Abstract
Purpose of Review
This systematic scoping review examines evidence from the last five years on sleep interventions in cognitive healthy older adults and those with mild cognitive impairment.
Recent Findings
Sleep disturbance has been identified as a potential early, modifiable risk factor for dementia, making it crucial to investigate if these interventions also enhance cognitive function and neurodegenerative biomarkers.
Summary
Since 2019, research on sleep interventions in older adults with or without cognitive impairment has gradually expanded, especially on non-pharmacological treatments including CBT-I, exercise, and multi-modal interventions, which show promise but require further study to confirm cognitive benefits. Pharmacological interventions have primarily focused on melatonin and orexin antagonists, with long-term safety remaining a concern. Tailored, clinically effective interventions that consider the presence of Alzheimer’s disease biomarkers, such as amyloid, tau, cerebrovascular disease, or alpha-synuclein in key sleep-related circuits, are essential to developing feasible, cost-effective, and scalable treatments for older adults with or without cognitive impairment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
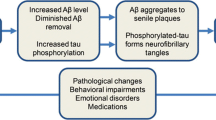
Dementia is a neurodegenerative disease that impairs cognitive ability, behaviour and functioning and is the 7th leading cause of death globally [1]. In the absence of a cure for dementia, research has recently shifted its focus to targeting modifiable risk factors that may prevent or delay cognitive decline [2]. Over the last decade, sleep and circadian (sleep–wake) disturbances have been increasingly recognised as possible risk factors for cognitive decline [3]. Sleep disturbances have shown to increase levels of beta-amyloid (Aβ) and phosphorylated tau (p-tau) [4], key pathophysiological features of Alzheimer’s disease (AD), and lead to worse global cognition. Although exact mechanisms are unknown, the likely bidirectional interrelationship between sleep and AD is mainly attributed to the role of sleep in learning and memory [5], synaptic plasticity [6], and glymphatic clearance of metabolic waste from the brain [7].
Sleep disturbance affects nearly one-third of adults in the general population and increases with age, with prevalence rates from 30–70% in older adults [8]. These disturbances include subjective complaints such as insomnia, and other sleep disorders such as obstructive sleep apnea (OSA) and excessive daytime sleepiness [9]. Older adults (aged over 65) are more at risk of disorders accompanied and/or exacerbated by poor sleep. Sleep–wake disturbances are a prominent feature of AD and other dementias [10], and are linked to poorer disease prognosis [11]. Converging evidence also suggests that sleep–wake disturbances manifest in the earlier stage of the dementia continuum, such as in individuals with mild cognitive impairment (MCI). MCI is a transitional phase between normal aging and dementia, marked by objectively impaired cognitive function that does not significantly interfere with daily activities, and carries a higher risk of progressing to dementia [12]. Around 50–63% of MCI patients subjectively report sleep–wake disturbances, with pronounced changes in sleep macro-architecture, including greater wake after sleep onset, reduced total sleep time, lower sleep efficiency, and longer sleep onset latency compared to healthy controls [13, 14]. Even earlier on the continuum, shorter sleep duration in healthy older adults in midlife, particularly at ages 50 and 60, has been associated with an increased risk of late-onset dementia, independent of sociodemographic, behavioural, cardiometabolic, and mental health factors [15]. This highlights an opportunity to target sleep disturbance in older adults as a potential strategy to prevent cognitive decline and dementia.
The objective of this systematic scoping review was to provide an overview of sleep interventions in older adults, with or without cognitive decline, over the past five years. Research in this area is rapidly progressing with several notable recent systematic reviews [16, 17], and we therefore sought to provide an updated systematic scoping review of empirical research published since January 2019. Our primary study aim was to document types of sleep interventions being evaluated in older adults with or without cognitive impairment. Second, we wanted to determine the impact of the sleep intervention on cognitive outcomes, neurodegenerative biomarkers, and brain structure and function using neuroimaging techniques, if assessed.
Methods
This scoping review employed a systematic search strategy and a narrative synthesis of findings [18]. Our objective was to map existing research on interventions for sleep disturbances and their effects on cognitive outcomes and neurodegeneration markers in older adults with or without cognitive impairment. The search was conducted using PubMed, Scopus, Web of Science, Embase and PsycInfo databases for English-language articles from 1 January 2019 to 1 March 2024 (see flow diagram in Fig. 1). Reference lists of relevant review articles were also screened. The search strategy was discussed and agreed upon informally by the multidisciplinary team. After removing duplicates, three authors (AS, SDK, ZMS) screened titles and abstracts against the inclusion criteria. The inclusion criteria were as follows:
-
1.
Population: Cognitively healthy older adults (mean age \(\ge\) 50 years) or those with MCI or preclinical AD, confirmed by examining the inclusion criteria or baseline scores against standardized diagnostic criteria.
-
2.
Intervention: Any form of pharmacological and/or non-pharmacological sleep intervention (any frequency and duration).
-
3.
Comparison: Any control arm, including passive (treatment as usual, wait-list) or active (matched placebo, active comparator).
-
4.
Outcome: Any validated measure evaluating changes in sleep (subjective or objective), cognitive function, neurodegenerative biomarkers, or neuroimaging data.
-
5.
Study design: Any controlled, interventional studies.
Articles were excluded if they met the following criteria: (i) published before 1 January 2019; (ii) included participants with comorbid medical conditions that could potentially influence treatment effects (e.g., cancer, cardiovascular or metabolic disorders); (iii) lacked essential data or statistical analyses to ascertain treatment effects; or (iv) pooled data of older and younger adult populations. The remaining full-text articles were categorized by intervention type. Data extraction performed by five authors (AS, SK, ZMS, BT, NC), each assigned to a specific intervention type. This review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping reviews (PRISMA-ScR) [19].
