), and it was observed that most particles were in the range of 100–400 nm. In the current study, the size of the fragments was measured by AFM microscopy, obtaining an average size of 1342 ± 464 nm in the control must (range 824–3180 nm, Fig. 3a) and 457 ± 140 nm in the UHPH must (range 235–744, Fig. 3b), which are relatively close to our previous results, and showing fragments in the UHPH product higher than 200 nm without nano-safety impact (which must be considered when the size is < 100 nm).
Microbial Loads
In the control must, Saccharomyces cerevisiae counts were 8 × 103 CFU/mL and non-Saccharomyces were 1.2·104 CFU/mL; therefore, they were globally approximately 4-log CFU/mL, which is the typical value in healthy grapes (Fleet, 2003) (Fig. 4a). In the UHPH-processed musts, neither Saccharomyces nor non-Saccharomyces yeasts were detected in 1 mL. This highly antimicrobial effectivity of UHPH follows previous works regarding grape musts (Bañuelos et al., 2020; Bevilacqua et al., 2018; Loira et al., 2018) and other juices (Bevilacqua et al., 2012; Calligaris et al., 2012; Patrignani et al., 2019 and 2020). Initial bacterial counts were 1.7 × 103 CFU/mL in the control must and undetected in the UHPH. Sporulated bacteria in 10 mL were four viable cells, undetected in UHPH musts. This result supports the high efficiency of UHPH controlling bacteria in grape juices compared with discontinuous pressurisation techniques, such as HHP, which is unable to eliminate them completely, even at pressures higher than 500 MPa/10 min (Morata et al., 2015). Also, UHPH is much more efficient than HPH, which normally needs multi-passes to reach inactivation lower than 2-log at 200 MPa (Szczepańska et al., 2021). Saccharomyces yeast starters were inoculated in both the control and UHPH musts at 5 × 107 CFU/mL, and the population of Saccharomyces and non-Saccharomyces yeasts was monitored every two days. It could be observed that non-Saccharomyces were undetected in the UHPH fermenters during the entire fermentation; however, they remained above or close to 4-log in the control fermentations (Fig. 4a) until day 7, when the ethanol was higher than 9% v/v (see Fig. 5a). The Saccharomyces populations were a little lower in the UHPH than in the control fermentations (but significant, p < 0.05) because the inoculated strain was the only one present.
A Saccharomyces yeast counts for the control (continuous black line) and UHPH treatments (continuous green line) during fermentation in 1-L flasks. Non-Saccharomyces counts for control (dashed black line) and UHPH treatments (undetected). B Evolution of fermentation in non-inoculated control and UHPH-processed musts by ethanol content calculated from the CO2 losses in the 100 mL fermenters. All the values are means and standard deviations of three independent fermentations
Additionally, triplicate samples of the UHPH and control musts were left to evolve without inoculation, and the fermentation was completed by the wild yeast population in the control musts in 50 days at 20 °C (Fig. 4b). The control wines reached 15% v/v in ethanol. The long duration was due to the high initial sugar content and, probably, due to the unsuitable fermentative ability of the wild yeast population. Fermentation was undetected in the uninoculated UHPH controls for longer than 50 days (Fig. 4b). The absence of fermentative activity in the long-term in the UHPH musts ensured the total elimination of yeasts, especially the damaged non-culturable cells that are sometimes observed in treatments by discontinuous HHP (Lado and Yousef, 2002).
Full elimination of fermentative yeasts allows the use of new fermentation biotechnologies, as the use of non-Saccharomyces yeasts and the yeast-bacteria allow co-inoculation to perform simultaneous alcoholic and malolactic fermentation (Bañuelos et al., 2016; Morata et al., 2017; Vaquero et al., 2021). In the UHPH must, there was full implantation of the inoculated yeast starter without any impact on the wild yeast or bacteria, which were completely eliminated. Therefore, UHPH helps to reduce the use of chemical additives as sulphites (Morata et al., 2017; Morata and Guamis., 2020; Christofi et al., 2020) and can help to delay fermentation processes at the winery, scheduling them at appropriate dates, favouring a better distribution of work and allowing non-stational wine production.
