Abstract
A comprehensive study using continuous pressure change technology (PCT) for the non-thermal processing of fresh pineapple juice on pilot scale was conducted (1 L/min, 50 MPa, argon, 3 min, <35 °C). The immediate effects of a single and a twofold PCT treatment on the most important quality parameters were examined and compared with those of fresh and thermally pasteurised (90 °C) juices. In comparison to the fresh juice, both PCT-treated samples exhibited slightly brighter and less yellowish colour (CIE L*a*b*). A significant reduction in the mean particle size resulted in diminished centrifugable pulp contents and enhanced cloud stability. Moreover, a slightly improved microbial quality (−0.9 to −1.2 log10 CFU/mL) in terms of total aerobic and yeast and mould counts was attained. Noteworthy, PCT retained a high bromelain activity (−3 to −15% reduction) and efficiently inactivated polyphenol oxidase. Water-soluble vitamins, phenolic compounds, and all further constituents assessed were mostly preserved. However, the high residual peroxidase activity (−10 to −23%) and microbial loads are likely to affect juice quality during storage. In contrast, thermal pasteurisation ensured a complete reduction in both microbial counts (−4.4 to −4.5 log10 CFU/mL) and effective inactivation of peroxidase. However, bromelain activity was strongly affected (−83%) by heat treatment, and colour was darkened and even less yellowish. Overall, this study highlighted the potential of PCT for the production of fresh-like pineapple juices; however, its current limitations were revealed as well.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pineapples (Ananas comosus [L.] Merr.) are one of the most popular tropical fruits consumed worldwide. In 2017, the global production of pineapples exceeded 27.4 million tons. According to the global import statistics, the major portion was consumed fresh and merely 7% were processed into juices and concentrates, respectively, among others (FAO 2019).
Pineapple juices are commonly thermally pasteurised aiming at the extension of their shelf life by inactivating pathogenic and spoilage microorganisms as well as enzymes. However, the fragile aroma, colour, and heat-sensitive micronutrients may be adversely affected (Lobo and Paull 2017; Miller and Silva 2016), impairing the ‘fresh’ and ‘natural’ character of the juice. However, the latter is exactly what consumers increasingly demand. In the European market, the share of cooled premium juices with superior sensory quality steadily grew during the past years whereas that of pasteurised juices storable at ambient temperatures gradually declined (AIJN 2019).
Innovative non-thermal technologies rendering both shelf life extension and retention of fresh-like quality traits possible are therefore increasingly in demand, and thus, of utmost importance for the juice industry. Pulsed light, ultraviolet light, pulsed electric fields, high-pressure, and supercritical carbon dioxide treatments have already been applied for non-thermal processing of pineapple juice or other liquid fruit products (Vollmer et al. 2020; Paniagua-Martínez et al. 2018; Koutchma 2009; Kincal et al. 2005; Norton and Sun 2008).
Pressure change technology (PCT) is an emerging non-thermal process, so far not sufficiently considered in previous studies. First promising results about batch, semi-continuous, and continuous PCT applications have been reported by Klingner et al. (2006), Bönsch et al. (2007), and Aschoff et al. (2016), respectively. PCT is based on a physical process. Juices or other liquid food products are put under moderate pressure in the presence of an inert gas. At pressures of up to 50 MPa for a short holding time, the gas dissolves in the liquid food products and diffuses into plant and microbial cells. Once the pressure is suddenly reduced to ambient conditions, the inert gas expands and compartmentalised structures, e.g. plant or microbial cells, are disrupted. Aschoff et al. (2016) stated that the mode of action is based on this dynamic decompression phase. In contrast, static technologies, such as high-pressure processing, are premised on much higher working pressures applied during a certain holding time, in addition to chemical effects (e.g. hydrolysis and acidification) when using carbon dioxide as the process gas (Balasubramaniam et al. 2015; Kincal et al. 2005).
The most obvious advantages of PCT are the low working temperatures (<40 °C) and the protective atmosphere due to the inert process gas. The exclusion of oxygen inhibits the destruction and oxidation of sensitive juice constituents. The successful reduction of diverse food-related microorganisms in cell suspensions has been demonstrated by Klingner et al. (2006) and Bönsch et al. (2007). This is particularly essential for the production of microbiologically safe juices (Miller and Silva 2016). Applying PCT operated with nitrogen at 50 MPa (40 °C, 5 min) to a Pseudomonas fluorescence suspension resulted in a significant reduction (−log N/N0 of 6.4) (Bönsch et al. 2007). However, the authors have also pointed out that the type of microorganism (gram− > yeast >> gram+), the applied pressure (5–25 MPa less effective than 50 MPa), the temperature (10, 25, or 40 °C), and the process gas (nitrogen, helium, argon, carbon dioxide, or nitrous oxide) determine the efficiency of this technology. Furthermore, continuous PCT studies (25/50 MPa, nitrogen, <40 °C) conducted by Aschoff et al. (2016) with freshly squeezed orange juice permitted a total plate count reduction of at least 3.4 log10 CFU/mL. Partial and even total inactivation of pectin methylesterase (PME) and peroxidase (POD), respectively, a significant reduction of the mean particle size, thus improving cloud stability, a negligible brightening and reinforcement of the yellow colour were additionally achieved. However, a slight reduction in micronutrients (carotenoids, vitamin C, and hesperidin) was observed. In addition, PCT offers opportunities for continuous processing and the optional recovery of the process gas.
The aim of the present study was to process fresh pineapple juice at pilot plant scale with a continuous PCT device applying one (PCT1) or two passages (PCT2), respectively, and to comprehensively investigate its immediate effects on relevant quality parameters. Physical traits, such as colour, particle size and distribution, and cloud stability as well as microbial loads (total aerobic plate count (TAPC), yeast and mould count (YMC)), presence of desired and detrimental enzymes (bromelain (BRM), PME, POD, and polyphenol oxidase (PPO)), and content of the most important micronutrients, inter alia vitamin C, B vitamins, and phenolic compounds, were assessed. In addition, both PCT-treated samples were compared with a fresh and thermally pasteurised (90 °C) pineapple juice. Hereby, the thermal pasteurisation represents the current state of the art applied in the industry, whereas the untreated juice serves as the reference nearest to natural. These reference points were used as relevant boundaries to range in our results.
Materials and Methods
Chemicals
All chemicals were purchased from Sigma-Aldrich (Steinheim, Germany, and Buchs, Switzerland), Merck (Darmstadt, Germany), VWR International (Leuven, Belgium, and Fontenay-sous-Bois, France), Th. Geyer (Renningen, Germany), Roth (Karlsruhe, Germany), Extrasynthèse (Genay, France), Fluka Chemie (Buchs, Switzerland), or Herbstreith & Fox (Neuenbürg, Germany). Ultrapure water was provided by an arium 611 UV (Sartorius, Göttingen, Germany) ultrapure water system and used throughout all experiments, if not stated otherwise.
Pineapple Juice Production
Approx. 210 kg of pineapples (Ananas comosus (L.) Merr. cv. ‘MD2’ (syn. ‘Extra Sweet’)), air-freighted from Ghana, was purchased from a local fruit distributor (Schumacher, Filderstadt-Bernhausen, Germany).
