Abstract
Purpose of review
This review outlines the relationship between sleep and the GI tract in health, before appraising the association between sleep and the GI tract in disease, namely disorders of gut-brain interaction (DGBI). We aim to explore whether sleep disturbance exacerbates DGBI symptoms or vice versa, and summarise the evidence for pharmacological and psychological treatment options.
Recent findings
Sleep disorders are more common in patients with irritable bowel syndrome (IBS) compared to healthy subjects, with a pooled prevalence of 37.6%. Sufficient evidence exists to support the use of melatonin to ameliorate overall IBS symptom severity and improve quality of life.
Summary
DGBIs are stress-sensitive disorders and simple lifestyle advice is recognised as first-line management. Sleep, a cornerstone of lifestyle management, appears to be the forgotten factor. Sleep disturbance (both duration and quality) has been associated with DGBI, namely IBS; however, further studies are required to determine whether treatment options targeted at sleep can lead to GI symptom improvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As humans, we spend approximately one-third of our lives asleep [4]. It is widely known that sleep serves a unique physiological function, without which we cannot survive [5]. The gastrointestinal (GI) tract obeys circadian rhythms and there is a known interplay between sleep disturbance and GI symptoms [1] which can be clearly understood through the lens of gut-brain interactions.
Disorders of gut-brain interaction (DGBI) are a group of conditions classified by chronic GI symptoms in any part of the GI tract, where the underlying aetiology relates to any combination of motility disturbance, altered mucosal and immune function, altered visceral function (e.g. hypersensitivity), altered gut microbiota and altered central or autonomic nervous system processing [6]. There are 33 DGBI spanning 6 regions of the GI tract, with no single cure. Globally, up to 40% of adults meet criteria for at least one DGBI with a female predominance [7]. In the UK, patients with DGBI have greater healthcare utilisation, GI medication use and surgical interventions [8]. One in three people with DGBI have multiple GI organ regions involved and this correlates with increased non-GI somatic symptoms, and reduced mental and physical quality of life [8].
Despite the high prevalence of DGBI and associated healthcare burden, pharmacological treatment options remain limited. This may be due to heterogeneity in the underlying aetiology of DGBIs and their complex pathophysiology. Irritable bowel syndrome (IBS) is the most common DGBI, affecting 5% of the global population [7] and accounts for one-third of all GI complaints presenting to UK primary care [9]. The IBS Global Impact Report (2018) [10] highlights that patients feel treatment efficacy is approximately 50–60%, with existing therapies only benefiting a subset of patients and side-effects precluding their long-term use. Patients with DGBI are twice as likely to resort to prescription pain medication than the general population [11] of which opiates represent an immediate threat to GI motility and function [12].
In the recently published British Society of Gastroenterology guidelines for IBS [13], first-line management is simple lifestyle advice. Although exercise, diet and relaxation are specifically discussed, sleep is not mentioned. If lifestyle advice fails, the algorithm points towards symptom-targeted pharmacological therapies where there is a high risk of reporting adverse events [14] resulting in non-compliance or drug discontinuation. Sleep is a cornerstone of lifestyle medicine [15] and appears to be the forgotten factor in DGBI management. The underlying mechanisms involved in the aetiology of DGBI cross-over with that (or those?) which drives sleep disturbance—i.e. immune, hormonal and nervous system disruption. Therefore, there is further scope and arguably, a clinical imperative, to explore the ways in which sleep interacts with these common and burdensome disorders.
This review will firstly outline the relationship between sleep and the GI tract in health, before appraising the association between sleep and the GI tract in disease, namely DGBI. We aim to explore whether sleep disturbance exacerbates DGBI symptoms or vice versa, and summarise the evidence for pharmacological and psychological treatment options.
Sleep and the GI tract in health
Sleep can be defined as “a complex reversible behavioural state whereby an individual is perceptually disengaged from and unresponsive to their environment” [16]. This complexity is characterised not only by the underlying physiology of the sleep state but also by the array of physiological functions that sleep serves.