Oenological Parameters in Grape Must: Effect of UHPH Processing on Must Composition
The general analysis of the standard oenological parameters was done by means of FTIR spectroscopy (Fig. 5). Fermentations proceed at a regular rate, reaching the highest potential alcoholic degree (15% v/v) in 25 days (Fig. 5a). The UHPH fermentations were slower than the controls (statistically significant, p < 0.05), but it must be considered that they were carried out in small volumes of 0.9 L and at 20 °C. Volatile acidity was controlled throughout all the fermentations at values close to or below 0.4 g/L, finishing in both the controls and UHPH at less than 0.25 g/L, without significant differences (p > 0.05), which is an optimal value for wine fermentation (Fig. 5b).
The pH was slightly higher in the UHPH musts, which is normal because of the higher extraction of salts and cations by the fragmentation of solid colloids (Fig. 5c). This has also been previously observed (Loira et al., 2018). However, the final values after fermentation did not have an oenological impact: pH 3.92 in the UHPH wines and 3.89 in the controls, although they were significant (p < 0.05). As a result of the pH values, acidity was somewhat higher in the control (3.37 g/L) than in the UHPH wines (3.20 g/L) but, again, without oenological impact (Fig. 5d). Finally, both wines finished dry, with residual sugars of < 1.6 g/L (Fig. 5e).
Antioxidant Power and Control of Oxidative Enzymes by UHPH
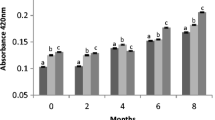
Oxidative damage by polyphenol oxidases (PPO) affects wine quality, producing detrimental colour and aroma quality (Hendrickx et al., 1998). The oxidation effect was monitored by the evolution of the hue (Fig. 6). The air exposure was significantly high (1 cm2/mL in cuvettes at room temperature) and produced an evolution to red-brown colour in the controls, while better colour stability was observed in the UHPH musts (Fig. 6). The control musts had a higher hue than the UHPH musts (p < 0.05). Using polynomic regression and future extrapolation, a high correlation could be observed (R2 > 0.99) with the measured values. After 6 days of exposure to air, the hue in the controls had noticeably increased (≈ × 6), and in the UHPH musts, it remained at lower values (≈ × 2). Therefore, the UHPH musts were much more stable, even with high aeration, at room temperature, and without SO2, which is in accordance with the findings reported in previous works (Bañuelos et al., 2020; Loira et al., 2018; Suárez-Jacobo et al., 2012). The powerful inactivation of PPO enzymes (≈ 90%, Loira et al., 2018) is an advantage of UHPH compared with discontinuous HHP, in which unclear enzyme inactivation is observed (Buckow et al., 2009), or HPH with inactivation not higher than 20% (Szczepańska et al., 2021). The antimicrobial, but also antioxidative, effect of UHPH opens the clear potential for using this technique to produce wines with low levels of SO2, or without it.
Evolution of the colour hue measured by the ratio of absorbances at 420 nm (yellow) and 520 nm (red). Oxidation was promoted by leaving the musts at room temperature in cuvettes with a surface exposure to air of 1 cm2/mL. Values are means with standard deviations (SDs) of triplicate samples. Values were approximated and extrapolated by polynomic regressions with a high r2 value (> 0.99). The real colour in 1 mm cuvettes is also shown, at 45 min and 48 h
Even when the initial antioxidant activity of the red musts was much higher compared to white musts processed in previous works (Bañuelos et al., 2020; Loira et al., 2018), due to the elevated content of polyphenols, the continuous treatment by UHPH at 300 MPa produced a 6.5% higher antioxidant activity (Trolox equivalents) in comparison with the untreated red must (control). The observed value in the UHPH-processed must was 7242 µmol/L compared to 6797 µmol/L in the unprocessed must (with significant differences p < 0.05), which is in accordance with previous research (Bañuelos et al., 2020; Loira et al.,; 2018; Patrignani et al., 2019; Velázquez-Estrada et al., 2013). This increased antioxidant activity is related to the better preservation of the flavonoids and other polyphenols by PPO inactivation.
