Abstract
Purpose of review
This review article aims to provide an overview of existing and emerging screening strategies for gastric cancer and discuss potential measures to improve its efficacy in countries with different risk profiles for the disease.
Recent findings
Recent developments in endoscopic technology, including image enhancement and computer-guided diagnosis, can improve further early cancer detection in countries with primary endoscopic screening. Moreover, accumulating data on upper endoscopy quality underlines the pivotal role of appropriate examination time and the vital role of premedication (sedation, defoaming agents, and antispasmodics). In regions with low-to-intermediate incidence, the “test-and-treat” approach for H. pylori infection seems feasible for gastric cancer screening and prevention. Possibly, a family-based approach to test for the condition within family households could further improve the efficacy of this approach. In addition, other non-invasive methods to identify at-risk individuals are being evaluated, such as breath testing, serological markers, and circulating tumor cells.
Summary
Gastric cancer screening practices vary widely globally based on incidence, local healthcare facilities, and funding. However, wide-ranged screening programs for gastric cancer may be feasible even in countries with low-to-intermediate incidence if the approach is tailored towards the local disease burden and focused on the pre-selection of at-risk individuals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The global incidence of gastric cancer (GC) has been dynamically changing within the last several decades. On one hand, GC incidence has seen a uniform decrease: a recent populational study estimated a continued reduction of 2–3% in GC incidence rates annually across five continents between 1998 and 2012 [1]. Indeed, in many regions, GC may begin to fall into the rare disease category [2]. On the other hand, however, the burden of GC remains high, with over 1 million estimated new cases in 2020 [3]. Moreover, a modeling study across 34 countries showed an alarming trend of increasing GC incidence within younger age groups (<50 years) [2].
These varying trends may be generally associated with the heterogenous nature of GC, including the critical distinction between non-cardia gastric cancer (NCGC; the overall dominant subtype [80%]) and cardia gastric cancer (CGC, constituting ~20%) [4]. While decreasing rates of Helicobacter pylori (H. pylori) infection and economic improvements have likely contributed to a corresponding decrease in NCGC incidence, increasing obesity and gastroesophageal reflux disease worldwide may be the cause for a continued rise in proximally located GC. Another element which should not be overlooked is the constantly changing definition and classification systems of gastroesophageal junction tumors [5•].
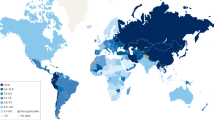
Regardless of its subtype, GC remains a highly lethal disease. It is the 3rd most common cause of cancer-related death worldwide [6] with an age-standardized 5-year survival range of 20–40% in most countries [7]. The reason for this poor survival rate is mainly due to advanced-stage disease at clinical presentation. Screening programs for GC may potentially improve those outcomes by identifying the disease in a premalignant or early stage, which would be amenable for minimally invasive endoscopic treatment [8]. However, due to its highly diverse epidemiology, a uniform strategy to screen and prevent GC does not seem feasible. This article discusses how potential strategies for GC screening may be improved in countries with different risk profiles for GC (Fig. 1).
Gastric cancer screening worldwide
Population-based screening programs for GC have been ongoing for over 20 years in Japan and South Korea. Both countries are characterized by a high incidence of GC, with an age-standardized rate (ASR) of 31.6 and 27.9 in 2020, respectively [9].
Japan launched its GC screening program in 1983, initially based on the upper gastrointestinal series (UGIS) [10, 11]. However, subsequent data began to show the favorable effect of primary endoscopic screening. For example, a community-based, case-control study evaluating mortality reduction in GC through endoscopic screening in 4 Japanese cities demonstrated a 30% reduction in GC mortality (odds ratio (OR): 0.70, 95% confidence interval (CI): 0.49–0.99) [12]. As a result of the accumulated data, endoscopy screening was accepted as a primary screening modality in Japan in 2016 and currently addresses citizens over 50 years of age [13].
South Korea began its screening program in 1999, and since 2005, it has offered UGIS or upper endoscopy every 2 years to individuals aged 40 and older [14,15,16]. A recent report showed a > 20% reduction in GC mortality within the screened population as compared to non-screened individuals (OR 0.79; 95% CI: 0.77–0.81) [17]. Overall, interest in the screening program is also growing, with an average increase of ~4% in yearly participation rate [18] (51.9% in 2016 [19]).