Results
A total of 35 full-text articles were reviewed and summarized (Table 1 and 2). Results are divided in two subsections: (i) non-pharmacological interventions, and (ii) pharmacological interventions, considering the type of interventions administered and the disease stage of participants. Of the 28 articles evaluating non-pharmacological treatments, interventions included cognitive behaviour therapy for insomnia (CBT-I) (six studies), light therapy (one study), exercise (14 studies), multi-modal intervention (two studies), mindfulness-based intervention (two studies), non-invasive brain stimulation (one study), and acoustic stimulation (two studies). Of the seven pharmacological intervention studies, one evaluated melatonin, five evaluated orexin antagonists, and one evaluated supplementation. No recent articles (2019-) on benzodiazepines or Z-drugs for treating sleep disturbances in older adults or individuals with MCI met inclusion criteria for this review.
The review encompassed research from 15 different countries, with notable concentration of studies from China (seven studies) and the USA (six studies). Most studies included cognitively healthy older adults (mean age \(\ge\) 50 years; 26 studies), while nine studies included individuals with MCI. Where data were provided, most studies had a majority of female participants (i.e., sample > 50% female). Nineteen studies assessed only subjective sleep measures, 13 studies included both subjective and objective sleep measures, and five studies examined only objective sleep measures. Nine studies examined cognitive outcomes, with one administering a discrete neuropsychological task, three using a brief measure of cognition only (e.g., the Montreal Cognitive Assessment (MoCA)), two administering comprehensive neuropsychological batteries, and three using a combination of both. Notably, no included study examined the effects of sleep interventions on neurodegenerative biomarkers or neuroimaging outcomes.
Cognitive and Behavioural Strategies and Bright Light Therapy
Four studies explored the effects of CBT-I, the gold-standard intervention for insomnia [20,21,22,23]. In a large study of 1,611 older adults with chronic insomnia, six 20-min sessions of digital CBT-I over 12 weeks significantly improved functional health, psychological well-being, and sleep-related quality of life compared to a sleep hygiene control group [22]. An RCT of digital CBT-I in adults with insomnia (mean age > 50 years) showed improvements in self-reported cognitive impairment post-treatment (d = 0.86) and at 6 months (d = 0.96), partially mediated by reduced insomnia severity and increased sleep efficiency [23]. Another study showed that 2-h weekly group CBT-I sessions for two months significantly increased the likelihood of remission of insomnia in older adults compared to the control group [21]. Those in the CBT-I group with sustained remission of insomnia also had an 82.6% decreased likelihood of experiencing incident or recurrent major depression compared to those in the control group without sustained remission of insomnia. A secondary analysis showed reduced expression of the cellular senescence blood-based biomarker p16INK4a in the CBT-I group at 24 months, suggesting long-term benefits for cellular aging [24]. Three studies utilizing abridged CBT-I methods, such as brief CBT-I and brief behavioural therapy for insomnia (BBT-I), reported positive effects on subjective sleep parameters, suggesting these shorter interventions may be just as effective [20, 25, 26].
No controlled interventional studies in the past five years examining CBT-I in MCI met criteria for this review. Nonetheless, a recent open-label RCT found that administering six weekly sessions of digital CBT-I was feasible in 40 older adults with MCI, with high adherence rates (79% completed > 4 out of the 6 CBT-I sessions) and an improvement on the Insomnia Severity Index after 12 weeks (NCT05568381). This suggests that digital delivery of standard CBT was acceptable and feasible in an MCI population, warranting further long-term studies in individuals with MCI. Although no studies have explored the effects of CBT-I on AD-related biomarkers, emerging studies (e.g., NCT0395210), aim to investigate the efficacy of CBT-I on cognitive function and its potential impact on Aβ accumulation in cognitively healthy older adults with insomnia, indicating a promising direction for future research.
Bright light therapy (BLT) is a non-pharmacological treatment aimed at treating the physiological changes associated with alternations of circadian rhythm in cognitively healthy older adults and those with dementia (for review, see [27]). Although some evidence supports its use to improve sleep disturbances in individuals with cognitive impairment or AD, several recent systematic reviews and meta-analyses indicate that the current evidence is at best equivocal [17, 28,29,30,31]. Larger, more rigorously conducted trials are needed before BLT can be recommended in clinical practice. Existing studies have administered heterogenous BLT protocols administered across different settings (institutions, nursing homes, or hospital settings) which raises the question whether BLT is an effective strategy in individuals with cognitive impairment or AD and highlights the need for early intervention. Juda et al. (2020) showed that a 5-week treatment with dynamic circadian lighting in cognitively healthy older adults did not significantly change subjective and actigraphy-derived measures of sleep; however, higher morning light exposure (between 6am-12 pm) resulted in less fragmented sleep and more stable rest-activity pattern [32].
Exercise-based Interventions
A 2022 systematic review and network meta-analysis of 35 RCTs found that muscle endurance training combined with walking improved sleep quality in older adults more than usual care (standardized mean difference, SMD = -2.2), but was less effective than face-to-face CBT-I (SMD = -4.7) [33]. Expanding on this, our review identified 14 studies on exercise interventions for sleep published since 2019. These studies align with recent systematic reviews showing that various exercise types (walking, aerobic exercise, moderate-to-high intensity interval training, resistance training, yoga, Tai Chi, and dance) improve both subjective and objective sleep outcomes.
No controlled interventional studies in the past five years on exercise interventions for MCI met our criteria. However, a recent systematic review and meta-analysis found moderate-to-high-quality evidence that exercise, regardless of intensity, benefits both subjective (e.g., Pittsburgh Sleep Quality Index) and objective sleep outcomes (e.g., polysomnography, actigraphy) in older adults with MCI or Alzheimer's disease and related dementias (AD/ADRD) [34]. A pre-registered trial (NCT03939286) will investigate the effects of exercise on brain structure, function, and metabolism using various brain imaging outcomes in patients with amnestic MCI and Alzheimer’s pathology.
Multi-modal Interventions
Three studies on non-pharmacological multi-modal interventions for sleep disturbance in MCI were identified. Han & Son (2023) found that an once weekly intervention combining walking, light exposure, and behavioural strategies for eight weeks, significantly improved subjective sleep quality and MoCA-Korean scores in MCI patients compared to a general education control group [35]. Weekly telephone coaching was provided to promote adherence to the intervention. Falck et al. (2020) showed that individualized BLT, physical activity, and sleep hygiene training improved subjective sleep quality but not actigraphy-derived sleep outcomes in older adults with probable MCI and sleep disturbance [36]. Adherence to treatment was not assessed. Current pre-registered trials will test various multi-modal lifestyle interventions on brain health including sleep, cognition, and AD-related biomarkers, such as inflammatory and neurodegenerative biomarkers and structural and functional brain imaging (for example, ACTRN12621001760864, NCT01041989, NCT03978052, NCT04364191).
