Abstract
Purpose of review
Medical devices made of substances are widely used in clinical practice, but they are considered to have less therapeutic efficacy than pharmacological agents. In this narrative review, we report a recent RCT experience of a medical device made of natural substances (Poliprotect) that proved as effective as the standard reference treatment.
The RCT addressed three questions: (1) Is Poliprotect useful in the initial treatment of heartburn and epigastric pain/burning as an alternative to the reference standard therapy with PPI, (2) is Poliprotect useful in the medium-term treatment of heartburn and epigastric pain/burning, and (3) is Poliprotect useful in the deprescribing phase of PPI?
Recent findings
Firstly, Poliprotect proved non-inferior to omeprazole for symptom relief in confirmed upper endoscopic negativity; secondly, the benefit remained unaltered after shifting to on-demand intake, with no gut microbiota variation; and thirdly, Poliprotect can be used to deprescribe the PPI treatment, avoiding the symptomatic worsening that accompanies the hyper secretive gastric acid rebound effect.
Summary
A medical device made of natural substances can be as effective as a pharmacological agent in the therapy of upper gastrointestinal symptoms in endoscopy-negative patients. Poliprotect effect started from the first day of treatment to improve in the following 2 weeks and to be maintained unaltered in the following 4 weeks with on-demand regimen. Finally, this medical device of natural substances showed high safety without affecting the gut microbiota.
Similar content being viewed by others
Introduction
About half and two-thirds of the patients complaining of, respectively, heartburn and dyspepsia with no lesions at upper endoscopy do not respond to reference standard PPI treatment [1, 2]. These chronic symptomatic conditions are currently considered disorders with multifactorial pathogenesis involving different mechanisms along the gut-brain axis, including increased epithelial permeability, pro-inflammatory immune responses, altered gastrointestinal motility, and visceral hypersensitivity.
Heartburn in non-erosive reflux disease is due to a reduced epithelial barrier function that alters the mucosal lining permeability. The consequently facilitated contact of the mucosa with irritants, for example, acid, pepsin, or bile acids, in the refluxate triggers the production of reactive oxygen species (ROS) which activates a pro-inflammatory immune response with the secretion of inflammatory mediators (IL8, IL-1β, and TNF) hence heightening the sensitization of peripheral nerves [3].
Several natural herbal medicines have been used for functional upper gastrointestinal symptoms, and a recent Cochrane Review [4] has reported that symptoms of dyspepsia are largely improved by peppermint and caraway oil and moderately improved by Curcuma longa and STW5 (Iberogast). They may improve with Lafoensia pacari, Nigella sativa, artichoke, Boesenbergia rotunda, Pistacia lentiscus, Enteroplant, Ferula asafoetida, ginger, Glycyrrhiza glabra, red pepper, Cudrania tricuspidata, jollab, and Pimpinella anisum. Molecules extracted from many of these herbal products, not different from medicines, act pharmacologically on multiple targets, and none has been demonstrated to improve gastroesophageal reflux symptomatology.
The digestive tract, not different from the skin and the respiratory system, has a fundamental function as a barrier to separate the organism from the outside environment of the lumen and has offered so far a great opportunity for medical devices made of substances (MDMS) that exert beneficial effects acting either within the lumen and/or on the epithelial barrier being, thus, devoid of any pharmacological, metabolic, and immunological effect on the organism.
So far in clinical practice, the main indications of MDMS have been for add-on treatment to pharmacological agents, the substitution of medicines that are either ineffective or causing unbearable side effects, and in several conditions in which medical treatment is not available or cannot be utilized. One or more of the abovementioned limitations of the medical agents often occur in the treatment of chronic or recurrent diseases that, by definition, defy an ultimate resolution. In addition, chronic diseases usually have multifactorial pathogenetic factors that may not be tackled alone by a single medicine that, by acting on one or few receptors, would limit its effect on a restricted number of the many underlying pathogenetic factors. Differently from pharmacological agents, an MDMS does not have a receptor effect, and, moreover, those made of natural substances, can be made of complex constituents which can synergically act on several different pathogenetic mechanisms.
Probably, the best example of a chronic gastroenterological condition in which MDMSs find a useful indication is for heartburn and functional dyspeptic symptoms.
MDMS that adhere to the gastrointestinal luminal epithelium—mucosal protective agents (MPA)—reinforce the mucosal barrier. In the esophagus and stomach, protect the epithelium from acid and non-acid luminal components. MPAs improve gastroesophageal reflux [5•] and FD symptoms [6] when added to a standard PPI treatment, and a recently published randomized controlled trial (RCT) has shown for the first time that an MPA made of natural substance, Poliprotect, is able to treat heartburn and epigastric pain or burning in upper endoscopy-negative patients [7••]. This review focuses on the recent updates on a medical device of natural substances in the therapy of upper gastrointestinal symptoms.