Normal sleep is defined by physiological parameters characterised by sleep studies, the most important of which is sleep efficiency (i.e. ratio of total sleep time to time in bed reported as a percentage) where normal is 85–90% [17]. Sleep disorders, of which there are more than 80 recognised, are conditions that disturb normal sleep patterns. Major types include insomnia, sleep-related breathing disorders (e.g. snoring and sleep apnoea), sleep-related movement disorders (e.g. restless leg syndrome), hypersomnia (which includes narcolepsy), circadian rhythm disorders and parasomnia.
Why we sleep is a fundamental question which has historically been regarded as scientifically elusive. Broadly speaking, there appears to be a metabolic cost of the waking state, universal to all creatures, such that energy conservation and repair must occur during the night. The sleep-wake state is a circadian rhythm—circa diem in Latin meaning “about a day” denoting a roughly 24-h cycle. It is governed by the suprachiasmatic nucleus (SCN) in the anterior hypothalamus—also known as our central circadian clock [18]. The SCN is aligned with light-dark cues from our external environment, whereby light detected via our optic retina regulates melatonin production by the pineal gland to initiate and maintain sleep. Yet circadian rhythms are intrinsic to nearly every aspect of our physiology—from cells to organs, and manifest as peripheral circadian clocks. The SCN communicates with peripheral clocks via neural and hormonal pathways such that all our biological functions operate roughly on a 24-h cycle, which assists in their performance at the most optimal time of day.
The GI tract obeys circadian rhythms
Every aspect of the GI tract displays circadian rhythmicity, from intestinal permeability to microbiota composition and GI motility. Beginning at the mouth, salivary production reduces by 50% during the night compared to the day [19, 20] and eating your main meal later in the evening shifts the diversity of the oral microbiome [21]. During sleep, there is a decreased number of oesophageal swallows [22] which can predispose susceptible individuals to nocturnal acid reflux [23]. Gastric emptying half-times have been shown to be significantly longer in the evening for solids but not liquids, when compared to morning [24]. More recently, the use of an ambulatory telemetric capsule system has demonstrated that reduced amplitude of gastric contractions correlates with depth of sleep [25]. There is a significant reduction in the activity of the enteric migrating motor complex during sleep [26] (waves of electrical/contractile activity that govern peristalsis) although this does not appear to correlate with depth of sleep [25]. Furthermore, basal colonic tone and contractile activity are suppressed during sleep [25, 27] such that nocturnal bowel movements are a recognised sign of organic GI disease [28]. Morning awakening, or indeed forced arousal from sleep, enhances the frequency of colonic contractions [27].
Beyond the profound inhibitory effect of sleep on GI motility, microbiota also display endogenous circadian rhythmicity [29]. The gut microbiota has a significant role to play in gut-brain interactions and the bi-directional cross-talk between the circadian clock and microbiota has been shown to play a role in metabolism and neural processing, as well as disease initiation an progression [30].
Gut-brain interactions
The GI tract contains the highest source of melatonin in the body, up to 400 times that of the pineal gland, produced by the enterochromaffin cells of GI mucosa [31]. This neuroendocrine hormone is responsible for sleep initiation and maintenance, yet plays a significant role in modulating the gut microbiota [32] and regulating GI motility [33], implicating its role in GI health. More recently, the gut-secreted neuropeptide CCHa1 has been identified as a mediator of circadian intercellular communications in fruit flies [34]. Interestingly, protein-rich diets are shown to potentiate CCHa1 secretion, thereby modulating brain activity during sleep [35].
The hypothalamic-pituitary-adrenal (HPA) axis is another key neuroendocrine pathway by which gut-brain interactions occur, whereby cortisol is liberated from the adrenal glands in response to stressful stimuli. Chronic stress alters gut-brain function via the downstream effects of a dysregulated HPA axis e.g. disruption of intestinal barrier integrity and subsequent increased intestinal permeability [36], which is implicated in the pathophysiology of DGBI. Sleep deprivation, which is considered a form of chronic stress, has been associated with high cortisol levels and maladaptive changes in the HPA axis [37, 38]. Lastly, both branches of the autonomic nervous system regulate gut function and are dysregulated by altered sleep-wake cycles [39, 40], also known as chronodisruption.