Mindfulness-based Interventions
Mindfulness-based interventions (MBI) have shown improvements in sleep quality, mental wellbeing, and cognition in older adults, including those with MCI and dementia (for reviews, see [37] and [38]). We identified two studies that met criteria for this review. Perini et al. (2023) found that eight weekly MBI sessions significantly improved subjective sleep outcomes, but not objective sleep measures, in cognitively healthy older adults with sleep disturbances, compared to an education-only control group, with effects sustained at 6-month follow-up [39]. Cai et al. (2022) found that eight weekly MBI sessions improved subjective sleep and general cognitive measures (e.g., MoCA), but not on discrete neuropsychological tests, in 75 MCI patients with sleep disturbance [40]. A recent systematic review and meta-analysis reported negligible effects of MBI on attention and memory, with a small effect on executive function (g = 0.14) in older adults with or without cognitive impairment [41]. It is hypothesized that longer lifetime experience with mindfulness practice and integration with existing therapies (e.g., CBT-I) may enhance cognitive benefits, particularly in specific cognitive domains.
Non-invasive Brain and Acoustic Stimulation Techniques
One study exploring non-invasive brain stimulation was included in the review. Lee et al. (2021) investigated the effects of low-frequency transcutaneous electric nerve stimulation (LF-TENS) compared to a sham device on sleep outcomes in 160 older adults with insomnia [42]. Significant improvement in subjective sleep quality was observed in participants aged > 60 years, but not in the middle-aged group (aged 40–60 years), suggesting age-specific effects. Current pre-registered trials are exploring other techniques, including transcranial electrical stimulation (NCT05771844) and transcranial alternating current stimulation (NCT05544201) on memory performance and neurodegenerative biomarkers.
Slow wave sleep (SWS)-enhancing technologies, such as acoustic stimulation, are being explored as a way to improve sleep and cognitive function in cognitively healthy older adults [43,44,45,46,47] and those with MCI [48]. Papalambros et al. (2019) found that one night of acoustic stimulation did not change PSG-derived sleep outcomes in amnestic MCI patients, but the magnitude of change in slow wave activity correlated positively with overnight memory recall on a verbal paired-associated task, suggesting a link between slow wave activity and memory consolidation [48]. Zeller et al. (2024) showed no effect of phase-locked acoustic stimulation (PLAS) versus sham on sleep outcomes in older adults with or without cognitive impairment [49]. However, PLAS induced significant electrophysiological responses and improved memory performance in both groups, with a delayed response in the cognitively impaired group. Stronger electrophysiological responses in the cognitively impaired group were significantly associated with improved Aβ ratios. These results suggest that PLAS could enhance SWS electrophysiology, memory, and amyloid burden in older adults with cognitive impairment, although longer interventions may be needed for more pronounced memory improvements.
OSA Therapies
Given the limited number of published RCTs and the prevalence of observational, cross-sectional, or population-based study designs exploring the effects of CPAP [50, 51], no controlled interventional studies met the inclusion criteria for this review. However, a systematic review of 11 studies (N = 60,840 OSA patients) found that CPAP therapy had a protective effect on the incidence of MCI and AD, suggesting OSA as a modifiable risk factor for cognitive decline [52]. Long-term research studies investigating whether CPAP delays the accumulation of AD pathology and cognitive and functional decline are currently underway. These include two multi-site RCTs: one in 180 older adults with moderate-to-severe OSA [53] [54] and another in 400 older adults with moderate-to-severe OSA (NCT05988385). Both trials will administer personalized OSA therapy by any combination (i.e., CPAP, oral appliance therapy, positional therapy) for dementia prevention. Additionally, three pre-registered trials will examine how CPAP affects brain health and cognition: one on Aβ deposition using PET imaging in those with subjective cognitive decline and MCI (NCT06150352); another on delaying progression of cognitive impairment in amnestic MCI (NCT03113461), and a third on the effect of CPAP withdrawal on brain waste clearance in OSA (NCT05606991).
Pharmacological Approaches for the Treatment of Sleep Disturbances
Melatonin
Exogenous melatonin is considered a safe alternative to hypnotic medications, with modest efficacy for insomnia and circadian rhythm disorders in older adults [55]. Duffy et al. (2022) tested 0.3 mg and 5 mg doses of melatonin for two weeks in 24 healthy older adults [56]. The higher dose showed more consistent improvements in sleep parameters than the lower dose and placebo. Although melatonin trials in older adults demonstrate a favorable safety profile, long-term safety data are limited, with most studies lasting less than 12 weeks (for review, see [55]). A pre-registered trial will examine the effects of 5 mg melatonin over a 9-month period in older adults with and without MCI, measuring actigraphy-derived sleep outcomes, AD biomarkers (p-tau, t-tau, Aβ-42 ratio) from cerebrospinal fluid (CSF), and cognitive outcomes through neuropsychological tests (NCT03954899). Melatonin, a potent antioxidant with potential anti-amyloid properties, is being increasingly studied at higher doses (> 10 mg) for various conditions, including cancer, cardiometabolic disease, and neurodegenerative disorders [57, 58]. One study will evaluate the feasibility of 25 mg melatonin for 12 weeks in 40 adults with MCI (aged 60–80 years) on measures of brain oxidative stress using magnetic resonance spectroscopy and blood-based biomarkers (ANZCTRN12619000876190).
Orexin Receptor Antagonists
Orexin receptor antagonists such as lemborexant, daridorexant, and suvorexant are being explored for treating insomnia in older adults. In one study, 38 cognitively healthy older adults (mean age > 50 years) received suvorexant, which reduced tau phosphorylation at threonine-181 (p-tau181) and Aβ levels (Aβ38, Aβ40, and Aβ42) in human CSF, despite no significant group differences in sleep outcome measures [59]. This suggests potential neuroprotective properties beyond its effects on sleep and underscores the need for long-term studies on cognitive function and neurodegenerative biomarkers. A pre-registered Phase II RCT of suvorexant (20 mg) in those aged > 65 years with amyloid pathology but no cognitive impairment will assess impact on the rate of Aβ accumulation via PET imaging (NCT04629547).