Fresh Juice
After one day of storage at ambient temperature, the entire batch of pineapples was sorted, decrowned, washed with tap water, peeled, and manually cut into quarters. The flesh was shredded using a grating mill (Bucher-Guyer, Niederweningen, Switzerland), and the juice extracted by finishing through a 1.6-mm sieve (Alberto Bertuzzi SpA, Brugherio, Italy). Large flesh particles were removed using a decanter (GEA Westfalia Separator, Oelde, Germany). Juice yield was 46% (w/w).
Thermally Pasteurised Juice
For thermal pasteurisation, the fresh juice was heated to a targeted filling temperature of 90 °C (Lobo and Paull 2017) at a continuous flow rate of 1.3 L/min applying a pilot plant scale tubular heat exchanger (Ruland Engineering & Consulting, Neustadt an der Weinstraße, Germany). After direct hot filling into 500-mL clear glass bottles and flushing the headspace of approx. 5% of the total volume with vapour using a filling and sealing machine (Schmalbach-Lubeca, Braunschweig, Germany), the juice was immediately cooled to ambient temperature in a water bath.
Pressure Change Technology-Treated Juices
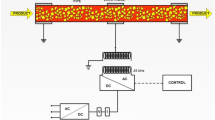
The fresh juice was transferred to the feed tank of a continuous PCT pilot plant located in a cleanroom with a controlled ambient temperature of 20 ± 1 °C (Fig. 1). At a continuous flow rate of 1.0 L/min, the juice was pressurised to 50 MPa within a few seconds (<10 s) by a high-pressure pump. Subsequently, the juice was homogeneously mixed with individually compressed argon applying a slight excess pressure of the gas (ca. 52 MPa) by an inline static mixer within the tubular PCT reactor. The gas flow rate was 20 g/min resulting in an argon to juice ratio of 20 g/L. The juice-argon mixture was kept constant at 50 MPa and released after 3 min through an exhaust valve into a filling tank previously flushed with nitrogen. The pineapple juice was bottled manually (PCT1). Once the first passage was entirely completed, the remaining juice was re-circulated from the filling to the feed tank following a further passage (PCT2) applying the above settings.
The initial temperature of the juice prior to both PCT treatments was 21.0 ± 2.8 °C. Throughout the following processes, the average juice temperature gradually increased. Pressurisation and mixing within the PCT reactor resulted in a slight increase to 22.0 ± 1.4 °C, while the main rise to 33.5 ± 0.7 °C was caused during the dynamic decompression phase. The latter rise in temperature may result from two individual effects that reversely occur during decompression of the juice-argon mixture at the exhaust valve. These include the cooling due to the sudden outgassing and rapidly expanding gas based on the Joule-Thomson effect and the predominating heating caused by shear forces.
Immediately after juice production, two representative samples of the four juice types each were taken, filled into containers headspace-flushed with nitrogen, and stored at −80 °C. Immediately prior to analyses, juices were thawed in a water bath (20 °C).
Analysis of Physicochemical Juice Properties
The pH value, the density, and the total soluble solids (TSS) were determined using a pH meter (inoLab pH 720, WTW, Weilheim, Germany), a density meter (DMA 48, Anton Paar, Graz, Austria), and a digital refractometer (RX-5000, Atago, Tokyo, Japan), respectively.
The titratable acidity (TA, expressed as citric acid in g/100 mL of juice) and the formol number (in mL 0.1 mol NaOH/100 mL juice) were analysed according to the methods of the International Federation of Fruit Juice Producers (IFU 1984, 1996a) using an automatic titration system (Titrino 718 STAT, Metrohm, Herisau, Switzerland). The titration solution was normalised using potassium hydrogen phthalate.
The total phenolic content (TPC) was assessed with the colorimetric Folin-Ciocalteu assay according to Singleton et al. (1999) with modifications. Briefly, 20 μL of centrifuged (14,100×g, 10 min, MiniSpin plus, Eppendorf, Hamburg, Germany) and filtered (polytetrafluoroethylene, 0.45 μm, Acrodisc CR 13, Pall, Ann Arbor, MI, USA) pineapple juice were mixed with 980 μL of ultrapure water and 100 μL of Folin-Ciocalteu reagent. After 3 min and the addition of 800 μL aqueous sodium carbonate solution (75 g/L), the mixture was incubated for 60 min at ambient temperature. Subsequently, the absorbance was measured at 760 nm using an UV/Vis spectrometer (Lambda 35, PerkinElmer, Singapore). Gallic acid was used for external linear calibration and TPC expressed as gallic acid equivalents (GAE) in mg/100 mL juice.
The centrifugable pulp content of the juices was determined according to IFU (1991) using a Heraeus Labofuge 400 R centrifuge (Thermo Fisher Scientific, Osterode, Germany).
5-(Hydroxymethyl)furfural (HMF) was analysed by UPLC-DAD following a modified procedure according to IFU (1996b). The pineapple juices were initially filtered through regenerated cellulose membranes (0.45 μm, Chromafil RC-45/15 MS, Macherey-Nagel, Düren, Germany) into amber glass vials. An Acquity H-class UPLC system equipped with an eλ photodiode array detector (all from Waters, Milford, MA, USA) was applied for quantitation. UPLC separation at 25 °C was achieved with a Kinetex reversed-phase C18 core-shell column (100 × 2.1 mm, 1.7-μm particle size, 100-Å pore size) and a guard column of the same material (both from Phenomenex, Torrance, CA, USA). The mobile phase consisted of ultrapure water/methanol (90:10, v/v). Applying an isocratic elution, total run time was 5 min at a flow rate of 0.35 mL/min. Injection volume was 3 μL. HMF was detected at 284 nm. Linear external calibration curves were prepared with ultrapure water and HMF-free pineapple juice, respectively. Limit of detection (LOD) and quantitation (LOQ) were ascertained based on the signal-to-noise (S/N) ratio of the calibration curves. LOD (S/N of 3:1) and LOQ (10:1) were determined at 0.001 and 0.016 mg/L in ultrapure water and 0.3 and 1.8 mg/L in pineapple juice, respectively.
Colour Measurement
CIE L*a*b* colour values were determined in triplicate using an UltraScan Vis spectrophotometer (HunterLab, Reston, VA, USA) in reflection mode and with standard illumination (D65/10°). L* (lightness), a* (green-red), and b* (blue-yellow) values were used to additionally calculate chromaticity (C*), hue angle (h°), and the total colour difference (∆E*).
Particle Size Analysis
Particle size distributions were analysed using a static light scattering particle analyser (Horiba LA-950, Retsch Technology, Haan, Germany). Mean particle diameters (d4,3); the particle diameters of the respective 10, 50, and 90% quantiles (d10, d50, and d90); and the particle size distributions are given as volume-weighted diameters.
Cloud Stability Monitoring
As described by Aschoff et al. (2016), cloud stability was monitored by filling 20 mL of pineapple juice into 25-mL graduated cylinders stored for two weeks at 4 °C. Sedimentation of cloud was visually inspected after 1, 2, 7, and 14 days.
Microbial Counts
Total aerobic plate count (TAPC) as well as yeast and mould count (YMC) were determined according to IFU (1996c,d) with modifications. In brief, serial dilutions were prepared using sterile saline solution containing 0.1% (w/v) peptone broth (VWR International). One hundred microlitres of juice and appropriate dilutions was spread out in triplicate on orange serum agar plates. For the cultivation of yeasts and moulds, the orange serum agar additionally contained 34 μg/mL of chloramphenicol (≥98%) to inhibit bacterial growth. All plates were incubated at 30 °C for 48 h. Both counts were expressed in colony-forming units (CFU)/millilitre of juice. Plates with <10 CFUs (<2 log10 CFU/mL) were not evaluated.