Chronodisruption
The most intuitive examples of acute circadian rhythm disruption in health are jet lag (also known as jet lag disorder) and shift work. Travellers’ constipation is both an anecdotal and studied phenomenon in healthy subjects [41]. Alterations in light-dark environmental cues have a direct impact on GI function by increasing intestinal permeability [42] and an indirect impact via changes to our food choices and eating patterns. The risk of developing IBS in shift workers has been linked to a western dietary pattern i.e. high consumption of processed food and snacks [43] as well as irregular eating habits [44, 45]—both of which are promoted by altered sleep-wake cycles. A recent meta-analysis has concluded that IBS was found to be 81% higher among shift workers than among non-shift workers [46].
Since the mechanisms governing sleep and GI health are inextricably linked, the implications of sleep disturbance in GI disease can thus be further examined. For the purposes of this review, we will briefly touch upon gastro-oesophageal reflux disease but focus primarily on the two most common DGBIs—functional dyspepsia (FD) and irritable bowel syndrome (IBS).
Sleep and the GI tract in disease
Gastro-oesophageal reflux disease (GORD)
There appears to be a bidirectional relationship between sleep disturbance and reflux symptoms. In cross-sectional studies, there is an association between daytime GORD and shorter sleep duration [47] as well as insomnia [48]. In a randomised cross-over study, sleep deprivation (1 night of <3 h sleep) has been proven to worsen acid perfusion sensitivity scores in patients with established erosive oesophagitis compared to healthy controls [49]. Further studies are needed to establish whether this experimentally induced oesophageal sensitivity has implications for patients with reflux hypersensitivity (i.e. sensitivity to physiological acid reflux) or functional heartburn (i.e. oesophageal sensitivity in the absence of physiological acid reflux). However, symptoms may also relate to eating patterns, as one study demonstrated that a dinner-to-bed time of less than 3 h is associated with 7.5 times increased likelihood of nocturnal GORD [50]. This study supports the current lifestyle advice we offer patients with nocturnal symptoms or hiatal hernias, to eat their dinner earlier in the evening.
Functional dyspepsia (FD)
Functional dyspepsia is defined as a premature sensation of fullness, discomfort, pain and/or early satiety following meals. A recent large scale population study in Australia (n = 1322) has concluded a 1.8 times increased likelihood of sleep disturbance in FD patients (both epigastric pain syndrome and postprandial distress syndrome subtypes) compared to the general population [51]—independent of age, gender and psychological distress. The prevalence of sleep disturbance is more frequent among FD patients who report co-morbid heartburn and/or regurgitation [52]. Subjectively reported sleep disturbance is also more common in patients with FD and co-morbid IBS compared to IBS alone [53]. This highlights the potential utility of sleep management in those patients with multiple DGBI who, as previously mentioned, suffer the worst quality of life.
Irritable bowel syndrome (IBS)
Sleep disturbance is more common in IBS patients compared to healthy subjects, with a pooled prevalence of 37.6% [2]. On average, IBS patients sleep more hours per day but feel less well-rested compared to healthy controls [54]. This may be explained by significantly higher sleep fragmentation associated with arousal and a shift to lighter sleep stages [55]. Patel et al. found IBS patients demonstrated more waking episodes during sleep and this was associated with greater abdominal pain and gastrointestinal distress, negatively correlated with general and IBS-specific QOL [54]. Notably, only 2.4% of patients in this study attributed their sleep disturbances to gastrointestinal symptoms implying a reverse causal relationship.
Buchanan et al. reported that insomnia significantly predicted next-day symptom exacerbation of abdominal pain, anxiety and fatigue in IBS patients with no impact on bowel habit. Interestingly, temporally-reversed analysis found no significant ability of IBS symptoms to predict sleep quality (within-subjects), suggesting that insomnia is an independent factor affecting GI symptoms [56]. Patients with sleep apnoea have been shown to have a four times increased odds of having IBS, although larger scale studies are required to confirm this association [57].
In the first study to measure the relationship between real-time reported GI symptoms and sleep quality in 80 IBS patients, Topan et al report that poor subjective sleep quality was associated with higher next day lower GI symptoms levels, but not vice versa. Objective sleep measures did not predict next day abdominal symptoms, potentially supporting the conclusion that it is the perception of sleep quality which is most influential [58].