Rosenberg et al. (2019) showed that 4-week treatment with lemborexant (5 or 10 mg) significantly improved sleep onset and maintenance compared to placebo and zolpidem in 1,006 adults with insomnia (aged \(\ge\) 55 years) and was well-tolerated [60]. Kärppä et al. (2020) tested the effects of 6-month treatment with lemborexant (5 or 10 mg) in adults with insomnia (mean age 54.4 years) [61]. Both doses improved subjective sleep parameters at 6 months, including sleep onset latency, wake after sleep onset (WASO), sleep efficiency (SE), and sleep quality, with low rates of adverse events. The effects on subjective sleep parameters were sustained at 12 months [62]. A secondary analysis confirmed these findings in a subgroup of older adults (\(\ge\) 65 years; n = 262) [63]. The safety and tolerability of lemborexant in elderly patients was similar to younger patients, with no age-based dose adjustments required. Previous studies reported no effects on postural stability [64] and driving performance [65], though 10 mg lemborexant negatively affected attention and memory in healthy older adults [64]. While preliminary work with lemborexant in AD patients with sleep disturbance is promising [66], more studies in older adults with cognitive impairment are needed. A pre-registered trial will examine the effect of lemborexant in cognitively healthy older adults with amyloid deposition on AD biomarkers including CSF and plasma markers of tau and Aβ accumulation, neurofilament light chain (NfL), and soluble triggering receptor expressed on myeloid cells 2 (sTREM2) (NCT06274528).
Zammit et al. (2020) assigned 58 adults (aged \(\ge\) 65 years) with insomnia to receive ascending doses of daridorexant and placebo over five two-night treatment periods [67]. Dose-dependent improvements in PSG-derived WASO and latency to persistent sleep (dose range 10–50 mg) were observed. Two subsequent Phase 3 RCTs (N = 1,854) showed that both 25 mg and 50 mg of daridorexant improved sleep outcomes, while 50 mg also improved daytime functioning, in adults with insomnia (mean age > 50 years) after 12 weeks [68]. A secondary analysis indicated that 50 mg of daridorexant was most effective for improving both sleep outcomes and daytime function, without increasing adverse events or next-morning residual effects [69]. The same participants joined a 40-week extension trial (n = 550) [70] showing 50 mg daridorexant consistently improved sleep and daytime functioning without next-morning sleepiness, withdrawal, or rebound effects. A pre-registered trial will test daridorexant for insomnia in MCI and mild-to-moderate AD, with outcomes including PSG, neuropsychological assessments, and neurodegenerative biomarker assays (NCT05924425).
Supplementation
One RCT examined the effects of a high-dose omega-3 and omega-6 fatty acids with antioxidant vitamins on sleep, cognitive function and functional capacity in 47 older adults with MCI over six months [71]. The supplementation group showed significant cognitive improvements on the ACE-R and MMSE compared to placebo, indicating potential for reducing cognitive and functional decline in MCI. A pre-registered RCT (NCT06029894) will explore the effects of a dietary supplement, citicoline, on sleep and cognition, including neuropsychological tests and changes in CSF-derived Aβ-42 and tau and p-tau levels.
Discussion
Since 2019, research on sleep interventions in older adults with or without cognitive impairment has significantly expanded, involving multiple countries and a higher proportion of female participants, reflecting increased representation of women in dementia research [72]. While most studies focused on subjective measures of sleep, an increasing number of studies included both subjective and objective measures, such as wearable devices. Few studies incorporated measures of cognition, with most using only gross measures of cognition and a subset administering discrete neuropsychological tasks. Additionally, few studies incorporated long-term follow-up to determine lasting clinical benefits and efficacy on sleep and cognitive outcomes, aligning with a recent meta-analysis calling for longer follow-up durations and use of supporting biomarkers [73]. Despite the growing interest in sleep, dementia, and neurodegenerative research [74], none of the included studies from the past five years examined the effect of sleep interventions on biomarkers of neurodegeneration (e.g., glial fibrillary acidic protein, NfL, p-tau217) or brain structure and function via neuroimaging. However, several emerging pre-registered trials include these outcomes, indicating a promising direction for future research.
Over the past five years, research has established non-pharmacological treatments such as CBT-I, exercise, and multi-modal interventions as the mainstay for treating sleep disturbances in older adults, with or without cognitive impairment. CBT-I remains a safe and effective method for improving sleep in this population, though further research is needed to optimize its delivery in older adults, including length and type of therapy (e.g., CBT-I, BBT-I) and mode of delivery (e.g., individual, group, or digital). Digital or web-based CBT-I interventions are particularly promising due to their accessibility, especially since the majority of individuals with MCI use e-tools like the internet and smartphones [75]. Indeed, a recent pilot RCT (N = 246) demonstrated that digital CBT-I is feasible and acceptable for those with MCI, showing a 5.9-point improvement in ISI scores at week 12 compared to the control group [76]. Similar results were observed in another pilot RCT involving a four-session multi-modal group intervention (“Sleep Well, Think Well”) for individuals with MCI [77]. Exercise intervention is a widely accessible intervention that can improve or protect against both functional and cognitive decline and improve depressive symptoms – a known risk factor for dementia [78]. A systematic review of 218 controlled studies (N = 14,170 participants) found that exercise was an effective treatment for depression, with walking/jogging, yoga, and strength training more effective than other exercises, and yoga somewhat more effective among older adults [79].
