Abstract
Purpose of review
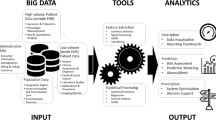
To survey the literature around “big data” and “artificial intelligence” use, its challenges, and examples in clinical and research domains within neurocritical care.
Recent findings
Innovations in digital data storage and integration technology have motivated the active development of data-driven tools for neurocritical care practice. There has been progress towards harmonization of data dictionaries within the field to boost generalizability of studies. Numerous collaborative groups have cultivated datasets that will enable further study of disease detection, prediction, and management of critically ill neurologic patients. Essential to the effective analysis of big data in neurocritical care are the increasing relationships between clinicians and data scientists. There are multiple challenges related to the efficient and ethical use of big data, including data complexity, database stewardship and governance, data quality, and safe implementation of data-driven conclusions. Continued efforts towards optimally harnessing data will be crucial in neurocritical care.
Summary
Critically ill neurologic patients generate an abundant amount of data in the course of routine management. Clinical research is evolving from benefiting the “average patient” to aiming to deliver more precise treatment to the individual patient. In neurocritical care, this has manifested through big data—for triage decisions, enhancing workflow, event detection, and outcome prediction.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Angus DC. Fusing randomized trials with big data: the key to self-learning health care systems? JAMA. 2015;314(8):767–8.
Office of the Press Secretary. FACT SHEET: Obama Administration Announces Key Actions to Accelerate Precision Medicine Initiative. https://obamawhitehouse.archives.gov/the-press-office/2016/02/25/fact-sheet-obama-administration-announces-key-actions-accelerate. Published 2016. Accessed August 18, 2019.
Frieden TR. Evidence for health decision making -beyond randomized, controlled trials. N Engl J Med. 2017;377(5):465–75.
Sagiroglu S, Sinanc D. Big data: a review. In. Proceeding of the 2013 international conference on collaboration technologies and systems (CTS): IEEE Computer Society; 2013:42–47.
The Office of the National Coordinator for Health Information Technology. Adoption of Electronic Health Record Systems among U.S. Non-Federal Acute Care Hospitals: 2008–2015. https://dashboard.healthit.gov/evaluations/data-briefs/non-federal-acute-care-hospital-ehr-adoption-2008-2015.php. Published 2016. Accessed August 18, 2019.
Rush B, Stone DJ, Celi LA. From big data to artificial intelligence: harnessing data routinely collected in the process of care. Crit Care Med. 2018;46(2):345–6.
Ramon J, Fierens D, Güiza F, et al. Mining data from intensive care patients. Adv Eng Inform. 2007;21(3):243–56.
Meyfroidt G, Güiza F, Ramon J, Bruynooghe M. Machine learning techniques to examine large patient databases. Best Pract Res Clin Anaesthesiol. 2009;23(1):127–43.
Bzdok D, Altman N, Krzywinski M. Points of significance: statistics versus machine learning. Nat Methods. 2018:1–7.
Fawcett T. ROC graphs: notes and practical considerations for data mining researchers Technical Report HPL-2003-4. HP Labs 2003.
Gail MH, Pfeiffer RM. Breast cancer risk model requirements for counseling, prevention, and screening. J Natl Cancer Inst. 2018;110(9):994–1002.
Kohavi R. A study of cross-validation and bootstrap for accuracy estimation and model selection. Paper presented at: Ijcai1995.
Lo BW, Macdonald RL, Baker A, Levine MA. Clinical outcome prediction in aneurysmal subarachnoid hemorrhage using Bayesian neural networks with fuzzy logic inferences. Comput Math Methods Med. 2013;2013:904860.
Zafar SF, Postma EN, Biswal S, et al. Electronic health data predict outcomes after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2018;28(2):184–93.
Zador Z, Sperrin M, King AT. Predictors of outcome in traumatic brain injury: new insight using receiver operating curve indices and Bayesian network analysis. PLoS One. 2016;11(7):e0158762.