Anorectal sensory-motor function has also been studied in a small group of IBS patients, whereby patients with sleep dysfunction were identified to have lower thresholds for urge rectal sensation and maximal anal sphincter squeeze pressure compared to those without sleep dysfunction [59].
Sleep and GI symptom management
Pharmacological treatment options (Table 1)
Short-term pharmacological treatment options for sleep disturbance include benzodiazepines (e.g. lorazepam) and benzodiazepine receptor agonists (e.g. zopiclone and zolpidem). Their mechanism of action as γ-amino butyric acid (GABA) receptor agonists is to modulate the central and autonomic nervous system, thereby influencing the gut-brain axis [60]. Benzodiazepines have been studied in the context of GI symptoms where anxiety predominates and were found to be safe and effective [61]. Yet despite their effective use as sedating medications, the risks of drug-tolerance, drug-dependency and their negative impact on sleep architecture precludes their long-term use [62]. There are no studies to date which have investigated the impact of these medications on the association between sleep quality and DGBI symptoms.
Long-term pharmacological treatment options include antidepressant medications and melatonin. A recent meta-analysis confirms that antidepressants (low-dose tricyclic antidepressants e.g. amitriptyline, and selective serotonin reuptake inhibitors e.g. citalopram) are efficacious in reducing symptoms of IBS patients; however, no specific sleep outcomes have been measured in randomised control trials [63]. Further studies are needed to assess whether these drugs, which are known to be sedating in nature, exert any of their impact on GI symptoms via sleep-related mechanisms. An up-to-date meta-analysis has concluded that sufficient evidence exists to support the use of melatonin to ameliorate overall IBS symptom severity and improve quality of life—see Table 1 for a summary of the studies included [3]. Probiotics such as VSL#3 have also been studied in relation to their potential mechanism of action in IBS via increasing salivary melatonin production [64]. The antioxidant ellagic acid (EA), a polyphenol found in fruits and nuts, has been studied as an oral supplement with the potential to impact sleep quality [65].
Psychological treatment options (Table 2)
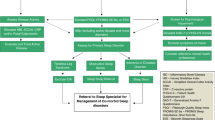
Cognitive behavioural therapy (CBT) and gut-directed hypnotherapy appear effective in the treatment of IBS [63]. CBT for insomnia (CBT-I) has also been trialled in over 118 studies, 87 of which were randomised controlled trials and demonstrates improvements in insomnia severity, sleep efficiency and subjective sleep quality [66]. One study has looked at the effect of CBT-I in college students with IBS and co-morbid insomnia, with favourable outcomes in both sleep and GI symptom scores [67]. A recent pilot study looked at brief behavioural therapy for insomnia (BBT-I) in IBS patients and concluded that it was a feasible intervention. Although there was no statistically significant improvement in IBS symptoms scores, 40% of the BBT-I group reported clinically meaningful symptom reduction vs. 17% of the control group [68] (Fig. 1).
Sleep and gut brain interactions in health and disease. GBI, gut-brain interactions; DGBI, disorders of gut brain interaction; SCN, suprachiasmatic nucleus; HPA, hypothalamic-pituitary axis; ANS, autonomic nervous system; IBS, irritable bowel syndrome; FD, functional dyspepsia; GI, gastrointestinal.
Digital delivery of CBT-I is as efficacious as face-to-face treatment [69] which has prompted the development of easily accessible smartphone applications [70]. Since digital CBT for IBS is also a validated treatment [71], the addition of sleep advice to easily accessible psychotherapy tools ought to be the subject of further studies.
Conclusions
DGBI are stress-sensitive disorders where simple lifestyle advice is recognised as first-line management. Sleep, a cornerstone of lifestyle management, appears to be the forgotten factor. Normal GI physiology is inextricably linked to sleep-wake cycles such that chronodisruption can generate GI symptoms. Sleep disturbance (both duration and quality) has been associated with DGBI, namely IBS and FD, however further studies are required to determine whether treatment options targeted at sleep can lead to GI symptom improvement.
References and Recommended Reading
Orr WC, et al. The effect of sleep on gastrointestinal functioning in common digestive diseases. Lancet Gastroenterol Hepatol. 2020;5(6):616–24.