Targeted screening
While primary endoscopy screening may be beneficial in regions with a high incidence of GC, population screening is neither feasible nor cost-effective in countries with low-intermediate incidence [20]. However, a targeted screening approach focusing on at-risk individuals may improve the efficacy of GC screening in such regions. Since H. pylori remains the primary risk factor for GC worldwide, accounting for ~90% of NCGC’s global burden [21], it is naturally recognized as a primary target of GC prevention strategies [22•].
“Test-and-treat” approach
The “test-and-treat” strategy for H. pylori infection has been established in several countries and has decreased GC incidence in some regions. A previous meta-analysis of randomized controlled trials postulated that eradication therapy reduced the incidence of GC (RR = 0.54; 95% CI 0.40–0.72) and GC-related mortality (RR = 0.61; 95% CI 0.40–0.92) in healthy Asian individuals [23]. Moreover, a recent report has shown that mass screening and eradication of H. pylori in the Matsu Islands of Taiwan has yielded an as yet statistically non-significant 25% reduction in the GC mortality rate (95% CI 14–51%, P = 0.18). However, if the trend continues, a significant reduction of 39% (95% CI 12–57%, P = .007) could be achieved by 2025 in this region [24].
In Western countries, the “test-and-treat” approach is commonly used as a workup for patients with dyspeptic symptoms [25••]. However, it is increasingly recognized as a feasible approach for GC prevention on a populational level. To illustrate this point, the recent European Union (EU) cancer screening recommendations for 2025 extended the existing screening programs to include GC and follow the screen-and-test strategies for H. pylori [26]. However, several challenges related to this concept must be addressed, including the growing H. pylori antibiotic resistance and possible adverse effects of wide-range antibiotic use.
The existing programs for testing and treating H. pylori infection are mostly individual-based. However, recently a family-based testing model has been discussed as a potentially more effective strategy for infection control.
Family-based strategy
H. pylori infection is known to occur in family-household clusters, and interfamilial transmission has been suggested as an important source for its spread [27, 28]. Therefore, identifying at-risk individuals within families could further increase the effectiveness of GC screening and prevention. The “family-based strategy” concept involves testing for H. pylori within the family members of each individual who tested positive for infection. If the selected individual tests negative, no further testing of family members is required. This way, the number of screened participants may be reduced, and the effectiveness of screening increases.
In a recent large-scale national study in China, a urea breath test (UBT) survey on 10,735 families showed family-based infection rates ranging from 50.3 to 85.1% [28]. A positive result for H. pylori in family members was found to be a significant risk factor for infection. For example, five infected family members increased the risk of household infection nearly three-fold (OR 2.72, 95% CI 1.86 to 4.00). Furthermore, a supplement modeling study on the same dataset by Zhang J et al. has shown that a “family-based” strategy would identify up to 12% more of H. pylori-infected individuals as compared to an individual “test-and-treat” approach [29]. Therefore, adopting a “family-based” strategy may identify infected individuals more rapidly and decrease the prevalence of H. pylori within a given population more effectively than the current individual-based random testing for infection. Such a strategy may be particularly appealing to economically developed countries with a high burden of GC, such as Central and Eastern Europe [29].
Non-invasive testing
Identifying at-risk individuals before endoscopy in a non-invasive and cost-effective way may be the key to improved GC screening. For example, several emerging non-endoscopic methods seek to identify patients with premalignant gastric conditions (atrophic gastritis (AG) and gastric intestinal metaplasia (GIM)) [30].
Serological markers
Pepsinogens are enzymes produced by the gastric mucosa and released into the gastric lumen and peripheral circulation. Pepsinogen I (PGI) is secreted mainly by chief cells in the fundic glands, whereas pepsinogen II (PGII) is secreted by pyloric cells [31]. When atrophic changes develop in the gastric body, levels of PGI decrease while PGII levels remain stable. Therefore, a low serum pepsinogen level or PGI/PGII ratio can be a helpful marker for atrophic gastritis. Pepsinogen testing may be further combined into a serological assay with gastrin-17 and H. pylori antibodies. In a meta-analysis including 20 studies (4241 participants), this panel test showed a sensitivity of 74.7% (95% CI, 62.0–84.3) and a specificity of 95.6% (95% CI, 92.6–97.4) for the detection of AG [32]. So far, the suboptimal sensitivity of pepsinogen testing remains a limitation for wide-ranged screening use. In particular, the British Society of Gastroenterology (BSG) does not recommend using serological biomarkers as a screening tool within the UK [33]. However, this assay’s non-invasiveness and high specificity make this method appealing for triage.