Polyphenol Content and Colour Intensity
The UHPH-treated and control musts showed similar initial contents of polyphenols, which are better stabilised in UHPH fermentations and remain at higher values, with a more stable trend at the end of fermentation (Fig. 7a). The content of total polyphenols in the controls was significantly lower at the end of fermentation, probably because of the effect of the PPOs in the absence of SO2. Usually, polyphenols show a typically increasing trend during red wine fermentation due to their better solubility in less polar solutions caused by the production of ethanol by yeasts (Morata et al., 2019a, 2019b). Conversely, anthocyanins and, therefore, colour show a decreasing trend because of their insolubilisation in higher ethanol contents. Previously, increased extraction of polyphenols by UHPH in kiwi juice has been observed (Patrignani et al., 2019). However, a reduction in phenolic compounds in mulberry juice treated by UHPH, but in different working conditions, in a multi-pass mode at 200 MPa, has also been found (Yu et al., 2014). Our results highlight the gentle but effective processing of grape juice in a single pass at 300 MPa.
Regarding colour intensity, as can be seen in Fig. 7b, because of the effect of UHPH, the colour was slightly weaker in the UHPH musts. However, there was a suitable evolution during fermentation and, in the end, the results of both fermentations were without significant differences (p < 0.05). Therefore, UHPH can be considered to be a protective technique in both total polyphenols as well as colour intensity (Fig. 7b) and appearance (Fig. 6).
Effect of UHPH on Anthocyanin Content
The grape anthocyanin contents (monoglucosides and acylated derivatives), together with the pyranoanthocyanins formed during fermentation, were analysed using LC-DAD (Table 1). A slightly higher concentration of anthocyanins can be observed in the UHPH musts, especially due to the selective protection of the UHPH in the acylated derivatives (Table 1), which show a 9.3% increment. This difference can be clearly observed in the peak areas for these derivatives in the LC-DAD chromatograms (Fig. 8). Acylated anthocyanins are especially interesting in wine colouration because they absorb at higher wavelengths compared to non-acylated ones (Mazza & Francis, 1995), producing bluish-red hues that are highly valued by consumers. The formation of stable pyranoanthocyanins during fermentation (Morata et al., 2019a, 2019b) was similar in the control and UHPH musts (Table 1). The similar contents indicate the protective effect on delicate pigments of this non-thermal technology, allowing the elimination of microorganisms and oxidative enzymes with a gentle effect on colour. This protective effect has been reported in other fruit pigments (Patrignani et al., 2019). The use of a single-pass 300 MPa UHPH process is more protective of colour than a multi-pass mode at 200 MPa (Yu et al., 2014).
The evolution of total anthocyanins and pyranoanthocyanins was monitored along with fermentation by LC-DAD, and similar trends in the insolubilisation of grape anthocyanins from controls and UHPH musts were observed (Fig. 9). Additionally, the formation of pyranoanthocyanins (vinylphenolic and vitisins) during fermentation by yeast showed a similar tendency in the controls and the UHPH.
Effect of UHPH on Fermentative Aroma
The fermentative volatiles had a normal profile in the wines (Table 2). However, it should be noted that the controls had a much higher content of higher alcohols than the UHPH wines (+ 78%). Higher alcohols are responsible for the winey flat smell of low-quality wines when they are present at concentrations higher than 350–400 mg/L. In the fermentation, both trial samples were under these values, but the control was closer to the threshold, which can shade and hide other floral and fruity aromas (de-la-Fuente-Blanco et al., 2016). Additionally, at moderate concentrations, they have a low impact on the sensory profile of wines (Ferreira et al., 2002). Concerning acetaldehyde, both samples showed a concentration found in the normal range of wines (Liu & Pilone, 2000). Ethyl acetate is responsible for fruity scents at low concentrations, but solvent odour at high amounts. The sensory threshold was around 12.3 mg/L (Culleré et al., 2019). So, in the case of the UHPH, the odour activity value (OAV) was 2.2, while it was 3.2 in the controls, thus, probably expressing more solvent-glue aroma in the last ones.