Wang B, Duan R, Duan L. Prevalence of sleep disorder in irritable bowel syndrome:a systematic review with meta-analysis. Saudi J Gastroenterol. 2018;24(3):141–50.
Chen KH, et al. The efficacy of exogenous melatonin supplement in ameliorating irritable bowel syndrome severity: a meta-analysis of randomized controlled trials. J Formos Med Assoc. 2023;122(3):276–85.
Colten HR, Altevogt BM. Sleep physiology. In: Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. National Acadamies Press (US); 2006.
Worley SL. The extraordinary importance of sleep: the detrimental effects of inadequate sleep on health and public safety drive an explosion of sleep research. Pharmacol Ther. 2018;43(12):758–63.
Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterol. 2016;150:1262–1279.e2.
Sperber AD, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome Foundation global study. Gastroenterol. 2021;160(1):99–114.e3.
Simons J, et al. Disorders of gut-brain interaction: highly prevalent and burdensome yet under-taught within medical education. United European Gastroenterol J. 2022;10(7):736–44.
Thompson WG, et al. Irritable bowel syndrome in general practice: prevalence, characteristics, and referral. Gut. 2000;46(1):78–82.
IGIR. IBS Global Impact Report. 2018; https://badgut-5q10xayth7t3zjokbv.netdna-ssl.com/wp-content/uploads/IBS-Global-Impact-Report.pdf
Luo Y, et al. Global patterns of prescription pain medication usage in disorders of gut-brain interactions. Neurogastroenterol Motil. 2022;35:e14457.
Sahota S, Fortun P. Disorders of gut-brain interaction and the long-term risks of opioids. Br J Hosp Med (Lond). 2019;80(10):C150–c154.
Vasant DH, et al. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut. 2021;70(7):1214–40.
Barberio B, et al. Adverse events in trials of licensed drugs for irritable bowel syndrome with constipation or diarrhea: systematic review and meta-analysis. Neurogastroenterol Motil. 2022;34(6):e14279.
Dedhia P, Maurer R. Sleep and health-a lifestyle medicine approach. J Fam Pract. 2022;71(Suppl 1 Lifestyle):S30–s34.
Carskadon WC, Dement MA. Normal human sleep: an overview. In: Principles and practice of sleep medicine. Fifth ed. Elsevier Saunders; 2011. p. 16–26.
Lichstein KL, et al. Quantitative criteria for insomnia. Behav Res Ther. 2003;41(4):427–45.
Mieda M. The central circadian clock of the suprachiasmatic nucleus as an ensemble of multiple oscillatory neurons. Neurosci Res. 2020;156:24–31.
Dawes C. Circadian rhythms in human salivary flow rate and composition. J Physiol. 1972;220(3):529–45.
Schneyer LH, et al. Rate of flow of human parotid, sublingual, and submaxillary secretions during sleep. J Dent Res. 1956;35(1):109–14.
Collado MC, et al. Timing of food intake impacts daily rhythms of human salivary microbiota: a randomized, crossover study. FASEB J. 2018;32(4):2060–72.
Sato K, Nakashima T. Human adult deglutition during sleep. Ann Otol Rhinol Laryngol. 2006;115(5):334–9.
Kanaly T, Shaheen NJ, Vaughn BV. Gastrointestinal physiology and digestive disorders in sleep. Curr Opin Pulm Med. 2009;15(6):571–7.
Goo RH, et al. Circadian variation in gastric emptying of meals in humans. Gastroenterol. 1987;93(3):515–8.
Haase AM, et al. Gastrointestinal motility during sleep assessed by tracking of telemetric capsules combined with polysomnography - a pilot study. Clin Exp Gastroenterol. 2015;8:327–32.
Kumar D, et al. Relationship between enteric migrating motor complex and the sleep cycle. Am J Phys. 1990;259(6 Pt 1):G983–90.
Scott SM. Manometric techniques for the evaluation of colonic motor activity: current status. Neurogastroenterol Motil. 2003;15(5):483–513.
Sood R, et al. Enhancing diagnostic performance of symptom-based criteria for irritable bowel syndrome by additional history and limited diagnostic evaluation. Am J Gastroenterol. 2016;111(10):1446–54.