Volatile organic compounds
Several breath tests are established in medical diagnostics, such as breathalyzers for ethanol detection, carbon-13 UBT for H. pylori infection, and hydrogen-methane testing in small intestinal bacterial overgrowth. Generally, breath testing (e-nose) technology uses electrical interfaces to measure the subtle carbon-containing volatile organic compounds (VOC) in exhaled air. This technology is up-and-coming for mass screening as it is non-invasive and may be conducted within primary care.
Several pilot studies have tested E-nose accuracy in detecting different types of cancer, including GC [34]. In a meta-analysis of 52 studies (3677 patients) on breath-test technology, the overall sensitivity and specificity for cancer detection amounted to 79% (95% CI, 77–81%) and 89% (95% CI, 88–90%), respectively. As expected, a high heterogeneity among studies was noted due to varying cancer origins, different measuring technologies used for VOC detection, and generally small sample sizes [34].
Within GC screening specifically, a VOC marker detection-based nanoarray technology has been promising in detecting precancerous conditions of the stomach [35]. This breath test could differentiate patients with severe GIM (Operative Link on Gastric Intestinal Metaplasia (OLGIM) stage III–IV [36]) from patients with normal gastric mucosa (OLGIM 0), with a respective specificity and sensitivity of 83% and 90%. The study’s authors postulate that the breath test could supplement endoscopy as a follow-up tool for the surveillance of high-risk patients. With its ease of use and high safety profile, E-Nose technology is an attractive tool for populational screening to identify at-risk individuals for GC. However, further standardized validation studies are required before its implementation into mass use.
Liquid biopsy
Following the formation of cancer, cells are shed from the primary tumor and fall into blood circulation. Recent years have seen an expansion in new methods for detecting circulating tumor markers based on whole-genome sequencing or DNA methylation patterns, enabling a real-time “liquid biopsy” of cancer. Among the many potential candidates, circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), and extracellular vesicles (EV) have been, so far, the most extensively investigated [37]. These studies fall beyond GC diagnosis and encompass several other clinical applications, such as predicting prognosis, monitoring early recurrence, and facilitating targeted treatment [38]. In addition, several of these tests have been combined into a single simultaneous liquid biopsy for multi-cancer screening, which aims to identify cancer occurrence and subtype. However, tests of this type remain limited concerning early cancer diagnosis.
For example, surface-enhanced Raman spectroscopy (SERS) was recently combined with artificial intelligence (AI) to simultaneously diagnose multiple cancer types (lung, breast, colon, liver, pancreas, and stomach, including early-stage tumors) by label-free analysis of plasma exosomes. The final integrated decision model showed a sensitivity of 90.2% at a specificity of 94.4% while predicting the tumor organ origin in 72% of positive patients. For early-stage GC alone, the test showed an accuracy of 0.99 (0.98–1.00) [39].
Improving endoscopic detection
High-resolution endoscopy with biopsy remains the gold standard in diagnosing gastric precancerous conditions and early GC. Although characterized by high accuracy in GC detection, it is a highly subjective and operator-dependent procedure, and the rate of missed upper GI neoplasms remains substantial [40,41,42,43,44,45,46,47,48]. Principally, high-quality endoscopy encompasses more than merely procedural aspects and should include pre-procedural measures and appropriate post-procedural care (Fig. 2). Indeed, if all three steps are considered and developed, an overall improvement could be made in the effectiveness of endoscopic GC screening [49].
Premedication
As proper bowel preparation continues to improve the diagnostic yield of colonoscopy, the role of measures undertaken to enhance mucosal visibility in the stomach is increasingly recognized. Two primary factors contribute to poor mucosal visualization in the stomach: residual mucus, bubbles and foam, and contractions of the stomach. Mucolytic and defoaming agents (e.g., pronase, N-acetylcysteine, simethicone) may help with the former, and antispasmodic agents (e.g., cimetropium bromide, scopolamine) aid the latter by suppressing peristalsis. However, very few studies address the utility of premedication in clinical practice.