Acetate esters have a powerful impact on the sensory profile because they have fruity or floral descriptors and low sensory thresholds. In this research, they did not show significant differences at the global level. However, 2-phenylethyl acetate showed a significantly higher concentration in the UHPH wines compared to the controls (× 1.6), both at higher sensory threshold values. 2-phenylethyl acetate is a key compound in wine fermentative aroma because of its floral impact with a rose petal descriptor (Morata et al., 2020). The high level of this ester in the UHPH wines could have been produced by the release of higher contents of the amino acid precursor from the grape cell fragments due to the intense impact forces. Higher contents of yeast-assimilable nitrogen were observed in the UHPH-processed musts (Loira et al., 2018).
Sensory Evaluation
The sensory attribute evaluation of the wines exhibited similar profiles, but significant differences were found between the control and UHPH wines, corresponding to the attributes’ global perception and aromatic quality (Fig. 10). The better aromatic quality and the probable consequence of a better global perception can be correlated with the lower concentrations of higher alcohols and the higher content of 2-phenylethyl acetate, which is representative of the floral esters with a rose petal descriptor. This molecule was above its sensory threshold in both cases (0.25 mg/L, Carrau et al., 2008), but at 63% higher in the UHPH than in the control wines. Both parameters were better valued in the UHPH wines. Concerning colour intensity and hue, the controls had the worst average values, but without statistical significance.
Conclusion
UHPH sterilisation eliminates wild microorganisms, avoiding spontaneous fermentation and favouring the implantation of yeast starters. The use of uncompetitive non-Saccharomyces yeasts can be strongly promoted, as can the use of yeast–bacteria co-inoculations. Additionally, the oxidative processes mediated by PPOs are inactivated following the denaturation of the enzyme structure by the impact and shearing forces during UHPH treatments. Colour is preserved, and oxidation is delayed because of the high air exposure in the absence of SO2. The results confirm that UHPH is a powerful tool in the production of healthier and more stable red wines without sulphites or other chemical additives. The intense forces produced by UHPH produce a different colloidal structure, with a lower average particle size, which has an impact on wine stability.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Bañuelos, M. A., Loira, I., Escott, C., Fresno, D., & J. M., Morata, A., Sanz, P. D., Otero, L., & Suárez-Lepe, J. A. (2016). Grape processing by high hydrostatic pressure: Effect on use of non-Saccharomyces in must fermentation. Food and Bioprocess Technology, 9, 1769–1778.
Bañuelos, M. A., Loira, I., Guamis, B., Escott, C., Del Fresno, J. M., Codina-Torrella, I., Quevedo, J. M., Gervilla, R., Rodríguez Chavarría, J. M., de Lamo, S., Ferrer-Gallego, R., Álvarez, R., González, C., Suárez-Lepe, J. A., & Morata, A. (2020). White wine processing by UHPH without SO2. Elimination of microbial populations and effect in oxidative enzymes, colloidal stability and sensory quality. Food Chemistry, 332, 127417. https://doi.org/10.1016/j.foodchem.2020.127417
Bevilacqua, A., Corbo, M. R., & Sinigaglia, M. (2012). Use of natural antimicrobials and high pressure homogenization to control the growth of Saccharomyces bayanus in apple juice. Food Control, 24, 109–115. https://doi.org/10.1016/j.foodcont.2011.09.011
Bevilacqua, A., Petruzzi, L., Perricone, M., Speranza, B., Campaniello, D., Sinigaglia, M., & Corbo, M. R. (2018). Nonthermal technologies for fruit and vegetable juices and beverages: Overview and advances. Comprehensive Reviews in Food Science and Food Safety, 17, 2–62. https://doi.org/10.1111/1541-4337.12299
Buckow, R., Weiss, U., & Knorr, D. (2009). Inactivation kinetics of apple polyphenol oxidase in different pressure–temperature domains. Innovative Food Science & Emerging Technologies, 10, 441–448. https://doi.org/10.1016/j.ifset.2009.05.005
Calligaris, S., Foschia, M., Bartolomeoli, I., Maifreni, M., & Manzocco, L. (2012). Study on the applicability of high-pressure homogenization for the production of banana juices. LWT- Food Science and Technology, 45, 117–121.