Poliprotect in the therapy of upper gastrointestinal symptoms
Poliprotect is a 100% natural product containing a polysaccharide fraction composed of a synergic molecular complex from Aloe vera, Malva sylvestris, and Althaea officinalis and of mineral limestone and nahcolite, embedded within the polysaccharides, and a flavonoid fraction from Glycyrrhiza glabra and Matricaria recutita. Such MDMS with the polysaccharide fraction adheres and strengthens the esophagogastric epithelium, with the antacids it buffers the acidic milieu within the polysaccharide, and with the flavonoid fraction exerts an anti-inflammatory and antioxidant effect. Poliprotect significantly decreased ethanol- and indomethacin-induced gastric mucosa lesions and damage to esophageal mucosal integrity induced by acid-pepsin-bile solution, as assessed by transepithelial electrical resistance and the ulcerogenic index, respectively. It also maintained 36% mucoadhesivity for at least 2 h and counteracted the oxidative stress induced by 2,2′-azobis(2-amidinopropane) dihydrochloride, in vitro, ([8], and unpublished proprietary data from the product’s technical dossier). This medical device made from natural substances, and hence with more components acting in synergy, enables to create a complex compound whose final action, to be regarded as non-pharmacological, has a protective epithelial barrier, as well as antacid and antioxidant effect.
The protocol of the RTC
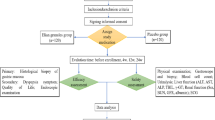
The protocol of this trial (Fig. 1) has been envisaged to clarify three relevant clinical questions. (1) Is Poliprotect useful in the initial treatment of heartburn and epigastric pain/burning as an alternative to the reference standard therapy with PPI, (2) is Poliprotect useful in the medium-term treatment of heartburn and epigastric pain/burning, and (3) is Poliprotect useful in the deprescribing phase of PPI? The omeprazole arm of the study protocol has been conceived so to mimic a frequently used real-world therapeutic sequence for the treatment of NERD and functional dyspeptic patients, i.e., omeprazole 20 mg qd for several days until symptom remission followed by an antireflux or an antacid on demand. Thus, the study assessed the role of Poliprotect (NeoBianacid®, 1.55 g, Aboca, Sansepolcro, Italy) as compared to the standard reference PPI therapy in the initial and medium-term treatment of heartburn and/or epigastric pain or burning in endoscopy-negative patients. It also enabled to assess the role of Poliprotect when PPI is deprescribed [7••].
Poliprotect vs omeprazole. Protocol of the controlled, randomized, double-blind, double-dummy trial after a 2-week screening [7••]. Pol, Poliprotect; OM, omeprazole; P, placebo; on D, on demand.
After a 2-week screening/washout period (V-1 to V0), 275 subjects who met the selection criteria were randomized, ensuring a 1:1 ratio, into either the PPI group (PPI verum + Poliprotect placebo) or the Poliprotect group (PPI placebo + Poliprotect verum) for a double-blind 4-week period (V0–V2). During this randomized, double-blind 4-week period, the Poliprotect treatment schedule was different in the first 2 weeks (V0–V1) compared to the following 2 weeks (V1–V2). In the first 2-week period of the protocol, the study assessed whether Poliprotect was not inferior to PPI, omeprazole 20 mg per day 30 min before breakfast, and the timing of onset of the symptomatic response. During this period, one tablet of Poliprotect had to be taken five times a day (30 min after breakfast, lunch, and dinner; midafternoon; before going to bed).
Findings of the RCT
Pain scores assessed as VAS were moderately severe and not significantly different between Poliprotect and PPI groups at baseline and, subsequently, promptly decreased already at 1, 3, and 7 days, and after 2 weeks of treatment, the mean VAS score was in the low severity range (Fig. 2a). Overall, the statistical analysis showed that Poliprotect was not inferior to the standard daily dose of PPI in the initial treatment of heartburn and epigastric pain or burning.
a VAS variation and use of rescue medicine from V0 to V1 during the first 2 weeks of the double-blind treatment during which Poliprotect was taken five times per day and omeprazole 20 mg per day. b VAS variation and use of rescue medicine from V1 to V2 during the third and fourth weeks of the double-blind treatment during which Poliprotect was taken on demand and omeprazole 20 mg per day. c VAS variation and use of rescue medicine in the 4 weeks during which patients of both arms used Poliprotect on demand.
In the following 2 weeks of the protocol, PPI therapy, omeprazole 20 mg per day, continued unaltered, whereas Poliprotect intake was on demand, defined as the product intake necessary to reach the healthy state of V1.