Zador Z, Huang W, Sperrin M, Lawton MT. Multivariable and bayesian network analysis of outcome predictors in acute aneurysmal subarachnoid hemorrhage: review of a pure surgical series in the post-international subarachnoid aneurysm trial era. Oper Neurosurg (Hagerstown). 2018;14(6):603–10.
de Toledo P, Rios PM, Ledezma A, Sanchis A, Alen JF, Lagares A. Predicting the outcome of patients with subarachnoid hemorrhage using machine learning techniques. IEEE Trans Inf Technol Biomed. 2009;13(5):794–801.
Rubbert C, Patil KR, Beseoglu K, et al. Prediction of outcome after aneurysmal subarachnoid haemorrhage using data from patient admission. Eur Radiol. 2018;28(12):4949–58.
van Donkelaar CE, Bakker NA, Birks J, et al. Prediction of outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2019;50(4):837–44.
Hale AT, Stonko DP, Brown A, et al. Machine-learning analysis outperforms conventional statistical models and CT classification systems in predicting 6-month outcomes in pediatric patients sustaining traumatic brain injury. Neurosurg Focus. 2018;45(5):E2.
Rau CS, Wu SC, Chien PC, et al. Prediction of mortality in patients with isolated traumatic subarachnoid hemorrhage using a decision tree classifier: a retrospective analysis based on a trauma registry system. Int J Environ Res Public Health. 2017;14(11).
Rau CS, Kuo PJ, Chien PC, Huang CY, Hsieh HY, Hsieh CH. Mortality prediction in patients with isolated moderate and severe traumatic brain injury using machine learning models. PLoS One. 2018;13(11):e0207192.
Gupta VP, Garton ALA, Sisti JA, et al. Prognosticating functional outcome after intracerebral hemorrhage: the ICHOP score. World Neurosurg. 2017;101:577–83.
Heo J, Yoon JG, Park H, Kim YD, Nam HS, Heo JH. Machine learning-based model for prediction of outcomes in acute stroke. Stroke. 2019;50(5):1263-1265. Deep learning improved prediction of long-term outcomes in ischemic stroke patients, over the ASTRAL score, an established integer-based score.
Lin J, Jiang A, Ling M, Mo Y, Li M, Zhao J. Prediction of neurologic deterioration based on support vector machine algorithms and serum osmolarity equations. Brain Behav. 2018;8(7):e01023.
van Os HJA, Ramos LA, Hilbert A, et al. Predicting outcome of endovascular treatment for acute ischemic stroke: potential value of machine learning algorithms. Front Neurol. 2018;9:784.
Abouzari M, Rashidi A, Zandi-Toghani M, Behzadi M, Asadollahi M. Chronic subdural hematoma outcome prediction using logistic regression and an artificial neural network. Neurosurg Rev. 2009;32(4):479–84.
Heino I, Frantzén J, Rinne J, et al. Risk factors for recurrent hematoma after surgery for acute traumatic subdural hematoma. World Neurosurg. 2019.
Oermann EK, Rubinsteyn A, Ding D, et al. Using a machine learning approach to predict outcomes after radiosurgery for cerebral arteriovenous malformations. Sci Rep. 2016;6:21161.
Ghassemi MM, Amorim E, Alhanai T, et al. Quantitative electroencephalogram trends predict recovery in hypoxic-ischemic encephalopathy. Crit Care Med. 2019.
•• Claassen J, Doyle K, Matory A, et al. Detection of brain activation in unresponsive patients with acute brain injury. N Engl J Med. 2019;380(26):2497–505. Using support vector machine with linear kernel on a large prospectively collected neurointensive care population, EEG features were utilized to classify patients with cognitive motor dissociation from unresponsive patients and systematically linked to long-term outcome.
Liu J, Xu H, Chen Q, et al. Prediction of hematoma expansion in spontaneous intracerebral hemorrhage using support vector machine. EBioMedicine. 2019;43:454–9.
Kasasbeh AS, Christensen S, Parsons MW, Campbell B, Albers GW, Lansberg MG. Artificial neural network computer tomography perfusion prediction of ischemic core. Stroke. 2019;50(6):1578–81.