Carrau, F. M., Medina, K., Farina, L., Boido, E., Henschke, P. A., & Dellacassa, E. (2008). Production of fermentation aroma compounds by Saccharomyces cerevisiae wine yeasts: Effects of yeast assimilable nitrogen on two model strains. FEMS Yeast Research, 8, 1196–1207. https://doi.org/10.1111/j.1567-1364.2008.00412.x
Christofi, S., Malliaris, D., Katsaros, G., Panagou, E., & Kallithraka, S. (2020). Limit SO2 content of wines by applying high hydrostatic pressure. Innovative Food Science & Emerging Technologies, 62, 102342. https://doi.org/10.1016/j.ifset.2020.102342
Comuzzo, P., & Calligaris, S. (2019). Potential applications of high pressure homogenization in winemaking: A review. Beverages, 5, 56. https://doi.org/10.3390/beverages5030056
Corrales, M., FernándezGarcía, A., Butz, P., & Tauscher, B. (2009). Extraction of anthocyanins from grape skins assisted by high hydrostatic pressure. Journal of Food Engineering, 90, 415–421. https://doi.org/10.1016/j.jfoodeng.2008.07.003
Culleré, L., López, R., & Ferreira, V. (2019). The instrumental analysis of aroma-active compounds for explaining the flavor of red wines. In: A. Morata (Ed.) Red wine technology, Ch. 20, (p. 287). London, UK: Academic Press, https://doi.org/10.1016/B978-0-12-814399-5.00020-7
de-la-Fuente-Blanco, A., Sáenz-Navajas, M.-P., & Ferreira, V. (2016). On the effects of higher alcohols on red wine aroma. Food Chemistry, 210, 107–114. https://doi.org/10.1016/j.foodchem.2016.04.021
EP2409583. Guamis-Lopez, B., Trujillo-Mesa, A.-J., Ferragut-Pérez, V., Quevedo-Terré, J. M., Lopez-Pedemonte, T., & Buffa-Dunat, M. N. (25.01.2012 Bulletin 2012/04). Title: Continuous system and procedure of sterilization and physical stabilization of pumpable fluids by means of ultra-high pressure homogenization. Designated Contracting States: AL AT BE BG CH CV CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO SE SI SK SM TR Designated Extension States: BA ME RS Applicant: Universidad Autónoma de Barcelona 08193 Barcelona (ES).
European Commission. (2020). https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:52020PC0570&from=EN retrieved 23/03/2021.
Ferreira, V., Ortín, N., Escudero, A., López, R., & Cacho, J. (2002). Chemical characterization of the aroma of Grenache rosé wines: Aroma extract dilution analysis, quantitative determination, and sensory reconstitution studies. Journal of Agricultural and Food Chemistry, 50, 4048–4054. https://doi.org/10.1021/jf0115645
Fleet, G. H. (2003). Yeast interactions and wine flavour (review article). International Journal of Food Microbiology, 86, 11–22.
Glories, Y. (1984). La couleur des vins rouges II. Connaisance de la Vigne et du Vin. pp 253–271.
Hendrickx, M., Ludikhuyze, L., Van Den Broeck, I., & Weemaes, C. (1998). Effects of high pressure on enzymes related to food quality. Trends in Food Science and Technology, 9, 197–203.