The benefit of Poliprotect improved further in the following 2 weeks of on-demand intake of 2–3 tablets per day, whereas the PPI benefit was maintained with a statistically significant greater intake of antacid rescue medicine (Magaldrate oral gel) as compared to the Poliprotect group (Fig. 2b). This result indicates that, whereas the PPI treatment diminishes its favorable effects after 2 weeks of treatment, Poliprotect maintains and improves its beneficial response even after reducing the daily dosage from 5 to 2–3 tablets per day.
For the remaining 4-week period (V2–V3) of the protocol, the blinding was removed, and all patients were administered Poliprotect verum only, on demand. In this phase of the study, Poliprotect showed to maintain, and still slightly improve, the benefit in both the patients who had started with Poliprotect therapy and in those who started with PPI treatment. The latter group, however, as expected for the acid rebound effect occurring at PPI withdrawal, maintained the symptomatic benefit with a statistically significant increase of antacid rescue medicine (Fig. 2c).
The outcome of these three consecutive periods of the trial responds to the three main clinical questions for which the study was made, and it appears to be of great interest for the practical clinical implication in the management of these patients. Firstly, Poliprotect can be prescribed in alternative to PPI as first-line treatment of heartburn and epigastric pain or burning, in the absence of red flags or in confirmed upper endoscopic negativity. Secondly, an initial beneficial treatment with Poliprotect can be maintained and progressively improved with an on-demand intake of 2–3 tablets per day for at least 6 weeks. Thirdly, of great relevance is also the observation that Poliprotect can be used to deprescribe the PPI treatment, avoiding the symptomatic worsening that accompanies the hyper secretive gastric acid rebound effect, a condition that often induces the patients to continue or resume PPI treatment.
The protocol also included the assessment of the intestinal microbiota by collecting the feces before starting and after 4 weeks of treatment. As compared to Poliprotect, which did not affect the intestinal microbiota, a significantly higher degree of microbiota variability from V0 to V2 was found in the PPI group with respect to the Bray–Curtis dissimilarity distance: in particular, a significant enrichment of the oral cavity species Streptococcus salivarius and Streptococcus sinensis and a significant increase over time in the relative abundance of Haemophilus parainfluenzae, Streptococcus dentisani, Streptococcus parasanguinis, and Veillonella dispar (p < 0.0001 for each) were observed in the PPI group.
The study confirmed [9, 10] that PPI treatment is associated with an increased abundance of oral cavity genera in the intestinal microbiota and, for the first time, shows that a microbiota change can take place after 4 weeks with the 20 mg daily dose of omeprazole in a patient population. The different effect of Poliprotect and PPI on the microbiota reflects the different modality the two products contrast gastric acidity. PPI reduces the secretion of acid and its antibacterial action on the ingested oral microbiota that can then colonize the intestine.
Unlike the hyposecretive action of PPIs, the buffering activity of Poliprotect is exerted by the bicarbonate minerals within the complex vegetable matrix adhered to the epithelial lining, without affecting the amount of intraluminal acid secretion and its antibacterial effect. It would therefore appear that Poliprotect mimics the esophagogastric mucus where the hydrogen ions moving from the lumen towards the epithelium meet and are buffered by bicarbonate ions moving from the epithelium towards the lumen.
Heartburn and epigastric pain/burning often overlap with other dyspeptic and intestinal symptoms, and this association was confirmed in the patients of the RCT. It is notable that the improvement in heartburn and epigastric pain/burning obtained with Poliprotect, not differently from PPI treatment, was accompanied by a parallel improvement in the associated dyspeptic and intestinal symptoms during 6 weeks of on-demand treatment with 2–3 tablets/day.
The trend in improvement throughout the treatment period was continuously progressive in the Poliprotect arm for all efficacy variables. The overall more favorable effect of Poliprotect as compared to PPI, which limits its effect on acid reduction, is likely due to an MDMS that provides the mucosa with a complex, mucus-like, adherent, antioxidant, pH-buffering matrix, thus limiting the stimulation of acid, bile, and other luminal sensitizers on the gastroesophageal epithelium. In large-scale surveys [11•], aimed at the post-marketing surveillance of Poliprotect, 3471 physicians and 848 patients did not report any serious AEs. In addition, physicians largely reported good tolerability in the Poliprotect-treated population, which included pregnant women and children. Post-market vigilance data reported an incidence rate < 1/10.000 of non-serious gastrointestinal and skin adverse effects of Poliprotect and no serious adverse effects. Based on the results of the mentioned Poliprotect vs omeprazole trial and considering such a high safety level, it is conceivable that Poliprotect might be used as first-line treatment for heartburn and epigastric pain/burning in patients without red flags and to substitute PPI in those conditions in which they are not recommended (Table 1).