Enzyme Assays
BRM activity was assessed spectrophotometrically following Jutamongkon and Charoenrein (2010) and expressed as casein digestive units (CDU).
PME, POD, and PPO were initially extracted from the pineapple juices according to Hirsch et al. (2008) with slight modifications. In brief, 20 g of pineapple juice was blended with 20 mg saponin, 600 mg polyvinylpolypyrrolidone, and 175.5 mg NaCl. After adjusting the pH value to 6.0 (10 and 1 M NaOH), the mixture was stirred (2 h, 4 °C) and subsequently centrifuged (25,000×g, 4 °C, 30 min, Suprafuge 22, Heraeus Instruments, Osterode, Germany). The supernatant was decanted, and the solid remainder washed twice with ultrapure water. The combined supernatant and washing water was made up to 40 mL with water. Boiled enzyme extracts were used as blanks. Extracts were stored at −80 °C until further analyses.
PME activity was determined by titration according to Hirsch et al. (2008) applying an automatic titration system (Titrino 907 STAT, Metrohm). Two millilitres enzyme extract and 58 mL substrate solution were employed for titrimetric analysis. The substrate solution (0.5%, w/v, pectin Classic CU 201, degree of esterification 70–74%, in 0.15 M NaCl) was prepared as detailed by Aschoff et al. (2016). PME activity, expressed in nkat/g of pineapple juice, was calculated as reported by Hirsch et al. (2008).
POD activity was assessed spectrophotometrically according to Hirsch et al. (2008) with slight modifications. Briefly, 0.4 mL of the enzyme extract diluted 1:2 with McIlvaine buffer (pH 6.5) if required was mixed with 1.1 mL of the substrate solution (12 mM tropolone and 3.3 mM hydrogen peroxide dissolved in McIlvaine buffer, pH 6.5). The absorbance was immediately recorded at 418 nm every 2 s for 10 min using a UV/Vis spectrophotometer (Lambda 35, PerkinElmer, Singapore). POD activity was expressed in nkat/g of pineapple juice.
PPO activity was analysed by spectrophotometry according to Baur et al. (2004) with slight modifications. Briefly, 1.5 mL of the reaction buffer (2 mM sodium dodecyl sulphate dissolved in McIlvaine buffer, pH 6.5), 0.2 mL of an L-proline solution (0.5 M in reaction buffer), and 0.1 mL of the enzyme extract were mixed. After adding 0.2 mL substrate solution (25 mM 4-methylcatechol in reaction buffer), the absorbance was immediately monitored every 2 s for 5 min using the above-mentioned UV/Vis spectrometer. PPO activity was expressed in nkat/g pineapple juice.
Analysis of Micronutrients and Sugars
Vitamin C
For the quantitation of vitamin C, i.e. ascorbic and dehydroascorbic acid (AA and DHAA), the sample preparation described by Aschoff et al. (2015) was applied using the buffers previously reported (Vollmer et al. 2020). For HPLC analysis, an Agilent 1100 Series system equipped with a G1315B diode array detector (DAD) was used (all from Agilent Technologies, Waldbronn, Germany). HPLC parameters, including column and mobile phase, were set as reported previously (Difonzo et al. 2019).
B Vitamins
Vitamins B1 (thiamin), B2 (riboflavin), B3 (niacin), B5 (pantothenic acid), and B6 (pyridoxine) were quantitated utilising microbiological microtiter plate tests (VitaFast, R-Biopharm, Darmstadt, Germany). Sample preparation and quantitation of B vitamins were conducted according to the supplier’s instructions. An aliquot of 1 mL pineapple juice was diluted with 20 mL citrate buffer (pH 4.5). Subsequently, 300 mg α-amylase from Aspergillus oryzae (~1.5 U/mg) and additional 10 mg acid potato phosphatase (0.5–3.0 U/mg) for the analysis of B2 and B6 were admixed following incubation for 1 h in the dark at 37 °C (incubator U 30ü, Memmert, Schwabach, Germany) with occasional shaking. After adding 19 mL of ultrapure water, the mixture was centrifuged at 4500×g and 20 °C for 10 min (Heraeus Labofuge 400 R). The supernatant was sterile-filtered (0.8/0.2 μm, polyethersulfone, Acrodisc Supor, Pall, Ann Arbor, MI, USA), and the filtrate immediately used for quantitation of B vitamins.
Phenolic Compounds, Furanones, and Other Metabolites
Prior to HPLC analysis, the pineapple juices were centrifuged (14,100×g, 10 min, MiniSpin plus, Eppendorf, Hamburg, Germany) and filtered through polytetrafluoroethylene membranes (0.45 μm, Acrodisc CR 13, Pall, Ann Arbor, MI, USA) into amber glass vials. Individual compounds were identified by HPLC-DAD-ESI-MSn analysis as detailed elsewhere (Vollmer et al. 2020; Difonzo et al. 2019; Steingass et al. 2015). For quantitation, the above-mentioned Acquity H-class UPLC system with an eλ photodiode array detector was used as reported previously (Difonzo et al. 2019). Detection wavelengths were 280 and 320 nm. Quantitation was achieved by external calibrations of adequate reference standards (Vollmer et al. 2020; Difonzo et al. 2019).
Sugars
Juices were pre-filtered (4–12 μm, cellulose, MN 615 ¼, Macherey-Nagel, Düren, Germany), diluted with ultrapure water (1:6250), and membrane filtered (0.45 μm, polyamide, Chromafil AO-45/15 MS, Macherey-Nagel, Düren, Germany) into amber glass vials. D-Glucose, D-fructose, and sucrose were quantitated by high-performance anion-exchange chromatography with pulsed amperometric detection (ICS-3000 ion chromatography system, Dionex, Sunnyvale, CA, USA) as described by Pöhnl et al. (2017).
Statistics
Two representative samples were drawn for each juice type and analysed in duplicate, if not stated otherwise. Results are displayed as means ± standard deviations. Statistically significant differences (p ≤ 0.05) among means were identified by one-way analysis of variance (ANOVA) and Tukey’s post-hoc multiple comparison test using SAS University Edition software (SAS Institute, Cary, NC, USA), assuming normal distribution and homogeneity of variances.
Unsupervised principal component analysis (PCA) and hierarchical cluster analysis (HCA) were calculated with Solo software release 8.2.1 (Eigenvector Research, Wenatchee, WA, USA) applying venetian blinds cross-validation method and Ward’s method of agglomeration, respectively. Data was pre-processed using the ‘autoscale’ function of the software.
Results and Discussion
General Quality Traits
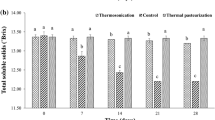
All pineapple juices displayed a constant pH value of 3.7 (Table 1). The relative density 20/20 (density), the TSS, the TA, and the formol number amounted to 1.045–1.048 (1.044–1.047 g/cm3), 11.7–11.9 °Brix, 0.5 g/100 mL, and 7.7–9.2 mL 0.1 mol NaOH/100 mL, respectively, thus complying with the AIJN (2016) reference guidelines for pineapple juice (relative density 20/20: ≥1.045; TSS: ≥11.2 °Brix; TA: 0.32–1.15 g/100 mL; formol number: 8–20 mL 0.1 mol NaOH/100 mL). The TSS/TA ratios of 24.5–24.7 g/g were within the range of 20–40 g/g reported for high-quality pineapple fruit (Lobo and Paull 2017). The consistent values determined among all juices indicated that neither concentrations nor dilutions occurred during processing.