Given the complex and multifactorial nature of cognitive decline in older adults, multidomain lifestyle interventions targeting several risk factors simultaneously show promise in improving sleep and cognitive decline. Large-scale, long-term RCTs, such as The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) and World-Wide FINGER (WW-FINGER), are in progress, with follow-ups of up to 11 years [80]. These studies involve the addition of metformin as a potential disease-modifying drug for AD and dementia, as well as the analysis of cognitive outcomes, sleep parameters, and genetic factors such as apolipoprotein E (APOE) [81]. Emerging non-pharmacological sleep interventions, including time-restricted feeding [82] and music-based interventions, are also being explored. Early evidence suggests that music-based interventions may improve sleep in older adults [83] and cognition among those with MCI and AD [82]. However, more research is needed to determine the type, duration, and dose of music required to produce lasting effects on sleep and cognition.
In acute cases of sleep disturbance or as adjuncts to non-pharmacological intervention, short-term pharmacological therapies may be offered. Melatonin is considered a safe alternative to hypnotic medications such as benzodiazepines and Z-drugs, which are not recommended due to their off-target effects and heightened risk of falls and injury [84, 85]. Orexin receptor antagonists are increasingly shown to be relatively safe and effective for improving sleep in older adults, without the need for aged-based dose adjustments. Preliminary studies suggest potential neuroprotective effects of suvorexant, emphasising the need for further long-term research to evaluate their impact on cognitive function and neurodegenerative biomarkers. The effects of AD-specific drugs on sleep are noteworthy. Memantine, an N-methyl-D-aspartate receptor antagonist (NMDA) receptor antagonist, has shown positive effects on sleep behavior in AD and dementia with Lewy bodies and Parkinson's disease dementia [86,87,88]. Anti-amyloid monoclonal antibodies (MABs), including donanemab and lecanemab, offer new avenues for research, particularly regarding their potential effects on sleep, which remain largely unexplored. Targeting amyloid burden early may reduce sleep disturbances and improve patient outcomes [89]. Future studies involving MABs and emerging pharmacological agents targeting neuroinflammation (e.g., NCT05904717) should incorporate validated objective and subjective measures of sleep-related outcomes.
The field of sleep-focused clinical trials is limited by an incomplete understanding of the neurobiology underlying sleep disturbances in older adults and those with MCI, and and how the regional distribution of neurodegenerative disease in the brain predisposes to and/or perpetuates sleep disorders and vice versa. Some studies suggest that distinct clusters of individuals attending memory clinics exhibit altered sleep neurophysiology, which correlates with distinct cognitive profiles and brain network connectivity [90]. Future research must consider the type of sleep disorder, environmental factors, and the neurodegenerative pathologies that contribute to sleep disturbances and cognitive decline. It is also pertinent that future research account for the heterogeneity of cognitive deficits in pre-dementia periods and tailor interventions accordingly, with insights into the neuroscience of sleep in MCI informing these efforts.
Conclusions
The field of sleep-focused clinical trials in older adults with or without cognitive impairment is progressing, but still needs robust evidence through well-designed studies targeting objective markers of sleep, cognition, and neurodegeneration. Personalized, clinically effective interventions, considering the presence of amyloid, tau, cerebrovascular disease, or alpha-synuclein in key sleep-related circuits, are needed to develop feasible, cost-effective, and scalable treatments in older adults with or without cognitive impairment.
Data Availability
No datasets were generated or analysed during the current study.
References
World Health Organisation. The top 10 Causes of Death. 2020. Accessed 20 May 2024: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–46.
Spira AP, Chen-Edinboro LP, Wu MN, Yaffe K. Impact of sleep on the risk of cognitive decline and dementia. Curr Opin Psychiatry. 2014;27(6):478–83.
Winer JR, Mander BA, Helfrich RF, Maass A, Harrison TM, Baker SL, et al. Sleep as a potential biomarker of tau and β-amyloid burden in the human brain. J Neurosci. 2019;39(32):6315–24.
Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004;44(1):121–33.
Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10(1):49–62.
Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–7.
Miner B, Kryger MH. Sleep in the aging population. Sleep Med Clin. 2020;15(2):311–8.
Canever JB, Zurman G, Vogel F, Sutil DV, Diz JBM, Danielewicz AL, et al. Worldwide prevalence of sleep problems in community-dwelling older adults: A systematic review and meta-analysis. Sleep Med. 2024
Zhang Y, Ren R, Yang L, Zhang H, Shi Y, Okhravi HR, et al. Sleep in Alzheimer’s disease: a systematic review and meta-analysis of polysomnographic findings. Transl Psychiatry. 2022;12(1):136.
Ju Y-ES, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology—a bidirectional relationship. Nat Rev Neurol. 2014;10(2):115–9.
Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–6.
McKinnon A, Terpening Z, Hickie IB, Batchelor J, Grunstein R, Lewis SJ, Naismith SL. Prevalence and predictors of poor sleep quality in mild cognitive impairment. J Geriatr Psychiatry Neurol. 2014;27(3):204–11.
D’Rozario AL, Chapman JL, Phillips CL, Palmer JR, Hoyos CM, Mowszowski L, et al. Objective measurement of sleep in mild cognitive impairment: A systematic review and meta-analysis. Sleep Med Rev. 2020;52: 101308.
Sabia S, Fayosse A, Dumurgier J, van Hees VT, Paquet C, Sommerlad A, et al. Association of sleep duration in middle and old age with incidence of dementia. Nat Commun. 2021;12(1):2289.
Blackman J, Swirski M, Clynes J, Harding S, Leng Y, Coulthard E. Pharmacological and non-pharmacological interventions to enhance sleep in mild cognitive impairment and mild Alzheimer’s disease: A systematic review. J Sleep Res. 2021;30(4): e13229.
O’Caoimh R, Mannion H, Sezgin D, O’Donovan MR, Liew A, Molloy DW. Non-pharmacological treatments for sleep disturbance in mild cognitive impairment and dementia: A systematic review and meta-analysis. Maturitas. 2019;127:82–94.
Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. JBI Evidence Implementation. 2015;13(3):141–6.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73.
Tanaka M, Kusaga M, Nyamathi AM, Tanaka K. Effects of brief cognitive behavioral therapy for insomnia on improving depression among community-dwelling older adults: A randomized controlled comparative study. Worldviews Evid Based Nurs. 2019;16(1):78–86.
Irwin MR, Carrillo C, Sadeghi N, Bjurstrom MF, Breen EC, Olmstead R. Prevention of incident and recurrent major depression in older adults with insomnia: a randomized clinical trial. JAMA Psychiat. 2022;79(1):33–41.