Paulose JK, et al. Human gut bacteria are sensitive to melatonin and express endogenous circadian rhythmicity. PLoS One. 2016;11(1):e0146643.
Gutierrez Lopez DE, et al. Circadian rhythms and the gut microbiome synchronize the host's metabolic response to diet. Cell Metab. 2021;33(5):873–87.
Bubenik GA, Brown GM, Grota LJ. Immunohistological localization of melatonin in the rat digestive system. Experientia. 1977;33(5):662–3.
Iesanu MI, et al. Melatonin-microbiome two-sided interaction in dysbiosis-associated conditions. Antioxidants. 2022;11(11):2244.
Merle A, et al. Effect of melatonin on motility pattern of small intestine in rats and its inhibition by melatonin receptor antagonist S 22153. J Pineal Res. 2000;29(2):116–24.
Fujiwara Y, et al. The CCHamide1 Neuropeptide expressed in the anterior dorsal neuron 1 conveys a circadian signal to the ventral lateral neurons in drosophila melanogaster. Front Physiol. 2018;9:1276.
Titos I, et al. A gut-secreted peptide suppresses arousability from sleep. Cell. 2023;186(7):1382–1397.e21.
Creekmore AL, et al. Chronic stress-associated visceral hyperalgesia correlates with severity of intestinal barrier dysfunction. Pain. 2018;159(9):1777–89.
Vgontzas AN, Chrousos GP. Sleep, the hypothalamic-pituitary-adrenal axis, and cytokines: multiple interactions and disturbances in sleep disorders. Endocrinol Metab Clin N Am. 2002;31(1):15–36.
Hirotsu C, Tufik S, Andersen ML. Interactions between sleep, stress, and metabolism: from physiological to pathological conditions. Sleep Sci. 2015;8(3):143–52.
Castro-Diehl C, et al. Sleep duration and quality in relation to autonomic nervous system measures: the multi-ethnic study of atherosclerosis (MESA). Sleep. 2016;39(11):1927–40.
Buijs RM, Escobar C, Swaab DF. The circadian system and the balance of the autonomic nervous system. Handb Clin Neurol. 2013;117:173–91.
Mearin F, et al. Traveler's constipation. Am J Gastroenterol. 2003;98(2):507–9.
Tran L, et al. Circadian misalignment by environmental light/dark shifting causes circadian disruption in colon. PLoS One. 2021;16(6):e0251604.
Buscail C, et al. Western dietary pattern is associated with irritable bowel syndrome in the French NutriNet cohort. Nutrients. 2017;9(9):986.
Bavani NG, et al. The relationship between meal regularity with irritable bowel syndrome (IBS) in adults. Eur J Clin Nutr. 2022;76:1315–22.
Nilholm C, et al. Irregular dietary habits with a high intake of cereals and sweets are associated with more severe gastrointestinal symptoms in IBS patients. Nutrients. 2019;11(6):1279.
Wang N, et al. Impact of shift work on irritable bowel syndrome and functional dyspepsia: a meta-analysis. Medicine. 2022;101(25):e29211.
Fujiwara Y, Arakawa T, Fass R. Gastroesophageal reflux disease and sleep disturbances. J Gastroenterol. 2012;47(7):760–9.
Jansson C, et al. A population-based study showing an association between gastroesophageal reflux disease and sleep problems. Clin Gastroenterol Hepatol. 2009;7(9):960–5.
Schey R, et al. Sleep deprivation is hyperalgesic in patients with gastroesophageal reflux disease. Gastroenterol. 2007;133(6):1787–95.
Fujiwara Y, et al. Association between dinner-to-bed time and gastro-esophageal reflux disease. Am J Gastroenterol. 2005;100(12):2633–6.
Koloski NA, et al. Sleep disturbances in the irritable bowel syndrome and functional dyspepsia are independent of psychological distress: a population-based study of 1322 Australians. Aliment Pharmacol Ther. 2021;54(5):627–36.
Vakil N, et al. Sleep disturbance due to heartburn and regurgitation is common in patients with functional dyspepsia. United European Gastroenterol J. 2016;4(2):191–8.