A maximum of 2 mg HMF/100 mL of pineapple juice is specified as an absolute quality requirement (AIJN 2016). HMF may be formed during intense thermal processing or during storage at elevated temperatures by acid-catalysed dehydration of reducing sugars or as an intermediate of the Maillard reaction of reducing sugars and amino compounds. HMF being absent in fresh, untreated juices represents a well-documented indicator of excessive heat treatment (Lozano 2006). During this study, HMF was not detected among any of the pineapple juices assessed (LOD: 0.03 mg/100 mL). The generation of HMF, which has been reported to depend on both the juice composition and the process parameters (Lozano 2006), may have been prevented due to good manufacturing practice and the omission of juice concentration.
Colour Profile
Lightness (L*) and yellowness (+b*) are the two main chromatic parameters characterising the colour of pineapple juice. As illustrated in Table 1, the fresh juice displayed average values of L* = 39.1 and b* = 10.5. The PCT-treated juices were slightly brighter and less yellowish as indicated by elevated L* and smaller b* values, respectively. Both effects were slightly more pronounced after the second PCT treatment. Such a brightening concomitantly with a slightly more intense yellow colour has also been observed by Aschoff et al. (2016) after treating fresh orange juice with PCT. The authors assumed that these alterations probably resulted from changes in light scattering owing to smaller particle sizes. The latter was also observed in our PCT-treated juices, as discussed in the ‘Particle Size, Centrifugable Pulp Content, and Cloud Stability’ section. In contrast, the thermally pasteurised juice turned out to be the darkest (L* = 37.9) and less yellowish (b* = 8.2) variant. The darkening may be attributed to non-enzymatic browning (Lozano 2006), possibly due to heating to 90 °C.
CIE L*a*b* colour values were in accordance with our visual impression. Overall, the calculated total colour differences (∆E*) to the fresh juice amounted to 2.8 for the thermally pasteurised and to 4.9 and 6.6 for the two PCT-treated juices, respectively. This clearly illustrated that the impact of the PCT treatments on the colour profile was significantly (p ≤ 0.05) higher compared with that of the thermal pasteurisation. However, consumers will decide about the final acceptance of the colour changes observed. The sensory analysis of PCT-treated fruit juices will be the subject of ongoing research.
Particle Size, Centrifugable Pulp Content, and Cloud Stability
Pineapple juices are inherently prone to poor cloud stability. Will (1995) and Will et al. (1999) have reported that this is mainly attributed to the presence of prevailing coarse (~100 μm) and the lack of fine cloud particles (~0.5 μm), in addition to the differing serum composition of pineapple juice (being rich in glucomannans and low in pectin) in comparison with those from other fruits. As the sedimentation velocity according to Stokes’ law increases with the square of the particle size, the latter is a crucial parameter determining cloud stability. Heavy and coarse cloud particles settle more quickly, resulting in the rapid separation of a clear upper serum and sediment at the bottom, which does not comply with consumers’ expectations (Will et al. 1999; Will 1995).
In our study, the particle sizes of all pineapple juices ranged between approx. 1 and 23 μm, displaying monomodal distributions (Fig. 2). Interestingly, the particle sizes of the fresh juice ranging between 2.6 and 13.2 μm were largely normally distributed. In contrast, both PCT-treated juices displayed a right-skewed distribution and a slightly narrower range, considerably shifted towards smaller particle sizes (PCT1: 1.2–8.8 μm; PCT2: 1.0–7.7 μm). These effects were also visible with regard to the volume-weighted particle diameters of the respective 10, 50, and 90% quantiles (significantly smaller d10, d50, and d90 values of the PCT-treated samples compared with the fresh juice, see Table 1). This shift towards smaller particle sizes may be attributed to mechanical stress during the decompression phase. The dissolved inert gas instantly expands through the exhaust valve, causing shear stress and resultantly, the disruption of juice particles. Interestingly, thermal pasteurisation also resulted in smaller, and concurrently, slightly larger particles than those of the fresh juice. Consequently, this right-skewed distribution was the broadest (1.5–22.8 μm) observed among all four juices. An increase in particle size after thermal treatments has also been observed by Baron et al. (2006) and Katiyo et al. (2017), as a result of enhanced Brownian motion and inter-particle forces (Genovese et al. 2007).
Overall, the fresh juice was characterised by a volume-weighted mean particle diameter (d4,3) of 5.5 μm, followed by 3.4 for the thermally pasteurised and 2.4 and 2.2 μm for the two PCT-treated juices, respectively (see Table 1). These smaller particle sizes may result in the clearly reduced centrifugable pulp content of both PCT-treated juices (see Table 1). After the first PCT treatment, traces of centrifugable pulp were still visible (<1.0%), whereas a second passage resulted in a complete depletion. In comparison, the centrifugable pulp contents of the fresh and the thermally pasteurised juice amounted to 4.5 and 2.5% of the total juice volume, respectively.
The cloud stability tests at 4 °C are illustrated in Fig. 3. Initially, the cloud particles were homogeneously suspended in all juices (day 0). In the fresh and thermally pasteurised juices, the onset of sedimentation was already visible after 6 h (not shown). The sedimentation in these two samples proceeded rapidly, resulting in a turbid serum due to suspended fine particles and a considerable sediment formation. By contrast, the PCT-treated juices still displayed a homogeneous, turbid phase. During the subsequent storage, the serum of the fresh and thermally pasteurised juices gradually clarified and cloud became more compact (days 7 and 14). This was more pronounced in the thermally pasteurised than in the fresh juice. Quick sedimentation of cloud in thermally pasteurised pineapple juices has also been pointed out by Will et al. (1999). In contrast, both PCT-treated juices displayed improved cloud stability, possibly resulting from the above-mentioned shifts towards reduced particle sizes and their narrowed distribution (Fig. 2). However, initial sedimentation was also observed in the PCT-treated samples as early as day 1 that further manifested with progressing storage. Will et al. (1999) have emphasised that particle sizes <1 μm are a prerequisite for satisfactory cloud stabilities. Thus, the particles in the PCT-treated samples were possibly still too large. However, their less compact sediment may be easily re-suspended prior to consumption, e.g. by shaking. As the viscosity is inversely proportional to the sedimentation velocity in Stokes’ law, the cloud stability may be further improved by thickening agents. The European regulation (EC) No 1333/2008 (European Union 2008) allows the admixture of up to 3 g pectin (E 440) per kg pineapple juice. Will (1995) and Will et al. (1999) have shown that this improved cloud stability, however, adversely affected the organoleptic properties of pineapple juice. The observed ‘homogenisation’ effect of the PCT treatment may permit to significantly reduce the allowed pectin dosage, and thus, not impairing the sensory appearance of PCT-treated pineapple juices.