Arvind V, Kim JS, Oermann EK, Kaji D, Cho SK. Predicting surgical complications in adult patients undergoing anterior cervical discectomy and fusion using machine learning. Neurospine. 2018;15(4):329–37.
Rohaut B, Doyle KW, Reynolds AS, et al. Deep structural brain lesions associated with consciousness impairment early after hemorrhagic stroke. Sci Rep. 2019;9(1):4174.
Maragkos GA, Enriquez-Marulanda A, Salem MM, Ascanio LC, Chida K, Gupta R, et al. Proposal of a grading system for predicting discharge mortality and functional outcome in patients with aneurysmal subarachnoid hemorrhage. World Neurosurg. 2019;121:e500–10.
Lublinsky S, Major S, Kola V, Horst V, Santos E, Platz J, et al. Early blood-brain barrier dysfunction predicts neurological outcome following aneurysmal subarachnoid hemorrhage. EBioMedicine. 2019;43:460–72.
Dijkland SA, Foks KA, Polinder S, et al. Prognosis in moderate and severe traumatic brain injury: a systematic review of contemporary models and validation studies. J Neurotrauma. 2019.
Lukić S, Ćojbasić Ž, Perić Z, et al. Artificial neural networks based early clinical prediction of mortality after spontaneous intracerebral hemorrhage. Acta Neurol Belg. 2012;112(4):375–82.
Alawieh A, Zaraket F, Alawieh MB, Chatterjee AR, Spiotta A. Using machine learning to optimize selection of elderly patients for endovascular thrombectomy. J Neurointerv Surg. 2019;11(8):847–51.
Witsch J, Kuohn L, Hebert R, et al. Early Prognostication of 1-year outcome after subarachnoid hemorrhage: the fresh score validation. J Stroke Cerebrovasc Dis. 2019;104280.
Fahey M, Crayton E, Wolfe C, Douiri A. Clinical prediction models for mortality and functional outcome following ischemic stroke: a systematic review and meta-analysis. PLoS One. 2018;13(1):e0185402.
Ntaios F, Ferrari L, Vemmos M. An integer-based score to predict functional outcome in acute ischemic stroke: the ASTRAL score. Neurology. 2012;78(24):1916–22.
Witsch J, Frey HP, Patel S, Park S, Lahiri S, Schmidt JM, et al. Prognostication of long-term outcomes after subarachnoid hemorrhage: the FRESH score. Ann Neurol. 2016;80(1):46–58.
Hernandes Rocha TA, Elahi C, Cristina da Silva N, et al. A traumatic brain injury prognostic model to support in-hospital triage in a low-income country: a machine learning-based approach. J Neurosurg. 2019:1–9.
•• Edlow BL, Chatelle C, Spencer CA, et al. Early detection of consciousness in patients with acute severe traumatic brain injury. Brain. 2017;140(9):2399-2414. Using fMRI to distinguish patients with cognitive motor dissociation from unresponsive patients, EEG features were also analysed (although not found to be significant) using support vector machine with linear kernel on a small subacute traumatic brain injured population.
Weijer C, Bruni T, Gofton T, Young GB, Norton L, Peterson A, et al. Ethical considerations in functional magnetic resonance imaging research in acutely comatose patients. Brain : a journal of neurology. 2016;139(Pt 1):292–9.
Dumont TM, Rughani AI, Tranmer BI. Prediction of symptomatic cerebral vasospasm after aneurysmal subarachnoid hemorrhage with an artificial neural network: feasibility and comparison with logistic regression models. World Neurosurg. 2011;75(1):57–63 discussion 25-58.
Dumont TM. Prospective assessment of a symptomatic cerebral vasospasm predictive neural network model. World Neurosurg. 2016;94:126–30.
Megjhani M, Terilli K, Frey HP, et al. Incorporating high-frequency physiologic data using computational dictionary learning improves prediction of delayed cerebral ischemia compared to existing methods. Front Neurol. 2018;9:122.
Park S, Megjhani M, Frey HP, et al. Predicting delayed cerebral ischemia after subarachnoid hemorrhage using physiological time series data. J Clin Monit Comput. 2018.