Elsenbruch S, et al. Behavioral and physiological sleep characteristics in women with irritable bowel syndrome. Am J Gastroenterol. 2002;97(9):2306–14.
Patel A, et al. Effects of disturbed sleep on gastrointestinal and somatic pain symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44(3):246–58.
Rotem AY, et al. Polysomnographic and actigraphic evidence of sleep fragmentation in patients with irritable bowel syndrome. Sleep. 2003;26(6):747–52.
Buchanan DT, et al. Sleep measures predict next-day symptoms in women with irritable bowel syndrome. J Clin Sleep Med. 2014;10(9):1003–9.
Ghiasi F, et al. Association of irritable bowel syndrome and sleep apnea in patients referred to sleep laboratory. J Res Med Sci. 2017;22:72.
Topan R, Vork L, Fitzke H, Pandya S, Keszthelyi D, Cornelis J, Ellis J, Van Oudenhove L, Van Den Houte M, Aziz Q. Poor subjective sleep quality predicts symptoms in irritable bowel syndrome using the experience sampling method. Am. J. Gastroenterol. 2023. https://doi.org/10.14309/ajg.0000000000002510
Chen CL, et al. Evidence for altered anorectal function in irritable bowel syndrome patients with sleep disturbance. Digestion. 2011;84(3):247–51.
Salari P, Abdollahi M. Systematic review of modulators of benzodiazepine receptors in irritable bowel syndrome: is there hope? World J Gastroenterol. 2011;17(38):4251–7.
Balon R, Sonino N, Rafanelli C. Benzodiazepines' role in managing gastrointestinal disorders. Psychother Psychosom. 2021;90(2):81–4.
Ashton H. The diagnosis and management of benzodiazepine dependence. Curr Opin Psychiatry. 2005;18(3):249–55.
Ford AC, et al. Effect of antidepressants and psychological therapies in irritable bowel syndrome: an updated systematic review and meta-analysis. Am J Gastroenterol. 2019;114(1):21–39.
Wong RK, et al. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig Dis Sci. 2015;60(1):186–94.
Mirzaie Z, et al. Improving effect of ellagic acid on sleep quality and gastrointestinal symptoms in patient with irritable bowel syndrome: randomized double-blind clinical trial. Turk J Gastroenterol. 2021;32(11):937–44.
van Straten A, et al. Cognitive and behavioral therapies in the treatment of insomnia: a meta-analysis. Sleep Med Rev. 2018;38:3–16.
Yang YY, Jun S. The effects of cognitive behavioral therapy for insomnia among college students with irritable bowel syndrome: a randomized controlled trial. Int J Environ Res Public Health. 2022;19(21):14174.
Ballou S, et al. Brief behavioral therapy for insomnia in patients with irritable bowel syndrome: a pilot study. Dig Dis Sci. 2020;65(11):3260–70.
Zachariae R, et al. Efficacy of internet-delivered cognitive-behavioral therapy for insomnia - A systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2016;30:1–10.
Yu JS, et al. Smartphone apps for insomnia: examining existing apps' usability and adherence to evidence-based principles for insomnia management. Transl Behav Med. 2019;9(1):110–9.
Everitt H, et al. Therapist telephone-delivered CBT and web-based CBT compared with treatment as usual in refractory irritable bowel syndrome: the ACTIB three-arm RCT. Health Technol Assess. 2019;23(17):1–154.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Rabia Topan declares that she has no conflict of interest.
S. Mark Scott declares that he has no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
New and Interesting Findings
• The gastrointestinal (GI) tract obeys circadian rhythms and there is a known interplay between sleep disturbance and GI symptoms [1].
• Sleep disorders are more common in IBS patients compared to healthy subjects, with a pooled prevalence of 37.6% [2].
• Sufficient evidence exists to support the use of melatonin to ameliorate overall IBS symptom severity and improve quality of life [3].
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Topan, R., Scott, S.M. Sleep: An Overlooked Lifestyle Factor in Disorders of Gut-Brain Interaction. Curr Treat Options Gastro 21, 435–446 (2023). https://doi.org/10.1007/s11938-023-00433-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11938-023-00433-1