Microbial Quality
In the fresh pineapple juice, TAPC and YMC amounted to 4.5 and 4.4 log10 CFU/mL, respectively (Table 2). Thermal pasteurisation reduced both counts below the detection limit (<2 log10 CFU/mL). In comparison, PCT resulted in average reductions of both counts by 0.9 and 1.1–1.2 log10 CFU/mL after the first and the second passage, respectively. The microbial quality was thus merely slightly improved by the PCT treatment, while Aschoff et al. (2016) reported a total reduction of the TAPC of initially 3.4 log10 CFU/mL after applying similar PCT settings (50 MPa, nitrogen, <40 °C, 2 L/min, 1.3 min) to orange juice. Bönsch et al. (2007) have demonstrated the efficient reduction (−log N/N0) of diverse food-related microorganisms in cell suspensions by PCT batch treatments (50 MPa) of up to 7.5. The latter authors stated that the inactivation efficiency is dependent on, inter alia, the type of microorganism (gram− > yeast >> gram+) and several process parameters, including working pressure, temperature, and the process gas. The combination of the latter three parameters determines the amount of gas dissolved in the liquid (Klingner et al. 2006). The juice matrix itself may have a significant effect as, e.g. salt (Sun et al. 2001) and sugar contents (Illera et al. 2019) influence the solubility of gases in liquids. In addition, the tissue and cell wall structure may affect the efficiency of the PCT treatment. Whereas orange endocarp consists of comparatively soft juice vesicles, the firm pineapple flesh is composed of cells rich in hemicellulose and cellulose (Vidal-Valverde et al. 1982). Consequently, the inactivation of microorganisms in pineapple juice by PCT requires further optimisation. The impact of the residual microbial loads on the shelf life and safety of the PCT-treated juices may be subject of continuative studies. Another possible reason for the comparatively inefficient reduction of plate counts observed herein may be the disintegration of multicellular microbial aggregates during PCT treatment. Similarly, Joyce et al. (2011) have observed such an effect after sonicating bacterial suspensions. This hypothesised disintegration of microbial cell aggregates, which possibly counteracts the reduction of the plate counts, merits further investigation.
Enzyme Activities
The activities of quality-relevant enzymes are listed in Table 2. Pineapples are well-known sources of the protease BRM (Lobo and Paull 2017; Mehrlich and Felton 1980). Its activity amounted to 106.3 ± 1.1 CDU in the fresh juice. Thermal pasteurisation caused a drastic drop to 17% of the initial activity, being in line with our previous study (Vollmer et al. 2020). In contrast, BRM activity was largely retained applying PCT (<35 °C) and merely reduced to 97 and 85% residual activity after one and two passages, respectively. Owing to the putative health-promoting effects of BRM (Lobo and Paull 2017; Maurer 2001), the maximum retention of this mixture of proteolytic enzymes is highly desirable with regard to the biofunctionality of the juice.
PME negatively affects cloud stability of fruit juices as the enzyme catalyses the demethylation of pectin. Free carboxyl groups arise, promoting the generation of coarse aggregates, cross-linked via bivalent cations resulting in undesired sedimentations and an overall quality loss (Terefe et al. 2014). In our study, PME activity was not detected among all pineapple juices. Low PME activities have previously been reported by Cautela et al. (2018) in fresh ‘Smooth Cayenne’ pineapple juice. The activity may thus depend on the cultivar. Pineapple juices contain low pectin levels (Will et al. 1999). The absence of the PME substrate renders high activities unlikely. PME is efficiently inactivated by thermal (Cautela et al. 2018; Terefe et al. 2014), but only partly by PCT treatment (<40 °C), as demonstrated for orange juice (Aschoff et al. 2016). Cultivars exerting none or only low PME activities, such as ‘MD2’, may be recommended for the production of fresh-like pineapple juices.
POD catalyses the oxidation of inter alia phenolic compounds in the presence of hydrogen peroxide. Undesirable discolouration and off-flavour formation may be the consequences, adversely affecting product quality (Terefe et al. 2014; Vámos-Vigyázó 1981). In pineapples, POD activity gradually declines with progressing ripening; however, it is still moderate in ripe fruit (Mehrlich and Felton 1980). In our fresh pineapple juice, POD activity amounted to ~7.0 nkat/g. An effective inactivation was achieved by thermal pasteurisation, being in agreement with literature (Vervoort et al. 2011). Due to its high thermal stability, POD often serves as an indicator of sufficient heat treatments during food processing, assuming that less thermally stable enzymes have already been inactivated (Miller and Silva 2016; Terefe et al. 2014). Non-thermal PCT application also reduced POD; however, the residual activities were still in the range of 77–90%, possibly limiting the shelf life of the respective juices. Conversely, Aschoff et al. (2016) have reported a complete inactivation of POD in orange juice after applying PCT with 25 MPa and 50 MPa, respectively. They suggested that shear forces occurring during the decompression phase are responsible for this effect. However, the initial POD activity of the fresh orange juice in the afore-mentioned study of merely 1.0 ± 0.1 nkat/g was considerably low compared with the 7.0 nkat/g determined herein.
PPO catalyses the hydroxylation and oxidation of phenolic compounds, resulting in yellow-coloured and reactive o-quinones. The latter polymerise in a non-enzymatic reaction, generating brown melanins impairing product colour (Terefe et al. 2014). In our study, PPO activity amounted to 3.2 ± 0.6 nkat/g in the fresh juice and was completely inactivated by both thermal pasteurisation and PCT. Concluding, PPO was more sensitive to the PCT treatment than POD and most likely inactivated by shear forces occurring at the exhaust valve during decompression. Future research may elucidate the reasons for the observed differences and underlying inactivation mechanisms. The inactivation of POD and PPO in pineapple puree by high-pressure and thermal treatments has already been studied by Chakraborty et al. (2015).
Micronutrients and Sugars
Vitamin C
All pineapple juices contained vitamin C at concentrations between 46.9 and 48.7 mg/100 mL, with a high proportion of ascorbic acid (84–90%) compared with dehydroascorbic acid (10–16%; Table 3). These vitamin C concentrations were almost as high as those reported for orange cv. ‘Navel Late’ juice (Aschoff et al. 2016). However, vitamin C contents may substantially vary among individual pineapple varieties (Lobo and Paull 2017). Thermally pasteurised juices from ‘Queen’ and ‘MD2’ pineapples have previously been reported to contain vitamin C levels of 7.7 and 50.6 mg/100 mL, respectively (Vollmer et al. 2020; Difonzo et al. 2019).
Surprisingly, thermal pasteurisation did not exert any adverse effects on vitamin C in our study, although it is known for being susceptible to heat (Miller and Silva 2016). In comparison, vitamin C contents declined by almost 60% during thermal pasteurisation (90 °C/5 min) at laboratory scale in our previous study (Vollmer et al. 2020). Noteworthy, vitamin C contents of 57.6–61.5 and 32.6–38.4 mg/100 mL have previously been reported for thermally pasteurised pineapple juice processed at pilot plant and laboratory scale, respectively (Difonzo et al. 2019).
Interestingly, the use of PCT completely retained vitamin C regardless of whether the pineapple juice was treated once or twice. Hereby, dehydroascorbic acid levels slightly increased at the expense of ascorbic acid (statistically not significant at p ≤ 0.05). Conversely, Aschoff et al. (2016) have observed a loss of 5–7% in vitamin C due to declining dehydroascorbic acid levels during PCT treatment of orange juice. Most likely, the inert working gas argon and, more importantly, flushing of the expansion tank with nitrogen that has not been implemented by Aschoff et al. (2016) may have prevented the degradation of vitamin C in our study.