Espie CA, Emsley R, Kyle SD, Gordon C, Drake CL, Siriwardena AN, et al. Effect of digital cognitive behavioral therapy for insomnia on health, psychological well-being, and sleep-related quality of life: a randomized clinical trial. JAMA Psychiat. 2019;76(1):21–30.
Kyle SD, Hurry ME, Emsley R, Marsden A, Omlin X, Juss A, et al. The effects of digital cognitive behavioral therapy for insomnia on cognitive function: a randomized controlled trial. Sleep. 2020;43(9):zsaa034.
Carroll JE, Olmstead R, Cole SW, Breen EC, Arevalo JM, Irwin MR. Remission of insomnia in older adults treated with cognitive behavioral therapy for insomnia (CBT-I) reduces p16INK4a gene expression in peripheral blood: secondary outcome analysis from a randomized clinical trial. GeroScience. 2023;45(4):2325–35.
Chan WS, Dautovich ND, McNamara JP, Stripling A, Dzierzewski JM, McCoy K, McCrae CS. Sleep discrepancy in a randomized controlled trial of brief behavioral therapy for chronic insomnia in older adults. Behav Sleep Med. 2021;19(2):221–31.
Polat F, Karasu F. Effect of sleep hygiene training given to elderly individuals on daytime sleepiness and fatigue: A randomized controlled trial. Perspect Psychiatr Care. 2022;58(4):2161–9.
Cibeira N, Maseda A, Lorenzo-López L, Rodriguez-Villamil JL, López-López R, Millan-Calenti JC. Application of light therapy in older adults with cognitive impairment: A systematic review. Geriatr Nurs. 2020;41(6):970–83.
Van Maanen A, Meijer AM, van der Heijden KB, Oort FJ. The effects of light therapy on sleep problems: a systematic review and meta-analysis. Sleep Med Rev. 2016;29:52–62.
Forbes D, Blake CM, Thiessen EJ, Peacock S, Hawranik P. Light therapy for improving cognition, activities of daily living, sleep, challenging behaviour, and psychiatric disturbances in dementia. Cochrane Database Syst Rev. 2014(2).
Mitolo M, Tonon C, La Morgia C, Testa C, Carelli V, Lodi R. Effects of light treatment on sleep, cognition, mood, and behavior in Alzheimer’s disease: a systematic review. Dement Geriatr Cogn Disord. 2019;46(5–6):371–84.
Tan JSI, Cheng LJ, Chan EY, Lau Y, Lau ST. Light therapy for sleep disturbances in older adults with dementia: a systematic review, meta-analysis and meta-regression. Sleep Med. 2022;90:153–66.
Juda M, Liu-Ambrose T, Feldman F, Suvagau C, Mistlberger RE. Light in the senior home: effects of dynamic and individual light exposure on sleep, cognition, and wellbeing. Clocks Sleep. 2020;2(4):557–7.
Hasan F, Tu Y-K, Lin C-M, Chuang L-P, Jeng C, Yuliana LT, et al. Comparative efficacy of exercise regimens on sleep quality in older adults: a systematic review and network meta-analysis. Sleep Med Rev. 2022;65:101673
Páez A, Frimpong E, Mograss M, Dang‐Vu TT. The effectiveness of exercise interventions targeting sleep in older adults with cognitive impairment or Alzheimer's disease and related dementias (AD/ADRD): A systematic review and meta‐analysis. J Sleep Res. 2024:e14189
Han EK, Son HK. The Effect of Sleep and Cognition Enhancement Multimodal Intervention for Mild Cognitive Impairment with Sleep Disturbance in the Community-Dwelling Elderly. Int J Ment Health Promot. 2023;25(11).
Falck RS, Davis JC, Best JR, Chan PC, Li LC, Wyrough AB, et al. Effect of a multimodal lifestyle intervention on sleep and cognitive function in older adults with probable mild cognitive impairment and poor sleep: a randomized clinical trial. J Alzheimer’s Dis. 2020;76(1):179–93.
Hazlett-Stevens H, Singer J, Chong A. Mindfulness-based stress reduction and mindfulness-based cognitive therapy with older adults: A qualitative review of randomized controlled outcome research. Clin Gerontol. 2019;42(4):347–58.
Shim M, Tilley JL, Im S, Price K, Gonzalez A. A systematic review of mindfulness-based interventions for patients with mild cognitive impairment or dementia and caregivers. J Geriatr Psychiatry Neurol. 2021;34(6):528–54.
Perini F, Wong KF, Lin J, Hassirim Z, Ong JL, Lo J, et al. Mindfulness-based therapy for insomnia for older adults with sleep difficulties: a randomized clinical trial. Psychol Med. 2023;53(3):1038–48.
Cai Z-Z, Lin R, Wang X-X, Yan Y-J, Li H. Effects of mindfulness in patients with mild cognitive impairment with insomnia: A double-blind randomized controlled trial. Geriatr Nurs. 2022;47:239–46.
Sanchez-Lara E, Lozano-Ruiz A, Perez-Garcia M, Caracuel A. Efficacy of mindfulness-based interventions in cognitive function in the elderly people: a systematic review and meta-analysis. Aging Ment Health. 2022;26(9):1699–709.
Lee HJ, Hong JK, Choi H, Chung S, Yoon I-Y. Age-Limited Effects of Low-Frequency Transcutaneous Electric Nerve Stimulation on Insomnia: A 4-Week Multi-Center, Randomized Controlled Study. Psychiatry Investig. 2022;19(6):451.
Diep C, Ftouni S, Manousakis JE, Nicholas CL, Drummond SP, Anderson C. Acoustic slow wave sleep enhancement via a novel, automated device improves executive function in middle-aged men. Sleep. 2020;43(1):zsz197.
Lustenberger C, Ferster ML, Huwiler S, Brogli L, Werth E, Huber R, Karlen W. Auditory deep sleep stimulation in older adults at home: a randomized crossover trial. Commun Med. 2022;2(1):30.