Leite, T. S., Augusto, P. E. D., & Cristianini, M. (2016). Frozen concentrated orange juice (FCOJ) processed by the high pressure homogenization (HPH) technology: Effect on the ready-to-drink juice. Food and Bioprocess Technology, 9, 1070–1078. https://doi.org/10.1007/s11947-016-1688-z
Liu, S.-Q., & Pilone, G. J. (2000). An overview of formation and roles of acetaldehyde in winemaking with emphasis on microbiological implications. International Journal of Food Science & Technology, 35, 49–61. https://doi.org/10.1046/j.1365-2621.2000.00341.x
Loira, I., Morata, A., Bañuelos, M. A., Puig-Pujol, A., Guamis, B., González, C., & Suárez-Lepe, J. A. (2018). Use of ultra-high pressure homogenization processing in winemaking: Control of microbial populations in grape musts and effects in sensory quality. Innovative Food Science and Emerging Technologies, 50, 50–56.
Martínez-Hernández, G. B., Álvarez-Hernández, M. H., & Artés-Hernández, F. (2019). Browning control using cyclodextrins in high pressure–treated apple juice. Food and Bioprocess Technology, 12, 694–703. https://doi.org/10.1007/s11947-019-2242-6
Mazza, G., & Francis, F. J. (1995). Anthocyanins in grapes and grape products. Critical Reviews in Food Science and Nutrition, 35, 341–371. https://doi.org/10.1080/10408399509527704
Morata, A., & Guamis, B. (2020). Use of UHPH to obtain juices with better nutritional quality and healthier wines with low levels of SO2. Frontiers in Nutrition, 7, 598286. https://doi.org/10.3389/fnut.2020.598286
Morata, A., Escott, C., Bañuelos, M. A., Loira, I., del Fresno, J. M., González, C., & Suárez-Lepe, J. A. (2020). Contribution of non-Saccharomyces yeasts to wine freshness. A Review. Biomolecules, 10, 34.
Morata, A., Escott, C., Loira, I., Del Fresno, J. M., González, C., & Suárez-Lepe, J. A. (2019a). Influence of Saccharomyces and non-Saccharomyces yeasts in the formation of pyranoanthocyanins and polymeric pigments during red wine making. Molecules, 24, 4490. https://doi.org/10.3390/molecules24244490
Morata, A., González, C., Tesfaye, W., Loira, I., & Suárez-Lepe, J. A. (2019). Maceration and fermentation: New technologies to increase extraction. In: A. Morata (Ed.) Red wine technology, Ch. 3, (p. 39). London, UK: Academic Press, https://doi.org/10.1016/B978-0-12-814399-5.00003-7
Morata, A., Loira, I., Vejarano, R., Bañuelos, M. A., Sanz, P. D., Otero, L., & Suárez-Lepe, J. A. (2015). Grape processing by high hydrostatic pressure: Effect on microbial populations, phenol extraction and wine quality. Food and Bioprocess Technology, 8, 277–286.
Morata, A., Loira, I., Vejarano, R., González, C., Callejo, M. J., & Suárez-Lepe, J. A. (2017). Emerging preservation technologies in grapes for winemaking. Trends in Food Science and Technology, 67, 36–43. https://doi.org/10.1016/j.tifs.2017.06.014
OIV. (2020). https://www.oiv.int/public/medias/7587/oiv-oeno-594b-2020-en.pdf retrieved 23/03/2021.