B Vitamins
Pineapples contain B vitamins, which are involved in many important functions in the human body (Lobo and Paull 2017). Table 3 summarises the concentrations of vitamin B1 (thiamin), B2 (riboflavin), B3 (niacin), B5 (pantothenic acid), and B6 (pyridoxine), together amounting to 401.7–441.6 μg/100 mL across all pineapple juices. The individual concentrations were substantially lower than those reported by the USDA (2019) for raw ‘MD2’ pineapples, possibly resulting from processing or seasonal fluctuations. The B vitamins were not significantly affected by thermal and PCT treatments. Some B vitamins have been reported as being sensitive to thermal processing (Miller and Silva 2016; Eitenmiller et al. 2008). However, with the exception of vitamin B1 and B6 model solutions subjected to high-pressure processing (99–100% retention) (Sancho et al. 1999), studies on the effects of non-thermal processing on B vitamins are still widely lacking (Zhang et al. 2011).
Phenolic Compounds, Furanones, and Other Metabolites
The concentrations of phenolic compounds, genuine pineapple furanones, L-tyrosine, and serotonin are displayed in Table 3. The most abundant constituents were S-coniferyl and S-sinapyl glutathione (12 and 13), in addition to their corresponding N-L-γ-glutamyl-L-cysteine (15 and 16) and L-cysteine (9 and 10) conjugates. Minor phenolic compounds included phenolic glycosides (7 and 8), esters (11 and 14), and one amide (17).
Total phenolic compounds in the fresh juice amounted to ~37.8 mg/100 mL. Thermal pasteurisation caused a slight, but significant (p ≤ 0.05), rise to 38.8 mg/100 mL, being in agreement with our previous results (Vollmer et al. 2020). Compared with this previous study, the stabilisation of phenolic compounds was considerably less pronounced. This may be attributed to the differences in production scales (laboratory vs. pilot plant scale) as already mentioned in the section about vitamin C. The content of phenolic compounds remained almost unaffected in both PCT-treated juices. In contrast, Aschoff et al. (2016) have reported significant losses of hesperidin during PCT treatment of orange juice. However, the high residual POD activities of our samples may promote the oxidation of phenolic compounds during prolonged storage.
The pineapple juices contained 4-hydroxy-2,5-dimethyl-3(2H)-furanone (HDMF, 3) and some glucosides thereof (4, 5, and 6), being consistent with a previous study (Vollmer et al. 2020). Owing to its ‘sweet and pineapple- and caramel-like’ odour and low odour threshold in water of 0.001 mg/100 mL (Tokitomo et al. 2005), HDMF may be of vital importance for the sensory quality of the pineapple juices assessed. The latter contained 3.9–4.0 mg/100 mL of this key odorant of fresh pineapples, irrespective of the treatments applied. These values were substantially lower than the 10.4–14.0 and 18.0–20.6 mg/100 mL reported previously for juices from ‘MD2’ and ‘Queen’ pineapples, respectively (Vollmer et al. 2020; Difonzo et al. 2019).
Sugars
The pineapple juices contained sucrose (6.6–6.8 g/100 mL), followed by D-glucose (1.7–1.8 g/100 mL) and D-fructose (1.4–1.5 g/100 mL), thus being largely within the typical ranges of pineapple juice (AIJN 2016). The proportions of sucrose (67–69%) and reducing sugars (32–33%) matched with those reported by Mehrlich and Felton (1980). The different treatments had no effect on the afore-mentioned three sugars. These findings were consistent with those of Vervoort et al. (2011), who have reported that neither thermal pasteurisation (72 °C/20 s) nor high-pressure processing (600 MPa/60 s/5 °C initial temperature) altered the sugar profile of orange juice.
Statistical Pattern Recognition
The first two PCs of the PCA, calculated on the basis of general quality traits, colour values, particle sizes (Table 1) in addition to microbial counts and enzyme activities (Table 2), explained 90% of the total variance (PC1: 56%, PC2: 34%) as illustrated in Fig. 4a,a'. The fresh juice samples formed one single cluster, located in the lower right quadrant. Its position and separation along PC1 were mainly related to the loadings of microbial counts (TAPC and YMC) and enzyme activities (BRM, POD, and PPO) as well as those related to the particle size distribution (d values). Both PCT-treated juices were closely clustered in the upper left quadrant. They formed one joint HCA cluster with the thermally pasteurised juice but were separated from the latter along PC2. Positioning of the PCT-treated juices was particularly influenced by the loadings of the lightness (L*) and the total colour difference (∆E*). The thermally pasteurised juice was separated by PC2 due to the elevated hue angle (h°), but also due to the complete inactivation of microorganisms and most enzymes. Their slightly elevated total soluble solids (TSS) further promoted this clustering, although the differences were rather small between the individual treatments.
Score plots of the principal component analysis (PCA) calculated on the basis of general juice quality traits, microbial loads, and enzyme activities (a) and levels of genuine juice constituents (b). The corresponding loading plots are displayed in a' and b'. Ellipses in the score plots illustrate clusters from hierarchical cluster analysis (HCA). Abbreviations are defined in Tables 1, 2, and 3.
The second PCA illustrated in Fig. 4b,b' was calculated on the basis of the genuine juice constituents (Table 3). The first two PCs explained 73% of the total variance of this data set (PC1: 49%, PC2: 24%). The thermally pasteurised juices formed one clear-cut cluster. The location of this cluster in the upper right quadrant was correlated with the position of the loadings of HDMF hexoside (4) and several phenolic compounds (8, 12, 13, and 14) that were found in slightly elevated concentrations in the thermally pasteurised juice (Table 3). The fresh and the PCT-treated samples formed one widespread cluster that was clearly separated from the thermally pasteurised juice. This differentiation was inter alia promoted by the elevated levels of dehydroascorbic acid (DHAA).
Concluding, multivariate statistics classified both PCT-treated juices to the fresh juice with regard to the phytochemical composition and to the thermally pasteurised juice with respect to the remaining physical and biochemical quality parameters.
Conclusions
To the best of our knowledge, continuous PCT was used for the first time for the non-thermal processing of pineapple juice on pilot scale. Our experiments not only emphasised the prospects of this innovative technology, but also revealed current limitations and the need for further process optimisation. In particular, the high retention of BRM activity and the significant reduction of the mean particle diameter, which substantially reduced centrifugable pulp contents and enhanced cloud stability, proved to be the most conspicuous advantages of PCT compared with thermal pasteurisation. However, the high residual POD activity and the unexpectedly small reduction of the microbial loads may adversely affect quality and limit the shelf life of PCT-treated pineapple juices. A second passage through the PCT device was less effective than expected. Hence, ongoing research may particularly focus on further optimisation of process parameters and the design of the PCT apparatus to overcome the above obstacles.
Complementing the results presented herein, future research may additionally assess the long-term preservation effects of PCT and the storage stability of the PCT-treated juices ensuring adequate shelf life and food safety, as well as the aroma-determining volatiles, the descriptive sensory profile, and the consumer acceptance of PCT-treated juices.