In a recent Korean screening program study, a retrospective propensity score-matched cohort of 20,835 paired individuals compared the outcomes of patients who either received or did not receive an antispasmodic agent (5 mg of cimetropium bromide i.v.) before gastroscopy [50]. The use of cimetropium bromide as premedication was associated with increased gastric neoplasm detection rates during endoscopic screening (OR 1.6 95% CI, 1.03–2.33; P = .04). Specifically, a more than two-fold higher rate of small gastric neoplasms (< 1 cm) was detected within the antispasmodic receivers (0.12% vs. 0.05%, P =.03). This increased yield in detection was most prominently seen within the gastric body, where a significantly higher rate of lesions was found (0.16% vs. 0.07%, P = .007%). These findings suggest that cimetropium bromide may be considered for premedication before upper endoscopy examination among individuals with no contraindications, especially in high-risk individuals.
Sedation
The clinical objectives of administering sedation for gastroscopy are as follows: to relieve patient anxiety and discomfort and thereby improve the quality of the procedures. However, the standards for the use of sedation are highly dependent upon local healthcare systems and legal frameworks and vary widely worldwide [51, 52]. For example, less than half of endoscopy procedures in Europe are performed under sedation, whereas in the USA, > 90% of endoscopies are performed with sedation. Among the few studies on the beneficial role of sedation, a large retrospective analysis from China compared the outcomes of patients who were examined under propofol-based sedation vs. no sedation. Involving four centers, the study analyzed 432,202 patients undergoing consecutive gastroscopies [53]. Sedation was associated with increased use of image-enhancement techniques (staining, virtual chromoendoscopy, magnification), more biopsies per patient (mean no. of biopsies per patient: 1.23 vs. 1.18, P < .001), and a longer inspection time (16.5 min. vs. 14.7 min.). Combined, these factors translated into higher detection rates of UGI early cancers (mainly flat IIb esophageal lesions) [53].
Inspection time
Procedural time is likely the most extensively studied quality indicator for upper endoscopy. An initial study from Singapore analyzed the association of routine upper GI examination time with detecting precancerous gastric lesions and cancers in the stomach. “Slow” endoscopists (> 7 min mean procedure time) were found more likely to detect high-risk lesions (GIM/AG, dysplasia, and cancers) when compared to “fast” endoscopists (OR 2.50; 95% CI, 1.52–4.12) [54]. The European Society of Gastrointestinal Endoscopy (ESGE) therefore, recommends a minimum 7-min procedural time for initial diagnostic UGI [55].
In a subsequent Korean study, 111,962 patients underwent screening gastroscopy by 14 board-certified endoscopists, 35.9% of which underwent conscious sedation with midazolam. During the first year of the study, the endoscopists were classified as fast and slow based on mean inspection time (withdrawal from the duodenum) for a normal examination without biopsy. A multivariable analysis showed that slower endoscopists were 50% more likely to detect gastric adenomas or carcinomas than fast endoscopists (OR 1.52; 95% CI, 1.17–1.97) [56].
Image-enhanced endoscopy (IEE)
Advanced imaging techniques have been emerging in recent years, with the intent to improve mucosal visualization and enhance fine structural and microvascular architecture (Fig. 3). The current spectrum of established image-enhancing (IE) techniques includes conventional chromoendoscopy, narrowed-spectrum endoscopy (such as narrow-band imaging NBI; Olympus Medical Systems, Tokyo, Japan) and blue laser imaging (BLI; Fujifilm, Tokyo, Japan), as well as i-Scan digital contrast (I-SCAN; Pentax, Tokyo, Japan), linked color imaging (LCI; Fujifilm, Tokyo, Japan), confocal laser endomicroscopy (CLE), and autofluorescence imaging (AFI) [57]. The state of clinical application of these technologies varies widely; however, several have an established role in routine practice and characterize a high-quality procedure.
For example, virtual chromoendoscopy is broadly used in surveillance premalignant conditions, and NBI may be particularly helpful in highlighting AG and discrete areas of GIM. Indeed, updated ESGE guidelines on managing epithelial precancerous conditions and lesions in the stomach (MAPS II) recommend that virtual chromoendoscopy (± magnification) be used to guide biopsy selection in diagnosing a premalignant stomach, helping to target the most representative areas for the disease [58].