Tanioka S, Ishida F, Nakano F, et al. Machine learning analysis of matricellular proteins and clinical variables for early prediction of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Mol Neurobiol. 2019.
Skoch J, Tahir R, Abruzzo T, Taylor JM, Zuccarello M, Vadivelu S. Predicting symptomatic cerebral vasospasm after aneurysmal subarachnoid hemorrhage with an artificial neural network in a pediatric population. Childs Nerv Syst. 2017;33(12):2153–7.
Roederer A, Holmes JH, Smith MJ, Lee I, Park S. Prediction of significant vasospasm in aneurysmal subarachnoid hemorrhage using automated data. Neurocrit Care. 2014;21(3):444–50.
Ramos LA, van der Steen WE, Sales Barros R, Majoie CBLM, van den Berg R, Verbaan D, et al. Machine learning improves prediction of delayed cerebral ischemia in patients with subarachnoid hemorrhage. J Neurointerv Surg. 2019;11(5):497–502.
Ramos LA, van der Steen WE, Sales Barros R, et al. Machine learning improves prediction of delayed cerebral ischemia in patients with subarachnoid hemorrhage. J Neurointerv Surg. 2018.
Kagiyama N, Sugahara M, Crago EA, et al. Neurocardiac injury assessed by strain imaging is associated with in-hospital mortality in patients with subarachnoid hemorrhage. JACC Cardiovasc Imaging. 2019.
Murad M, Farhad K, Kalijah T, Park S. Heart rate variability as a biomarker of neurocardiogenic injury after subarachnoid hemorrhage. In. .
Jaja BNR, Schweizer TA, Claassen J, et al. The SAFARI score to assess the risk of convulsive seizure during admission for aneurysmal subarachnoid hemorrhage. Neurosurgery. 2018;82(6):887–93.
Du B, Xiong W, Wu J, Zhang L, Tao D. Stacked convolutional denoising auto-encoders for feature representation. IEEE Trans Cybern. 2017;47(4):1017–27.
Bengio Y, Courville A, Vincent P. Representation learning: a review and new perspectives. IEEE Trans Pattern Anal Mach Intell. 2013;35(8):1798–828.
Ribeiro MT, Singh S, Guestrin C. Why should i trust you?: explaining the predictions of any classifier. Paper presented at: Proceedings of the 22nd ACM SIGKDD international conference on knowledge discovery and data mining2016.
•• Donald R, Howells T, Piper I, et al. Forewarning of hypotensive events using a Bayesian artificial neural network in neurocritical care. J Clin Monit Comput. 2019;33(1):39–51. Bayesian artificial neural network model was able to predict episodes of hypotension in neurocritical care patients, using minute-to-minute blood pressure and heart rate.
Yu Y, Guo D, Lou M, Liebeskind D, Scalzo F. Prediction of hemorrhagic transformation severity in acute stroke from source perfusion MRI. IEEE Trans Biomed Eng. 2018;65(9):2058–65.
Chan KL, Leng X, Zhang W, et al. Early identification of high-risk TIA or minor stroke using artificial neural network. Front Neurol. 2019;10:171.
Gultepe E, Green JP, Nguyen H, Adams J, Albertson T, Tagkopoulos I. From vital signs to clinical outcomes for patients with sepsis: a machine learning basis for a clinical decision support system. J Am Med Inform Assoc. 2014;21(2):315–25.
Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O'Neal PV, Keane KA, et al. The Richmond agitation–sedation scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–44.
Nagaraj SB, McClain LM, Zhou DW, Biswal S, Rosenthal ES, Purdon PL, et al. Automatic classification of sedation levels in ICU patients using heart rate variability. Crit Care Med. 2016;44(9):e782–9.
Sun H, Nagaraj SB, Akeju O, Purdon PL, Westover BM. Brain monitoring of sedation in the intensive care unit using a recurrent neural network. Conf Proc IEEE Eng Med Biol Soc. 2018;2018:1–4.