Schneider J, Lewis PA, Koester D, Born J, Ngo H-VV. Susceptibility to auditory closed-loop stimulation of sleep slow oscillations changes with age. Sleep. 2020;43(12):zsaa111.
Wunderlin M, Zeller CJ, Senti SR, Fehér KD, Suppiger D, Wyss P, et al. Acoustic stimulation during sleep predicts long-lasting increases in memory performance and beneficial amyloid response in older adults. Age Ageing. 2023;52(12):afad228.
Wunderlin M, Zeller CJ, Wicki K, Nissen C, Züst MA. Acoustic stimulation during slow wave sleep shows delayed effects on memory performance in older adults. Front Sleep. 2024;2:1294957.
Papalambros NA, Weintraub S, Chen T, Grimaldi D, Santostasi G, Paller KA, et al. Acoustic enhancement of sleep slow oscillations in mild cognitive impairment. Ann Clin Transl Neurol. 2019;6(7):1191–201.
Zeller CJ, Wunderlin M, Wicki K, Teunissen CE, Nissen C, Züst MA, Klöppel S. Multi-night acoustic stimulation is associated with better sleep, amyloid dynamics, and memory in older adults with cognitive impairment. GeroScience. 2024:1–16.
Jiang X, Wang Z, Hu N, Yang Y, Xiong R, Fu Z. Cognition effectiveness of continuous positive airway pressure treatment in obstructive sleep apnea syndrome patients with cognitive impairment: a meta-analysis. Exp Brain Res. 2021;239:3537–52.
Fernandes M, Placidi F, Mercuri NB, Liguori C. The importance of diagnosing and the clinical potential of treating obstructive sleep apnea to delay mild cognitive impairment and Alzheimer’s disease: a special focus on cognitive performance. J Alzheimer’s Dis Rep. 2021;5(1):515–33.
Shieu MM, Zaheed AB, Shannon C, Chervin RD, Conceicao A, Paulson HL, et al. Positive airway pressure and cognitive disorders in adults with obstructive sleep apnea: a systematic review of the literature. Neurology. 2022;99(4):e334-e46.
Hoyos CM, Cross NE, Terpening Z, D’Rozario AL, Yee BJ, LaMonica H, et al. Continuous positive airway pressure for cognition in sleep apnea and mild cognitive impairment: a pilot randomized crossover clinical trial. Am J Respir Crit Care Med. 2022;205(12):1479–82.
Hoyos CM, Phillips CL, Marshall NS, Yaffe K, Martins R, Lagopoulos J, et al. REducing Sleep Apnoea for the PrEvention of Dementia (REShAPED): Protocol for a multi-site feasibility randomised controlled trial. Contemp Clin Trials. 2024;137: 107424.
Tuft C, Matar E, Menczel Schrire Z, Grunstein RR, Yee BJ, Hoyos CM. Current insights into the risks of using melatonin as a treatment for sleep disorders in older adults. Clin Interv Aging. 2023:49–59.
Duffy JF, Wang W, Ronda JM, Czeisler CA. High dose melatonin increases sleep duration during nighttime and daytime sleep episodes in older adults. J Pineal Res. 2022;73(1): e12801.
Schrire ZM, Phillips CL, Duffy SL, Marshall NS, Mowszowski L, La Monica HM, et al. Feasibility of 3-month melatonin supplementation for brain oxidative stress and sleep in mild cognitive impairment: protocol for a randomised, placebo-controlled study. BMJ Open. 2021;11(2): e041500.
Menczel Schrire Z, Phillips CL, Chapman JL, Duffy SL, Wong G, D’Rozario AL, et al. Safety of higher doses of melatonin in adults: a systematic review and meta-analysis. J Pineal Res. 2022;72(2): e12782.
Lucey BP, Liu H, Toedebusch CD, Freund D, Redrick T, Chahin SL, et al. Suvorexant acutely decreases tau phosphorylation and Aβ in the human CNS. Ann Neurol. 2023;94(1):27–40.
Rosenberg R, Murphy P, Zammit G, Mayleben D, Kumar D, Dhadda S, et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Netw Open. 2019;2(12):e1918254-e.
Kärppä M, Yardley J, Pinner K, Filippov G, Zammit G, Moline M, et al. Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE 2. Sleep. 2020;43(9):zsaa123.
Yardley J, Kärppä M, Inoue Y, Pinner K, Perdomo C, Ishikawa K, et al. Long-term effectiveness and safety of lemborexant in adults with insomnia disorder: results from a phase 3 randomized clinical trial. Sleep Med. 2021;80:333–42.
Arnold V, Ancoli-Israel S, Dang-Vu TT, Mishima K, Pinner K, Malhotra M, Moline M. Efficacy of Lemborexant in Adults≥ 65 Years of Age with Insomnia Disorder. Neurol Ther. 2024:1–18.
Murphy P, Kumar D, Zammit G, Rosenberg R, Moline M. Safety of lemborexant versus placebo and zolpidem: effects on auditory awakening threshold, postural stability, and cognitive performance in healthy older participants in the middle of the night and upon morning awakening. J Clin Sleep Med. 2020;16(5):765–73.
Vermeeren A, Jongen S, Murphy P, Moline M, Filippov G, Pinner K, et al. On-the-road driving performance the morning after bedtime administration of lemborexant in healthy adult and elderly volunteers. Sleep. 2019;42(4):zsy260.
Moline M, Thein S, Bsharat M, Rabbee N, Kemethofer-Waliczky M, Filippov G, et al. Safety and efficacy of lemborexant in patients with irregular sleep-wake rhythm disorder and Alzheimer’s disease dementia: results from a phase 2 randomized clinical trial. J Prev Alzheimers Dis. 2021;8:7–18.
Zammit G, Dauvilliers Y, Pain S, Sebök Kinter D, Mansour Y, Kunz D. Daridorexant, a new dual orexin receptor antagonist, in elderly subjects with insomnia disorder. Neurology. 2020;94(21):e2222–32.
Mignot E, Mayleben D, Fietze I, Leger D, Zammit G, Bassetti CL, et al. Safety and efficacy of daridorexant in patients with insomnia disorder: results from two multicentre, randomised, double-blind, placebo-controlled, phase 3 trials. Lancet Neurol. 2022;21(2):125–39.