Additionally, magnifying endoscopy combined with narrow band imaging (NBI) can help to visualize both the microvascular and microsurface patterns. In a meta-analysis including 44 studies on IE endoscopy for gastric preneoplastic conditions, NBI obtained a pooled sensitivity and specificity of 0.79 (95% CI 0.72–0.85) and 0.91 (95% CI 0.88–0.94) on a per-patient basis for detection of GIM. A tubulovillous pattern was the most accurate marker to detect GIM and was effectively assessed without high magnification. For dysplasia, NBI showed a sensitivity and specificity of 0.87 (95% CI 0.84–0.89) and 0.97 (95% CI 0.97–0.98) on per-biopsy basis, respectively. The use of magnification improved the performance of NBI in characterizing early gastric cancer, especially when vessel plus surface classification was applied [59].
Artificial intelligence (AI)
AI platforms using deep-learning algorithms are increasingly incorporated into endoscopy equipment, aiding in detecting and characterizing neoplastic lesions to such an extent that it requires its own review article. However, another exciting branch of AI research is quality control: providing real-time detection and feedback on the inspection process (e.g., identifying the blind spots missed during endoscopic evaluation or indicating low-quality images that require recapturing) may, in effect, aid in improving the quality and standardizing the procedure [37]. For example, an AI system based on deep convolutional neural networks called WISENSE significantly reduced the blind spot rate during an upper endoscopy (5.86% vs. 22.46% in the control group, P < .001), with a mean difference of −15.39% (95% CI −19.23 to −11.54) [60].
Since the standardization and quality assessment of gastroscopy remains challenging, utilizing AI in endoscopic screening appears promising—however, future research before being introduced into an organized screening program is required.
Conclusions
GC screening practices vary widely across the globe, based on incidence as well as local healthcare facilities and funding. The efficacy of screening programs in regions where primary endoscopy remains the standard practice may be improved by incorporating appropriate premedications to the patients, increased use of IE techniques, and operator training (including inspection time guidelines). A promising role of AI is increasingly seen both in improved detection and procedure quality control.
In regions with low-intermediate incidence, the screening programs should be tailored to identify at-risk individuals before endoscopic intervention. The two main risk factors for GC include H. pylori infection and a family history of GC. Focusing screening programs on these individuals could increase the effectiveness of GC screening and, eventually, GC prevention. Accumulating evidence suggests that focusing on “family-based” clusters may improve upon “test-and-treat” strategies. However, this has not yet been studied in Western populations.
In general, GC screening is an elusive research field, and there is room for wide-ranged populational studies. Multiple strategies should be considered, creating a tailored approach to the needs of the local population, the incidence of disease, and the healthcare systems. Only then can further studies improve upon the data collected by each screening program.
Abbreviations
- AG:
-
Atrophic gastritis
- AI:
-
Artificial intelligence
- ASR:
-
Age-standardized rate (incidence)
- CGC:
-
Cardia gastric cancer
- CI:
-
Confidence interval
- CTC:
-
Circulating tumor cell(s)
- ctDNA:
-
Circulating tumor DNA
- EV:
-
Extracellular vesicles
- GC:
-
Gastric cancer
- GIM:
-
Gastric intestinal metaplasia
- NCGC:
-
Non-cardia gastric cancer
- OLGIM:
-
Ooperative Llink on Gastric Iintestinal Mmetaplasia
- OR:
-
Odds ratio
- PG:
-
Pepsinogen (I and II)
- UBT:
-
Urea breath test
- UGIS:
-
Upper gastrointestinal series
- VOC:
-
Volatile organic compounds
References
Papers of particular interest, published recently, have been highlighted as • Of importance •• Of major importance
Li M, Park JY, Sheikh M, Kayamba V, Rumgay H, Jenab M, et al. Population-based investigation of common and deviating patterns of gastric cancer and oesophageal cancer incidence across populations and time. Gut. 2023;163(3):649–658.e2.
Arnold M, Park JY, Camargo MC, Lunet N, Forman D, Soerjomataram I. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut. 69:823.
Morgan E, Arnold M, Camargo MC, Gini A, Kunzmann AT, Matsuda T, et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020–40: a population-based modelling study. EClinicalMedicine. 2022;47
Arnold M, Ferlay J, Van Berge Henegouwen MI. Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. 2020;69:1564–71.
Sugano K, Spechler SJ, El-Omar EM, McColl KEL, Takubo K, Gotoda T, et al. Kyoto international consensus report on anatomy, pathophysiology and clinical significance of the gastro-oesophageal junction. Gut. 2022:1488–514. This article offers a comprehensive summary of current knowledge on the gastroesophageal junction.
Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. 2020;
Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival: analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers during 2000–2014 from 322 population-based registries in 71 countries (CONCORD-3). Lancet. 2018;391:1023.
Suzuki H, Oda I, Abe S, Sekiguchi M, Mori G, Nonaka S, et al. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer. 2016;19:198–205.
Morgan E, Soerjomataram I, Rumgay H, Coleman HG, Thrift AP, Vignat J, et al. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: new estimates from GLOBOCAN 2020. Gastroenterology. 163(3):649–658.e2.
Tsubono Y, Hisamichi S. Screening for gastric cancer in Japan. Gastric Cancer. 2000;3:9–18.
Hamashima C. Cancer screening guidelines and policy making: 15 years of experience in cancer screening guideline development in Japan. Jpn J Clin Oncol. 2018;48:278–86.
Hamashima C, Ogoshi K, Okamoto M, Shabana M, Kishimoto T, Fukao A. A community-based, case-control study evaluating mortality reduction from gastric cancer by endoscopic screening in Japan. PloS One. 2013;8:e79088. https://doi.org/10.1371/journal.pone.0079088.
Hamashima C, Kato K, Miyashiro I, Nishida H, Takaku R, Terasawa T, et al. Update version of the Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol. 2018;48:673–83.
Suh YS, Lee J, Woo H, Shin D, Kong SH, Lee HJ, et al. National cancer screening program for gastric cancer in Korea: Nationwide treatment benefit and cost. Cancer. 2020;126:1929–39.
Lee KS, Oh DK, Han MA, Lee HY, Jun JK, Choi KS, et al. Gastric cancer screening in Korea: report on the national cancer screening program in 2008. Cancer Res Treat. 2011;43:83–8. https://doi.org/10.4143/crt.2011.43.2.83.
Choi KS, Jun JK, Lee HY, Park S, Jung KW, Han MA, et al. Performance of gastric cancer screening by endoscopy testing through the National Cancer Screening Program of Korea. Cancer Sci. 2011;
Jun JK, Choi KS, Lee HY, Suh M, Park B, Song SH, et al. Effectiveness of the Korean National Cancer Screening Program in reducing gastric cancer mortality. Gastroenterology. 2017;152:1319–1328.e7.
Lee S, Jun JK, Suh M, Park B, Noh DK, Jung KW, et al. Gastric cancer screening uptake trends in Korea: results for the National Cancer Screening Program from 2002 to 2011: a prospective cross-sectional study. Medicine. Medicine. 2015;94:e533.
Ryu JE, Choi E, Lee K, Jun JK, Suh M, Jung KW, et al. Trends in the performance of the Korean National Cancer Screening Program for gastric cancer from 2007 to 2016. Cancer Res Treat. 2022;54:842–9.
Januszewicz W, Turkot MH, Malfertheiner P, Regula J. A global perspective on gastric cancer screening: which concepts are feasible, and when? Cancers. 2023;15
Plummer M, Franceschi S, Vignat J, Forman D, De Martel C. Global burden of gastric cancer attributable to pylori. Int J Cancer. 2015;136:487–90.
Liou JM, Malfertheiner P, Lee YC, Sheu BS, Sugano K, Cheng HC, et al. Screening and eradication of Helicobacter pylori for gastric cancer prevention: the Taipei global consensus. Gut. 2020;69:2093–112. Important article which summarizes the role of H. pylori eradication in prevention of gastric cancer
Ford AC, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer: systematic review and meta-analysis. Gut. 2020;69:2113–21.
Chiang TH, Chang WJ, Chen SLS, Yen AMF, Fann JCY, Chiu SYH, et al. Mass eradication of Helicobacter pylori to reduce gastric cancer incidence and mortality: A long-term cohort study on Matsu Islands. Gut. 2021;70:243–50.
Malfertheiner P, Megraud F, Rokkas T, Gisbert JP, Liou J-M, Schulz C, et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022;71:1724–62. The current guidelines on the management of Helicobacter pylori infection worldwide
European Commission 2022 European Health Union: new approach on cancer screening. https://ec.europa.eu/commission/presscorner/detail/en/ip_22_7548
Ding SZ, Du YQ, Lu H, Wang WH, Cheng H, Chen SY, et al. Chinese consensus report on family-based Helicobacter pylori infection control and management (2021 Edition). Gut. 2022;71:238–53.