Conclusions
In conclusion, starting from the first day of treatment, a medical device made of natural substances proved non-inferior to omeprazole in the relief of heartburn, epigastric pain, and burning in the initial 2 weeks, and even better on demand (on average 2–3 tablets/day) compared to omeprazole in the subsequent 2 weeks. In addition, Poliprotect on demand counteracted the predictable worsening of symptoms that follows the suspension of PPI treatment. Furthermore, the MPA is a 100% natural product and therefore biodegradable by definition, with no impact on the environment [12], and in the present RCT, it showed high safety without affecting the gut microbiota.
Data Availability
Data are available upon request to Enrico Stefano Corazziari.
Change history
23 March 2024
A Correction to this paper has been published: https://doi.org/10.1007/s11938-024-00444-6
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Fass R, Sifrim D. Management of heartburn not responding to proton pump inhibitors. Gut. 2009;58(2):295–309.
Moayyedi P, Soo S, Deeks J, Delaney B et al. Pharmacological interventions for non–ulcer dyspepsia. Cochrane Database Syst Rev. 2011;2011(2):CD001960. https://doi.org/10.1002/14651858.CD001960.pub4.
Fass R, Boeckxstaens GE, El-Serag H, Rosen R, Sifrim D, Vaezi MF. Gastro-oesophageal reflux disease. Nat Rev Dis Primers. 2021;7(1):55. https://doi.org/10.1038/s41572-021-00287-w.
Báez G, Vargas C, Arancibia M, et al. Non-Chinese herbal medicines for functional dyspepsia. Cochrane Database Syst Rev. 2023;(6). Art. No.: CD013323. https://doi.org/10.1002/14651858.CD013323.pub2.
Savarino V, Pace F, Scarpignato C. Randomised clinical trial: mucosal protection combined with acid suppression in the treatment of non-erosive reflux disease - efficacy of Esoxx, a hyaluronic acid-chondroitin sulphate based bioadhesive formulation. Aliment Pharmacol Ther. 2017;45:631–42. This RTC demonstrated that the synergistic effect of a MPA with PPI treatment suggests that mucosal protection added to acid suppression could improve symptoms and quality of life in patients with non-erosive gastroesophageal reflux.
Ko SJ, Park J, Kim MJ, et al. Effects of the herbal medicine Rikkunshito, for functional dyspepsia: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2021;36:64–74.
Corazziari ES, Gasbarrini A, D’Alba L et al. Poliprotect vs omeprazole in the relief of heartburn, epigastric pain, and burning in patients without erosive esophagitis and gastroduodenal lesions. A randomized controlled trial. Am J Gastroenterol. 2023. https://doi.org/10.14309/ajg.0000000000002360. This recent published RTC demonstrated for the first time that an MPA made of natural substances, Poliprotect, is able to treat heartburn and epigastric pain or burning in upper endoscopy-negative patients.
Liguor,i G, Baldi, F, Di Simone MP. Mo1175 - Topical protection of esophageal mucosa: in vitro evaluation of a medical device made of natural substances in comparison with sodium alginate. Gastroenterology. 2018;154(6):S–696. https://doi.org/10.1016/S0016-5085(18)32456-9.
Shi YC, Cai ST, Tian YP, et al. Effects of proton pump inhibitors on the gastrointestinal microbiota in gastroesophageal reflux disease. Genomics Proteomics Bioinforma. 2019;17:52–63.
Imhann F, Bonder MJ, Vich Vila A, et al. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:740–8.
Cioeta R, Muti P, Rigoni M, et al. Effectiveness and tolerability of Poliprotect, a natural mucosal protective agent for gastroesophageal reflux disease and dyspepsia: surveys from patients, physicians, and pharmacists. Front Drug Saf Regul. 2022;2. In these large-scale surveys, aimed at the post-marketing surveillance of Poliprotect, did not report any serious AEs of the MPA.
EMA. Guideline on the environmental risk assessment of medicinal products for human use EMEA/CHMP/SWP/4447/00 Rev. 1 [pdf] 2018 [cited 2022 june]; Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-environmental-risk-assessment-medicinal-products-human-use-revision-1_en.pdf.
Author information
Authors and Affiliations
Contributions
Conceptualization, writing, review, and editing the manuscript text, figures, and table: ESC and ER; supervision: ESC. The authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Enrico Stefano Corazziari reports personal fees from Aboca S.p.A., personal fees from Coloplast S.p.A., personal fees from Sofar S.p.A., personal fees from GE HealthCare, outside the submitted work. Emanuela Ribichini reports personal fees from Aboca S.p.A. outside the submitted work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The paper should be open access.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Corazziari, E.S., Ribichini, E. Therapy of Upper Gastrointestinal Symptoms: Experience with a Medical Device Made of Natural Substances. Curr Treat Options Gastro 22, 15–22 (2024). https://doi.org/10.1007/s11938-024-00440-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11938-024-00440-w