Fietze I, Bassetti CL, Mayleben DW, Pain S, Seboek Kinter D, McCall WV. Efficacy and safety of daridorexant in older and younger adults with insomnia disorder: a secondary analysis of a randomised placebo-controlled trial. Drugs Aging. 2022;39(10):795–810.
Kunz D, Dauvilliers Y, Benes H, García-Borreguero D, Plazzi G, Seboek Kinter D, et al. Long-term safety and tolerability of daridorexant in patients with insomnia disorder. CNS Drugs. 2023;37(1):93–106.
Stavrinou P, Andreou E, Aphamis G, Pantzaris M, Ioannou M, Patrikios I, Giannaki C. The effects of a 6-month high dose omega-3 and omega-6 polyunsaturated fatty acids and antioxidant vitamins supplementation on cognitive function and functional capacity in older adults with mild cognitive impairment. Nutrients. 2020;12(2):325.
Pinho-Gomes A-C, Gong J, Harris K, Woodward M, Carcel C. Dementia clinical trials over the past decade: are women fairly represented? BMJ Neurol Open. 2022;4(2).
Franks KH, Rowsthorn E, Nicolazzo J, Boland A, Lavale A, Baker J, et al. The treatment of sleep dysfunction to improve cognitive function: A meta-analysis of randomized controlled trials. Sleep Med. 2023;101:118–26.
Harenbrock J, Holling H, Reid G, Koychev I. A meta-analysis of the relationship between sleep and β-Amyloid biomarkers in Alzheimer’s disease. Biomarkers Neuropsychiatr. 2023;9: 100068.
LaMonica HM, English A, Hickie IB, Ip J, Ireland C, West S, et al. Examining internet and eHealth practices and preferences: survey study of Australian older adults with subjective memory complaints, mild cognitive impairment, or dementia. J Med Internet Res. 2017;19(10): e358.
Hoyos C, Espinosa N, Marshall N, LaMonica H, Gordon C, Rainey-Smith S, et al. 0441 Sleep Disturbance in MCI: A Pilot Study of a Cognitive Behavioural Therapy Digital Intervention (SUCCEED). Sleep. 2024;47(Supplement_1):A189-A.
Naismith SL, Pye J, Terpening Z, Lewis S, Bartlett D. “Sleep Well, Think Well” group program for mild cognitive impairment: a randomized controlled pilot study. Behav Sleep Med. 2018.
Kaup AR, Byers AL, Falvey C, Simonsick EM, Satterfield S, Ayonayon HN, et al. Trajectories of depressive symptoms in older adults and risk of dementia. JAMA Psychiatr. 2016;73(5):525–31.
Noetel M, Sanders T, Gallardo-Gómez D, Taylor P, del Pozo Cruz B, Van Den Hoek D, et al. Effect of exercise for depression: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2024;384.
Rosenberg A, Mangialasche F, Ngandu T, Solomon A, Kivipelto M. Multidomain interventions to prevent cognitive impairment, Alzheimer’s disease, and dementia: From FINGER to World-Wide FINGERS. J Prev Alzheimers Dis. 2020;7:29–36
Kivipelto M, Barbera M, de Jager CA, Lehtisalo J, Levak N, Middleton LT, et al. From FINGER to MET‐FINGER: metformin and lifestyle intervention for multimodal precision prevention of dementia. Alzheimer’s Dement. 2022;18:e061539
Ezzati A, Pak VM. The effects of time-restricted eating on sleep, cognitive decline, and Alzheimer’s disease. Exp Gerontol. 2023;171: 112033.
Petrovsky DV, Ramesh P, McPhillips MV, Hodgson NA. Effects of music interventions on sleep in older adults: A systematic review. Geriatr Nurs. 2021;42(4):869–79.
Scharner V, Hasieber L, Sönnichsen A, Mann E. Efficacy and safety of Z-substances in the management of insomnia in older adults: a systematic review for the development of recommendations to reduce potentially inappropriate prescribing. BMC Geriatr. 2022;22(1):87.
Markota M, Rummans TA, Bostwick JM, Lapid MI. Benzodiazepine use in older adults: dangers, management, and alternative therapies. Mayo Clin Proceed: Elsevier; 2016. p. 1632–9.
Larsson V, Aarsland D, Ballard C, Minthon L, Londos E. The effect of memantine on sleep behaviour in dementia with Lewy bodies and Parkinson’s disease dementia. Int J Geriatr Psychiatry. 2010;25(10):1030–8.
Ishikawa I, Shinno H, Ando N, Mori T, Nakamura Y. The effect of memantine on sleep architecture and psychiatric symptoms in patients with Alzheimer’s disease. Acta Neuropsychiatrica. 2016;28(3):157–64.
Mori K, Yoshida M, Tomita K, Nakamura M, Yoshimura T. Sleep-Prolonging Effect of Memantine for Short Periods and Low Doses. Neurosci Behav Physiol. 2021;51(7):1027–31.
Kimura N, Sasaki Y, Masuda T, Ataka T, Eguchi A, Kakuma T, Matsubara E. Objective sleep was longitudinally associated with brain amyloid burden in mild cognitive impairment. Ann Clin Transl Neurol. 2023;10(12):2266–75.
Orlando IF, O’Callaghan C, Lam A, McKinnon AC, Tan JB, Michaelian JC, et al. Sleep spindle architecture associated with distinct clinical phenotypes in older adults at risk for dementia. Mol Psychiatry. 2023:1–10.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This research received no external funding.
Author information
Authors and Affiliations
Contributions
A.S. and S.L.N. contributed to the manuscript’s conception and design. A.S., Z.M.S., S.D.K., B.A.T., and N.C. contributed to article screening and data extraction. A.S. wrote the main manuscript text. All authors critically revised and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Suraev, A., Kong, S.D., Menczel Schrire, Z. et al. Current and Emerging Sleep Interventions for Older Adults with or without Mild Cognitive Impairment. Curr Treat Options Neurol (2024). https://doi.org/10.1007/s11940-024-00808-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s11940-024-00808-4