Zhou XZ, Lyu NH, Zhu HY, Cai QC, Kong XY, Xie P, et al. Large-scale, national, family-based epidemiological study on Helicobacter pylori infection in China: the time to change practice for related disease prevention. Gut. 2023;
Zhang J, Deng Y, Liu C, Wang H, Ren H, Chen S, et al. ‘Family-based’ strategy for Helicobacter pylori infection screening: an efficient alternative to ‘test and treat’ strategy. Gut. 2023; https://doi.org/10.1136/gutjnl-2023-329696.
Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M. A model for gastric cancer epidemiology. The Lancet. 1975;306:58–60.
Samloff IM, Liebman WM. Cellular localization of the group II pepsinogens in human stomach and duodenum by immunofluorescence. Gastroenterology. 1973;65:36–42.
Zagari RM, Rabitti S, Greenwood DC, Eusebi LH, Vestito A, Bazzoli F. Systematic review with meta-analysis: diagnostic performance of the combination of pepsinogen, gastrin-17 and anti-Helicobacter pylori antibodies serum assays for the diagnosis of atrophic gastritis. Aliment Pharmacol Ther. 2017:657–67. https://doi.org/10.1111/apt.14248.
Banks M, Graham D, Jansen M, Gotoda T, Coda S, Di Pietro M, et al. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut. 2019;68:1545–75.
MHMC S, Al-Difaie Z, Brandts L, Peeters A, Van Grinsven B, Bouvy ND. Diagnostic performance of electronic noses in cancer diagnoses using exhaled breath: a systematic review and meta-analysis. JAMA Netw Open. 2022;5:e2219372.
Amal H, Leja M, Funka K, Skapars R, Sivins A, Ancans G, et al. Detection of precancerous gastric lesions and gastric cancer through exhaled breath. Gut, BMJ. 65:400–7.
Yue H, Shan L, Bin L. The significance of OLGA and OLGIM staging systems in the risk assessment of gastric cancer: a systematic review and meta-analysis. Gastric Cancer. 2018:579–87.
Diaz LA, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014:579–86. https://doi.org/10.1200/JCO.2012.45.2011.
Ma S, Zhou M, Xu Y, Gu X, Zou M, Abudushalamu G, et al. Clinical application and detection techniques of liquid biopsy in gastric cancer. Mol Cancer. 2023;22:1–23. https://doi.org/10.1186/s12943-023-01715-z.
Shin H, Choi BH, Shim O, Kim J, Park Y, Cho SK, et al. Single test-based diagnosis of multiple cancer types using exosome-SERS-AI for early stage cancers. Nat Commun. 2023;14:1644.
Chadwick G, Groene O, Riley S, Hardwick R, Crosby T, Hoare J, et al. Gastric cancers missed during endoscopy in England. Clin Gastroenterol Hepatol. 2015;13:1264–1270.e1.
Yalamarthi S, Witherspoon P, McCole D, Auld CD. Missed diagnoses in patients with upper gastrointestinal cancers. Endoscopy. 2004;36:874–9.
Raftopoulos SC, Segarajasingam DS, Burke V, Ee HC, Yusoff IF. A cohort study of missed and new cancers after esophagogastroduodenoscopy. Am J Gastroenterol. 2010;105:1292–7.
Delgado Guillena PG, Morales Alvarado VJ, Jimeno Ramiro M, Rigau Cañardo J, Ramírez Salazar C, García Rodríguez A, et al. Gastric cancer missed at esophagogastroduodenoscopy in a well-defined Spanish population. Dig Liver Dis. 2019;51:1123–9.
FWD T, Wray N, Sidhu R, Hopper A, Mc Alindon M. Factors associated with oesophagogastric cancers missed by gastroscopy: a case-control study. Frontline Gastroenterol. 2020;11:194–201.
Khalil Q, Gopalswamy N, Agrawal S. Missed esophageal and gastric cancers after esophagogastroduodenoscopy in a midwestern military veteran population. South Med J. 2014;107:225–8.
Menon S, Trudgill N. How commonly is upper gastrointestinal cancer missed at endoscopy? A meta-analysis. Endosc Int Open. 2014;2:E46–50. https://doi.org/10.1055/s-0034-1365524.
Rodríguez de Santiago E, Hernanz N, Marcos-Prieto HM, De-Jorge-Turrión MÁ, Barreiro-Alonso E, Rodríguez-Escaja C, et al. Rate of missed oesophageal cancer at routine endoscopy and survival outcomes: a multicentric cohort study. United European Gastroenterol J. 2019;7:189–98. https://doi.org/10.1177/2050640618811477.