Oliete, B., Potin, F., Cases, E., & Saurel, R. (2019). Microfluidization as homogenization technique in pea globulin-based emulsions. Food and Bioprocess Technology, 12, 877–882. https://doi.org/10.1007/s11947-019-02265-3
Patrignani, F., & Lanciotti, R. (2016). Applications of high and ultra high pressure homogenization for food safety. Frontiers in Microbiology, 7, 1132. https://doi.org/10.3389/fmicb.2016.01132
Patrignani, F., Mannozzi, C., Tappi, S., Tylewicz, U., Pasini, F., Castellone, V., Riciputi, Y., Rocculi, P., Romani, S., Caboni, M. F., Gardini, F., Lanciotti, R., & Dalla Rosa, M. (2019). (Ultra) high pressure homogenization potential on the shelf-life and functionality of kiwifruit juice. Frontiers in Microbiology, 10, 246. https://doi.org/10.3389/fmicb.2019.00246
Patrignani, F., Siroli, L., Braschi, G., & Lanciotti, R. (2020). Combined use of natural antimicrobial based nanoemulsions and ultra high pressure homogenization to increase safety and shelf-life of apple juice. Food Control, 111, 107051. https://doi.org/10.1016/j.foodcont.2019.107051
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine, 26, 1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3
Ribéreau-Gayon, J., Peynaud, E., Sudraud, P., & Ribéreau-Gayon, P. Tratado de enología. Ciencias y técnicas del vino. Análisis y control de los vinos (Tomo I), 2nd ed.; Hemisferio Sur: Buenos Aires, 1980.
Suárez-Jacobo, Á., Saldo, J., Rüfer, C. E., Guamis, B., Roig-Sagués, A. X., & Gervilla, R. (2012). Aseptically packaged UHPH-treated apple juice: Safety and quality parameters during storage. Journal of Food Engineering, 109, 291–300. https://doi.org/10.1016/j.jfoodeng.2011.09.007
Sulaiman, A., Soo, M. J., Yoon, M. M. L., Farid, M., & Silva, F. V. M. (2015). Modeling the polyphenoloxidase inactivation kinetics in pear, apple and strawberry purees after High Pressure Processing. Journal of Food Engineering, 147, 89–94. https://doi.org/10.1016/j.jfoodeng.2014.09.030
Szczepańska, J., Skąpska, S., & Marszałek, K. (2021). Continuous high-pressure cooling-assisted homogenization process for stabilization of apple juice. Food and Bioprocess Technology, 14, 1101–1117. https://doi.org/10.1007/s11947-021-02611-4
Vaquero, C., Loira, I., Heras, J. M., Carrau, F., González, C., & Morata, A. (2021). Biocompatibility in ternary fermentations with Lachancea thermotolerans, other non-Saccharomyces and Saccharomyces cerevisiae to control pH and improve the sensory profile of wines from warm areas. Frontiers in Microbiology, 12, 832; https://doi.org/10.3389/fmicb.2021.656262
Velázquez-Estrada, R. M., Hernández-Herrero, M. M., Rüfer, C. E., Guamis-López, B., & Roig-Sagués, A. X. (2013). Influence of ultra high pressure homogenization processing on bioactive compounds and antioxidant activity of orange juice. Innovative Food Science & Emerging Technologies, 18, 89–94. https://doi.org/10.1016/j.ifset.2013.02.005
Ypsicon the company that produce the UHPH technology: https://www.ypsicon.com/
Yu, Y., Xu, Y., Wu, J., Xiao, G., Fu, M., & Zhang, Y. (2014). Effect of ultra-high pressure homogenisation processing on phenolic compounds, antioxidant capacity and anti-glucosidase of mulberry juice. Food Chemistry, 153, 114–120. https://doi.org/10.1016/j.foodchem.2013.12.038
Zamora, A., & Guamis, B. (2015). Opportunities for ultra-high-pressure homogenisation (UHPH) for the food industry. Food Engineering Reviews, 7, 130–142.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work was funded by the Ministerio de Ciencia, Innovación y Universidades project RTI2018-096626-B-I00.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vaquero, C., Escott, C., Loira, I. et al. Cabernet Sauvignon Red Must Processing by UHPH to Produce Wine Without SO2: the Colloidal Structure, Microbial and Oxidation Control, Colour Protection and Sensory Quality of the Wine. Food Bioprocess Technol 15, 620–634 (2022). https://doi.org/10.1007/s11947-022-02766-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-022-02766-8