Sanz-García A, Pérez-Romero M, Pastor J, Sola RG, Vega-Zelaya L, Vega G, et al. Potential EEG biomarkers of sedation doses in intensive care patients unveiled by using a machine learning approach. J Neural Eng. 2019;16(2):026031.
Bricolo A, Turazzi S, Faccioli F, Odorizzi F, Sciaretta G, Erculiani P. Clinical application of compressed spectral array in long-term EEG monitoring of comatose patients. Electroencephalogr Clin Neurophysiol. 1978;45(2):211–25.
Karnaze DS, Marshall LF, Bickford RG. EEG monitoring of clinical coma: the compressed spectral array. Neurology. 1982;32(3):289–92.
Johansen AR, Jin J, Maszczyk T, Dauwels J, Cash SS, Westover MB. EPILEPTIFORM spike detection via convolutional neural networks. Proc IEEE Int Conf Acoust Speech Signal Process. 2016;2016:754–8.
Thomas J, Comoretto L, Jin J, Dauwels J, Cash SS, Westover MB. EEG classification via convolutional neural network-based interictal epileptiform event detection. Conf Proc IEEE Eng Med Biol Soc. 2018;2018:3148–51.
Bagheri E, Jin J, Dauwels J, Cash S, Westover MB. A fast machine learning approach to facilitate the detection of interictal epileptiform discharges in the scalp electroencephalogram. J Neurosci Methods. 2019.
Shoeb A, Kharbouch A, Soegaard J, Schachter S, Guttag J. A machine-learning algorithm for detecting seizure termination in scalp EEG. Epilepsy & behavior: E&B. 2011;22(Suppl 1):S36–43.
Struck AF, Rodriguez-Ruiz AA, Osman G, Gilmore EJ, Haider HA, Dhakar MB, et al. Comparison of machine learning models for seizure prediction in hospitalized patients. Ann Clin Transl Neurol. 2019;6(7):1239–47.
Swinburne NC, Schefflein J, Sakai Y, et al. Machine learning for semi-automated classification of glioblastoma, brain metastasis and central nervous system lymphoma using magnetic resonance advanced imaging. Ann Transl Med. 2019;7(11):232.
Scalzo F, Xiao H. Semi-supervised detection of intracranial pressure alarms using waveform dynamics. Physiol Meas. 2013;34(4):465–78. https://doi.org/10.1088/0967-3334/34/4/465 IOP Publishing, Accessed 27 Mar 2020.
Kim HC, Rhim JK, Ahn JH, et al. Machine learning application for rupture risk assessment in small-sized intracranial aneurysm. J Clin Med. 2019;8(5).
Rosenthal ES, Biswal S, Zafar SF, et al. Continuous electroencephalography predicts delayed cerebral ischemia after subarachnoid hemorrhage: A prospective study of diagnostic accuracy. Ann Neurol. 2018;83(5):958–69.
Baumgartner C, Koren JP. Seizure detection using scalp-EEG. Epilepsia. 2018;59(Suppl 1):14–22.
Gotman J. Automatic seizure detection: improvements and evaluation. Electroencephalogr Clin Neurophysiol. 1990;76(4):317–24.
Pauri F, Pierelli F, Chatrian GE, Erdly WW. Long-term EEG-video-audio monitoring: computer detection of focal EEG seizure patterns. Electroencephalogr Clin Neurophysiol. 1992;82(1):1–9.
Gabor AJ, Leach RR, Dowla FU. Automated seizure detection using a self-organizing neural network. Electroencephalogr Clin Neurophysiol. 1996;99(3):257–66.
Gabor AJ. Seizure detection using a self-organizing neural network: validation and comparison with other detection strategies. Electroencephalogr Clin Neurophysiol. 1998;107(1):27–32.
Wilson SB, Scheuer ML, Emerson RG, Gabor AJ. Seizure detection: evaluation of the reveal algorithm. Clin Neurophysiol. 2004;115(10):2280–91.
Saab ME, Gotman J. A system to detect the onset of epileptic seizures in scalp EEG. Clin Neurophysiol. 2005;116(2):427–42.