Januszewicz W, Witczak K, Wieszczy P, Socha M, Turkot MH, Wojciechowska U, et al. Prevalence and risk factors of upper gastrointestinal cancers missed during endoscopy: a nationwide registry-based study. Endoscopy. 2022;54:653–60.
Januszewicz W, Kaminski MF. Quality indicators in diagnostic upper gastrointestinal endoscopy. Therap Adv Gastroenterol. 2020;13:175628482091669. https://doi.org/10.1177/1756284820916693.
Kim SY, Park JM, Cho HS, Cho YK, Choi MG. Assessment of cimetropium bromide use for the detection of gastric neoplasms during esophagogastroduodenoscopy. JAMA Netw Open. 2022;5:E223827.
Early DS, Lightdale JR, Vargo JJ, Acosta RD, Chandrasekhara V, Chathadi KV, et al. Guidelines for sedation and anesthesia in GI endoscopy. Gastrointest Endosc. 2018;87:327–37. https://doi.org/10.1016/j.gie.2017.07.018.
Dumonceau JM, Riphaus A, Beilenhoff U, Vilmann P, Hornslet P, Aparicio JR, et al. European curriculum for sedation training in gastrointestinal endoscopy: Position statement of the european society of gastrointestinal endoscopy (ESGE) and European society of gastroenterology and endoscopy nurses and associates (ESGENA). Endoscopy. 2013;45:496–504.
Zhou J, Li Z, Ji R, Wang P, Zhang A, Wu K, et al. Influence of sedation on the detection rate of early cancer and precancerous lesions during diagnostic upper gastrointestinal endoscopies: a multicenter retrospective study. Am J Gastroenterol. 2021;116:1230–7.
Teh JL, Tan JR, Lau LJF, Saxena N, Salim A, Tay A, et al. Longer examination time improves detection of gastric cancer during diagnostic upper gastrointestinal endoscopy. Clin Gastroenterol Hepatol. 2015;13:480–487.e2.
Bisschops R, Areia M, Coron E, Dobru D, Kaskas B, Kuvaev R, et al. Performance measures for upper gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy. 2016;48:843–64.
Park JM, Huo SM, Lee HH, Lee B-I, Song HJ, Choi M-G. Longer observation time increases proportion of neoplasms detected by esophagogastroduodenoscopy. Gastroenterology. 2017;153:460–469.e1. https://doi.org/10.1053/j.gastro.2017.05.009.
East JE, Vleugels JL, Roelandt P, Bhandari P, Bisschops R, Dekker E, et al. Advanced endoscopic imaging: European Society of Gastrointestinal Endoscopy (ESGE) Technology Review. Endoscopy. 2016;48:1029–45.
Pimentel-Nunes P, Libânio D, Marcos-Pinto R, Areia M, Leja M, Esposito G, et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Port. Endoscopy. 2019;51:365–88.
Rodríguez-Carrasco M, Esposito G, Libânio D, Pimentel-Nunes P, Dinis-Ribeiro M. Image-enhanced endoscopy for gastric preneoplastic conditions and neoplastic lesions: a systematic review and meta-analysis. Endoscopy. 2020;52:1048–65.
Wu L, Zhang J, Zhou W, An P, Shen L, Liu J, et al. Randomised controlled trial of WISENSE, a real-time quality improving system for monitoring blind spots during esophagogastroduodenoscopy. Gut. 2019;68:2161–9.
Acknowledgements
The first author would like to thank Sophia Munoz for her significant help with the English editing of the text.
Author information
Authors and Affiliations
Contributions
WJ: literature review, drafting the manuscript; MHT: literature review, drafting the manuscript; JR: supervision of the work, drafting the manuscript; All authors have approved the final submitted draft of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
Wladyslaw Januszewicz declares that he has no conflict of interest. Maryla Helena Turkot declares that she has no conflict of interest. Jaroslaw Regula declares that he has no conflict of interest.
Disclaimer
All figures included in the manuscript are original and never published before.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Januszewicz, W., Turkot, M.H. & Regula, J. How to Improve the Efficacy of Gastric Cancer Screening?. Curr Treat Options Gastro 21, 241–255 (2023). https://doi.org/10.1007/s11938-023-00430-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11938-023-00430-4