Abbreviations
- a * :
-
Green-red
- AA:
-
Ascorbic acid
- ANOVA:
-
Analysis of variance
- TPC:
-
Total phenolic content
- b * :
-
Blue-yellow
- BRM:
-
Bromelain
- C * :
-
Chromaticity
- CDU:
-
Casein digestive unit
- DHAA:
-
Dehydroascorbic acid
- ∆E* :
-
Total colour difference
- ESI:
-
Electrospray ionisation
- GAE:
-
Gallic acid equivalent
- h ° :
-
Hue angle
- HCA:
-
Hierarchical cluster analysis
- HDMF:
-
4-Hydroxy-2,5-dimethyl-3(2H)-furanone
- HMF:
-
5-(Hydroxymethyl)furfural
- HPLC-/UPLC-DAD:
-
High-/ultra-performance liquid chromatography-diode array detection
- L * :
-
Lightness
- MSn :
-
Multi-stage mass spectrometry
- PCA:
-
Principal component analysis
- PCT:
-
Pressure change technology
- PME:
-
Pectin methylesterase
- POD:
-
Peroxidase
- PPO:
-
Polyphenol oxidase
- TA:
-
Titratable acidity
- TSS:
-
Total soluble solids
- TAPC:
-
Total aerobic plate count
- YMC:
-
Yeast and mould count
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantitation
References
AIJN (2016). 6.5 Reference guideline for pineapple juice. In Code of practice for evaluation of fruit and vegetable juices (pp. 1–5). Brussels: Association of the Industry of Juices and Nectars from Fruits and Vegetables of the European Union.
AIJN (2019). Liquid fruit. Market report 2019. http://www.aijn2019report.com/aijn2019report/homepage. Accessed 9 December 2019.
Aschoff, J. K., Kaufmann, S., Kalkan, O., Neidhart, S., Carle, R., & Schweiggert, R. M. (2015). In vitro bioaccessibility of carotenoids, flavonoids, and vitamin C from differently processed oranges and orange juices [Citrus sinensis (L.) Osbeck]. Journal of Agricultural and Food Chemistry, 63(2), 578–587. https://doi.org/10.1021/jf505297t.
Aschoff, J. K., Knoblauch, K., Hüttner, C., Vásquez-Caicedo, A. L., Carle, R., & Schweiggert, R. M. (2016). Non-thermal pasteurization of orange (Citrus sinensis (L.) Osbeck) juices using continuous pressure change technology (PCT): A proof-of-concept. Food and Bioprocess Technology, 9(10), 1681–1691. https://doi.org/10.1007/s11947-016-1754-6.
Balasubramaniam, V. M. B., Martínez-Monteagudo, S. I., & Gupta, R. (2015). Principles and application of high pressure-based technologies in the food industry. Annual Review of Food Science and Technology, 6(1), 435–462. https://doi.org/10.1146/annurev-food-022814-015539 .
Baron, A., Dénes, J.-M., & Durier, C. (2006). High-pressure treatment of cloudy apple juice. LWT - Food Science and Technology, 39(9), 1005–1013. https://doi.org/10.1016/j.lwt.2006.02.016 .
Baur, S., Klaiber, R. G., Koblo, A., & Carle, R. (2004). Effect of different washing procedures on phenolic metabolism of shredded, packaged iceberg lettuce during storage. Journal of Agricultural and Food Chemistry, 52(23), 7017–7025. https://doi.org/10.1021/jf048961a .
Bönsch, K., Wecks, M., Ondruschka, J., & Staudt, R. (2007). The effect of a new pressure change technology (PCT) on microorganisms: An innovate concept for food safety. Chemical Engineering & Technology, 30(6), 755–757. https://doi.org/10.1002/ceat.200600402.
Cautela, D., Castaldo, D., & Laratta, B. (2018). Thermal inactivation of pectin methylesterase in pineapple juice. Journal of Food Measurement and Characterization, 12(4), 2795–2800. https://doi.org/10.1007/s11694-018-9894-1 .
Chakraborty, S., Rao, P. S., & Mishra, H. N. (2015). Kinetic modeling of polyphenoloxidase and peroxidase inactivation in pineapple (Ananas comosus L.) puree during high-pressure and thermal treatments. Innovative Food Science and Emerging Technologies, 27, 57–68. https://doi.org/10.1016/j.ifset.2014.11.003.
Difonzo, G., Vollmer, K., Caponio, F., Pasqualone, A., Carle, R., & Steingass, C. B. (2019). Characterisation and classification of pineapple (Ananas comosus [L.] Merr.) juice from pulp and peel. Food Control, 96, 260–270. https://doi.org/10.1016/j.foodcont.2018.09.015.
Eitenmiller, R. R., Ye, L., & Landen, W. O. (2008). Vitamin analysis for the health and food sciences (2nd ed.). Boca Raton: CRC Press.
FAO (2019). FAOSTAT. Food and Agriculture Organization of the United States. http://www.fao.org/faostat/en/#data. Accessed 14 July 2019.
Genovese, D. B., Lozano, J. E., & Rao, M. A. (2007). The rheology of colloidal and noncolloidal food dispersions. Journal of Food Science, 72(2), R11–R20. https://doi.org/10.1111/j.1750-3841.2006.00253.x .
Hirsch, A. R., Förch, K., Neidhart, S., Wolf, G., & Carle, R. (2008). Effects of thermal treatments and storage on pectin methylesterase and peroxidase activity in freshly squeezed orange juice. Journal of Agricultural and Food Chemistry, 56(14), 5691–5699. https://doi.org/10.1021/jf073007+ .
IFU (1984). Analysis no. 30: Determination of formol number. In Methods of analysis (pp. 1–2). Zug: International Federation of Fruit Juice Producers.
IFU (1991). Analysis no. 60: Determination of centrifugable pulp. In Methods of analysis (pp. 1–2). Zug: International Federation of Fruit Juice Producers.
IFU (1996a). Analysis no. 3: Titratable acidity. In Methods of analysis (pp. 1–4). Zug: International Federation of Fruit Juice Producers.
IFU (1996b). Analysis no. 69: Determination of hydroxymethylfurfural (HPLC). In Methods of analysis (pp. 1–3). Zug: International Federation of Fruit Juice Producers.
IFU (1996c). Analysis no. 2: Total count of potential spoilaging microorganisms of fruits and related products. In Methods of analysis (pp. 1–5). Zug: International Federation of Fruit Juice Producers.
IFU (1996d). Method no. 4: Moulds count procedure. In Methods of analysis (pp. 1–15). Zug: International Federation of Fruit Juice Producers.
Illera, A. E., Sanz, M. T., Beltrán, S., & Melgosa, R. (2019). High pressure CO2 solubility in food model solutions and fruit juices. The Journal of Supercritical Fluids, 143, 120–125. https://doi.org/10.1016/j.supflu.2018.07.009.
Joyce, E., Al-Hashimi, A., & Mason, T. J. (2011). Assessing the effect of different ultrasonic frequencies on bacterial viability using flow cytometry. Journal of Applied Microbiology, 110(4), 862–870. https://doi.org/10.1111/j.1365-2672.2011.04923.x .
Jutamongkon, R., & Charoenrein, S. (2010). Effect of temperature on the stability of fruit bromelain from Smooth Cayenne pineapple. Kasetsart Journal - Natural Science, 44, 943–948.
Katiyo, W., Yang, R., & Zhao, W. (2017). Effects of combined pulsed electric fields and mild temperature pasteurization on microbial inactivation and physicochemical properties of cloudy red apple juice (Malus pumila Niedzwetzkyana (Dieck)). Journal of Food Safety, 37(4), e12369. https://doi.org/10.1111/jfs.12369.
Kincal, D., Hill, W. S., Balaban, M. O., Portier, K. M., Wei, C. I., & Marshall, M. R. (2005). A continuous high pressure carbon dioxide system for microbial reduction in orange juice. Journal of Food Science, 70(5), M249–M254. https://doi.org/10.1111/j.1365-2621.2005.tb09979.x .