Kuhlmann L, Burkitt AN, Cook MJ, Fuller K, Grayden DB, Seiderer L, et al. Seizure detection using seizure probability estimation: comparison of features used to detect seizures. Ann Biomed Eng. 2009;37(10):2129–45.
Meier R, Dittrich H, Schulze-Bonhage A, Aertsen A. Detecting epileptic seizures in long-term human EEG: a new approach to automatic online and real-time detection and classification of polymorphic seizure patterns. J Clin Neurophysiol. 2008;25(3):119–31.
Kelly KM, Shiau DS, Kern RT, Chien JH, Yang MC, Yandora KA, et al. Assessment of a scalp EEG-based automated seizure detection system. Clin Neurophysiol. 2010;121(11):1832–43.
Hopfengärtner R, Kerling F, Bauer V, Stefan H. An efficient, robust and fast method for the offline detection of epileptic seizures in long-term scalp EEG recordings. Clin Neurophysiol. 2007;118(11):2332–43.
Hopfengärtner R, Kasper BS, Graf W, Gollwitzer S, Kreiselmeyer G, Stefan H, et al. Automatic seizure detection in long-term scalp EEG using an adaptive thresholding technique: a validation study for clinical routine. Clin Neurophysiol. 2014;125(7):1346–52.
Minasyan GR, Chatten JB, Chatten MJ, Harner RN. Patient-specific early seizure detection from scalp electroencephalogram. J Clin Neurophysiol. 2010;27(3):163–78.
Hartmann MM, Fürbass F, Perko H, et al. EpiScan: online seizure detection for epilepsy monitoring units. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:6096–9.
Fürbass F, Hartmann M, Perko H, et al. Combining time series and frequency domain analysis for a automatic seizure detection. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:1020–3.
Fürbass F, Ossenblok P, Hartmann M, Perko H, Skupch AM, Lindinger G, et al. Prospective multi-center study of an automatic online seizure detection system for epilepsy monitoring units. Clin Neurophysiol. 2015;126(6):1124–31.
Sansevere AJ, Hahn CD, Abend NS. Conventional and quantitative EEG in status epilepticus. Seizure. 2019;68:38–45.
Williamson CA, Wahlster S, Shafi MM, Westover MB. Sensitivity of compressed spectral arrays for detecting seizures in acutely ill adults. Neurocrit Care. 2014;20(1):32–9.
Abend NS, Dlugos DJ, Hahn CD, Hirsch LJ, Herman ST. Use of EEG monitoring and management of non-convulsive seizures in critically ill patients: a survey of neurologists. Neurocrit Care. 2010;12(3):382–9.
Swisher CB, Sinha SR. Utilization of quantitative EEG trends for critical care continuous EEG monitoring: a survey of neurophysiologists. J Clin Neurophysiol. 2016;33(6):538–44.
Vespa PM, Nuwer MR, Juhász C, Alexander M, Nenov V, Martin N, et al. Early detection of vasospasm after acute subarachnoid hemorrhage using continuous EEG ICU monitoring. Electroencephalogr Clin Neurophysiol. 1997;103(6):607–15.
Claassen J, Hirsch LJ, Kreiter KT, du EY, Connolly ES, Emerson RG, et al. Quantitative continuous EEG for detecting delayed cerebral ischemia in patients with poor-grade subarachnoid hemorrhage. Clin Neurophysiol. 2004;115(12):2699–710.
Simpao AF, Ahumada LM, Gálvez JA, Rehman MA. A review of analytics and clinical informatics in health care. J Med Syst. 2014;38(4):45.
Wagshul ME, Eide PK, Madsen JR. The pulsating brain: a review of experimental and clinical studies of intracranial pulsatility. Fluids Barriers CNS. 2011;8(1):5.
Eide PK. A new method for processing of continuous intracranial pressure signals. Med Eng Phys. 2006;28(6):579–87.
Hu X, Xu P, Scalzo F, Vespa P, Bergsneider M. Morphological clustering and analysis of continuous intracranial pressure. IEEE Trans Biomed Eng. 2009;56(3):696–705.