Klingner, E., Harting, P., Steiger, H.-J., Wecks, M., & Bönsch, K. (2006). Unter Druck gelöste Gase als Zerstörpotenzial für Mikroorganismen: Ein alternatives Verfahren zur Haltbarmachung von Flüssigkeiten. Chemie Ingenieur Technik, 78(11), 1731–1737. https://doi.org/10.1002/cite.200600091 .
Koutchma, T. (2009). Advances in ultraviolet light technology for non-thermal processing of liquid foods. Food and Bioprocess Technology, 2(2), 138–155. https://doi.org/10.1007/s11947-008-0178-3 .
Lobo, M. G., & Paull, R. E. (Eds.). (2017). Handbook of pineapple technology: Production, postharvest science, processing and nutrition. Chichester: Wiley.
Lozano, J. E. (2006). Fruit manufacturing: Scientific basis, engineering properties, and deteriorative reactions of technological importance. New York: Springer.
Maurer, H. R. (2001). Bromelain: Biochemistry, pharmacology and medical use. Cellular and Molecular Life Sciences, 58(9), 1234–1245. https://doi.org/10.1007/PL00000936.
Mehrlich, F. P., & Felton, G. E. (1980). Pineapple juice. In P. E. Nelson & D. K. Tressler (Eds.), Fruit and vegetable juice processing technology (3rd ed., pp. 180–221). Westport: AVI Publishing.
Miller, F. A., & Silva, C. L. M. (2016). Thermal treatment effects in fruit juices. In S. Rodrigues & F. A. N. Fernandes (Eds.), Advances in fruit processing technologies (pp. 363–386, Contemporary food engineering). Boca Raton: CRC Press.
Norton, T., & Sun, D.-W. (2008). Recent advances in the use of high pressure as an effective processing technique in the food industry. Food and Bioprocess Technology, 1(1), 2–34. https://doi.org/10.1007/s11947-007-0007-0 .
Paniagua-Martínez, I., Ramírez-Martínez, A., Serment-Moreno, V., Rodrigues, S., & Ozuna, C. (2018). Non-thermal technologies as alternative methods for Saccharomyces cerevisiae inactivation in liquid media: A review. Food and Bioprocess Technology, 11(3), 487–510. https://doi.org/10.1007/s11947-018-2066-9.
Pöhnl, T., Böttcher, C., Schulz, H., Stürtz, M., Widder, S., Carle, R., & Schweiggert, R. M. (2017). Comparison of high performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD) and ultra-high performance liquid chromatography with evaporative light scattering (UHPLC-ELSD) for the analyses of fructooligosaccharides in onion (Allium cepa L.). Journal of Food Composition and Analysis, 63, 148–156. https://doi.org/10.1016/j.jfca.2017.08.001.
European Union (2008). Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives (consolidated version of 28 October 2019), L 354.
Sancho, F., Lambert, Y., Demazeau, G., Largeteau, A., Bouvier, J.-M., & Narbonne, J.-F. (1999). Effect of ultra-high hydrostatic pressure on hydrosoluble vitamins. Journal of Food Engineering, 39(3), 247–253. https://doi.org/10.1016/S0260-8774(98)00143-5 .
Singleton, V. L., Orthofer, R., & Lamuela-Raventós, R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymology, 299, 152–178. https://doi.org/10.1016/S0076-6879(99)99017-1.
Steingass, C. B., Glock, M. P., Schweiggert, R. M., & Carle, R. (2015). Studies into the phenolic patterns of different tissues of pineapple (Ananas comosus [L.] Merr.) infructescence by HPLC-DAD-ESI-MSn and GC-MS analysis. Analytical and Bioanalytical Chemistry, 407(21), 6463–6479. https://doi.org/10.1007/s00216-015-8811-2.
Sun, R., Hu, W., & Duan, Z. (2001). Prediction of nitrogen solubility in pure water and aqueous NaCl solutions up to high temperature, pressure, and ionic strength. Journal of Solution Chemistry, 30(6), 561–573. https://doi.org/10.1023/A:1010339019489 .
Terefe, N. S., Buckow, R., & Versteeg, C. (2014). Quality-related enzymes in fruit and vegetable products: Effects of novel food processing technologies, part 1: High-pressure processing. Critical Reviews in Food Science and Nutrition, 54(1), 24–63. https://doi.org/10.1080/10408398.2011.566946.
Tokitomo, Y., Steinhaus, M., Büttner, A., & Schieberle, P. (2005). Odor-active constituents in fresh pineapple (Ananas comosus [L.] Merr.) by quantitative and sensory evaluation. Bioscience, Biotechnology, and Biochemistry, 69(7), 1323–1330. https://doi.org/10.1271/bbb.69.1323.
USDA (2019). Food database: Pineapple, raw, extra sweet variety. https://fdc.nal.usda.gov/fdc-app.html#/food-search. Accessed 24 January 2020.
Vámos-Vigyázó, L. (1981). Polyphenol oxidase and peroxidase in fruits and vegetables. Critical Reviews in Food Science and Nutrition, 15(1), 49–127. https://doi.org/10.1080/10408398109527312 .
Vervoort, L., van der Plancken, I., Grauwet, T., Timmermans, R. A. H., Mastwijk, H. C., Matser, A. M., Hendrickx, M. E., & van Loey, A. (2011). Comparing equivalent thermal, high pressure and pulsed electric field processes for mild pasteurization of orange juice. Innovative Food Science and Emerging Technologies, 12(4), 466–477. https://doi.org/10.1016/j.ifset.2011.06.003 .
Vidal-Valverde, C., Herranz, J., Blanco, I., & Rojas-Hidalgo, E. (1982). Dietary fiber in Spanish fruits. Journal of Food Science, 47(6), 1840–1845. https://doi.org/10.1111/j.1365-2621.1982.tb12895.x .
Vollmer, K., Chakraborty, S., Bhalerao, P. P., Carle, R., Frank, J., & Steingass, C. B. (2020). Effect of pulsed light treatment on natural microbiota, enzyme activity, and phytochemical composition of pineapple (Ananas comosus [L.] Merr.) juice. Food and Bioprocess Technology, 13(7), 1095–1109. https://doi.org/10.1007/s11947-020-02460-7.
Will, F. (1995). Trubzusammensetzung und Trübungsstabilität von Ananassäften. Flüssiges Obst, 62(6), 258–262.
Will, F., Hagemann, S., Dietrich, H., & Zimmer, E. (1999). Modellversuche zur Herstellung von trübungsstabilen Ananassäften. Deutsche Lebensmittel-Rundschau, 95(8), 310–317.
Zhang, H. Q., Barbosa-Cánovas, G. V., Balasubramaniam, V. M., Dunne, C. P., Farkas, D. F., & Yuan, J. T. C. (2011). Nonthermal processing technologies for food (IFT Press series). Chichester: Blackwell Publishing.
Acknowledgements
We gratefully acknowledge Klaus Mix (University of Hohenheim) for his excellent assistance during pineapple juice production, and we thank Tobias Pöhnl for his helpful support in sugar analysis. We are also grateful to Domenico Albrich and Katharina Henn for their contributions to the analytical work as part of their master programme.
Funding
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vollmer, K., Santarelli, S., Vásquez-Caicedo, A.L. et al. Non-thermal Processing of Pineapple (Ananas comosus [L.] Merr.) Juice Using Continuous Pressure Change Technology (PCT): Effects on Physical Traits, Microbial Loads, Enzyme Activities, and Phytochemical Composition. Food Bioprocess Technol 13, 1833–1847 (2020). https://doi.org/10.1007/s11947-020-02520-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-020-02520-y