Megjhani M, Alkhachroum A, Terilli K, et al. An active learning framework for enhancing identification of non-artifactual intracranial pressure waveforms. Physiol Meas. 2019;40(1):015002.
Lee SB, Kim H, Kim YT, et al. Artifact removal from neurophysiological signals: impact on intracranial and arterial pressure monitoring in traumatic brain injury. J Neurosurg. 2019:1–9.
Kuang H, Najm M, Chakraborty D, Maraj N, Sohn SI, Goyal M, et al. Automated ASPECTS on noncontrast CT scans in patients with acute ischemic stroke using machine learning. AJNR Am J Neuroradiol. 2019;40(1):33–8.
Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11–21.
Maegerlein C, Fischer J, Mönch S, Berndt M, Wunderlich S, Seifert CL, et al. Automated calculation of the Alberta stroke program early CT score: feasibility and reliability. Radiology. 2019;291(1):141–8.
Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, et al. Bayesian analysis of neuroimaging data in FSL. NeuroImage. 2009;45(1 Suppl):S173–86.
McCoy D, Dupont S, Gros C, Cohen-Adad J, Huie RJ, Ferguson A, et al. Convolutional neural network–based automated segmentation of the spinal cord and contusion injury: deep learning biomarker correlates of motor impairment in acute spinal cord injury. Am J Neuroradiol. 2019;40(4):737–44.
Rodriguez A, Ramsey M, Kohne M, Moberg D. Review of medical device connectivity in neurocritical care. iProc. 2015;1(1):e12.
Vesin A, Azoulay E, Ruckly S, Vignoud L, Rusinovà K, Benoit D, et al. Reporting and handling missing values in clinical studies in intensive care units. Intensive Care Med. 2013;39(8):1396–404.
Little RJ, Rubin DB. Statistical analysis with missing data. 793: John Wiley & Sons; 2019.
Johnson AE, Ghassemi MM, Nemati S, Niehaus KE, Clifton DA, Clifford GD. Machine learning and decision support in critical care. Proc IEEE Inst Electr Electron Eng. 2016;104(2):444–66.
Nouira K, Trabelsi A. Intelligent monitoring system for intensive care units. J Med Syst. 2012;36(4):2309–18.
Imhoff M, Bauer M, Gather U, Löhlein D. Statistical pattern detection in univariate time series of intensive care on-line monitoring data. Intensive Care Med. 1998;24(12):1305–14.
Sivaganesan A, Manley GT, Huang MC. Informatics for neurocritical care: challenges and opportunities. Neurocrit Care. 2014;20(1):132–41.
Vorwerk J, Oostenveld R, Piastra MC, Magyari L, Wolters CH. The FieldTrip-SimBio pipeline for EEG forward solutions. Biomed Eng Online. 2018;17(1):37.
Riley GF. Administrative and claims records as sources of health care cost data. Med Care. 2009;47(7 Suppl 1):S51–5.
Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol. 2014;10(1):44–58.
Witsch J, Bruce E, Meyers E, Velazquez A, Schmidt JM, Suwatcharangkoon S, et al. Intraventricular hemorrhage expansion in patients with spontaneous intracerebral hemorrhage. Neurology. 2015;84(10):989–94.
Lantigua H, Ortega-Gutierrez S, Schmidt JM, et al. Subarachnoid hemorrhage: who dies, and why? Crit Care. 2015;19:309.
NINDS. NINDS Common Data Elements. https://www.commondataelements.ninds.nih.gov/. Accessed July 1, 2019.
Maas AI, Menon DK, Steyerberg EW, Citerio G, Lecky F, Manley GT, et al. Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI): a prospective longitudinal observational study. Neurosurgery. 2015;76(1):67–80.
Yuh EL, Cooper SR, Mukherjee P, Yue JK, Lingsma HF, Gordon WA, et al. Diffusion tensor imaging for outcome prediction in mild traumatic brain injury: a TRACK-TBI study. J Neurotrauma. 2014;31(17):1457–77.
Piper I, Chambers I, Citerio G, Enblad P, Gregson B, Howells T, et al. The brain monitoring with information technology (BrainIT) collaborative network: EC feasibility study results and future direction. Acta Neurochir. 2010;152(11):1859–71.
Gaspard N, Foreman BP, Alvarez V, Cabrera Kang C, Probasco JC, Jongeling AC, et al. New-onset refractory status epilepticus: etiology, clinical features, and outcome. Neurology. 2015;85(18):1604–13.
Hirsch LJ, Brenner RP, Drislane FW, So E, Kaplan PW, Jordan KG, et al. The ACNS subcommittee on research terminology for continuous EEG monitoring: proposed standardized terminology for rhythmic and periodic EEG patterns encountered in critically ill patients. J Clin Neurophysiol. 2005;22(2):128–35.
Critical Care EEG Monitoring Research Consortium. Mission Statement of the CCEMRC. https://www.acns.org/research/critical-care-eeg-monitoring-research-consortium-ccemrc. Published 2019. .
American Heart Association. Precision Medicine Platform. https://precision.heart.org/about. Published 2016. Accessed July 1, 2019.
Alkhachroum AM, Rubinos C, Kummer BR, Parikh NS, Chen M, Chatterjee A, et al. Risk of seizures and status epilepticus in older patients with liver disease. Epilepsia. 2018;59(7):1392–7.
Jalbert JJ, Isaacs AJ, Kamel H, Sedrakyan A. Clipping and coiling of unruptured intracranial aneurysms among Medicare beneficiaries, 2000 to 2010. Stroke. 2015;46(9):2452–7.
Parikh NS, Merkler AE, Jesudian A, Kamel H. Association between cirrhosis and aneurysmal subarachnoid hemorrhage. Ann Clin Transl Neurol. 2019;6(1):27–32.
Knopman J, Link TW, Navi BB, Murthy SB, Merkler AE, Kamel H. Rates of repeated operation for isolated subdural hematoma among older adults. JAMA Netw Open. 2018;1(6):e183737.
Martin A, Chen ML, Chatterjee A, Merkler AE, Chung CD, Wu X, et al. Specialty classifications of physicians who provide neurocritical care in the United States. Neurocrit Care. 2019;30(1):177–84.
Morris NA, May TL, Motta M, Agarwal S, Kamel H. Long-term risk of seizures among cardiac arrest survivors. Resuscitation. 2018;129:94–6.
Morris NA, Chatterjee A, Adejumo OL, Chen M, Merkler AE, Murthy SB, et al. The risk of Takotsubo cardiomyopathy in acute neurological disease. Neurocrit Care. 2019;30(1):171–6.
Saeed M, Villarroel M, Reisner AT, Clifford G, Lehman LW, Moody G, et al. Multiparameter intelligent monitoring in intensive care II: a public-access intensive care unit database. Crit Care Med. 2011;39(5):952–60.
Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3:160035.
Price WN II. Black-box medicine. Harv JL & Tech. 2014;28:419.
Price WN, II. Medical malpractice and black-box medicine. In: Cohen IG, ed. Big Data, Health Law, and Bioethics. Cambridge University Press, Forthcoming; 2017.
Kaji DA, Zech JR, Kim JS, Cho SK, Dangayach NS, Costa AB, et al. An attention based deep learning model of clinical events in the intensive care unit. PLoS One. 2019;14(2):e0211057.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ayham Alkhachroum declares no conflict of interest.
Kalijah Terilli declares no conflict of interest.
Murad Megjhani reports grants from American Heart Assocation Award, during the conduct of the study.
Soojin Park reports grants from National Institutes of Health, during the conduct of the study.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Critical Care Neurology
Rights and permissions
About this article
Cite this article
Alkhachroum, A., Terilli, K., Megjhani, M. et al. Harnessing Big Data in Neurocritical Care in the Era of Precision Medicine. Curr Treat Options Neurol 22, 15 (2020). https://doi.org/10.1007/s11940-020-00622-8
Published:
DOI: https://doi.org/10.1007/s11940-020-00622-8