Abstract
Purpose of Review
3D printing (3DP) technology has emerged as a valuable tool for surgeons and cardiovascular interventionalists in developing and tailoring patient-specific treatment strategies, especially in complex and rare cases. This short review covers advances, primarily in the last three years, in the use of 3DP in the diagnosis and management of heart failure and related cardiovascular conditions.
Recent Findings
Latest studies include utilization of 3DP in ventricular assist device placement, congenital heart disease identification and treatment, pre-operative planning and management in hypertrophic cardiomyopathy, clinician as well as patient education, and benchtop mock circulatory loops.
Summary
Studies reported benefits for patients including significantly reduced operation time, potential for lower radiation exposure, shorter mechanical ventilation times, lower intraoperative blood loss, and less total hospitalization time, as a result of the use of 3DP. As 3DP technology continues to evolve, clinicians, basic science researchers, engineers, and regulatory authorities must collaborate closely to optimize the utilization of 3D printing technology in the diagnosis and management of heart failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Opinion Statement
The integration of 3D printing (3DP) technology in the diagnosis and management of heart failure represents a significant leap in cardiovascular medicine. By harnessing the power of 3DP, medical professionals can create precise, patient-specific models of the heart, enabling enhanced diagnostic accuracy and personalized treatment strategies. One of the most significant advantages of 3DP in heart failure management is its ability to generate detailed anatomical replicas of the heart based on patient-specific imaging data, such as magnetic resonance imaging (MRI) or computed tomography (CT) scans. These 3D printed models provide clinicians with a tangible visualization of the patient's cardiac anatomy, allowing for a deeper understanding of the underlying pathology and facilitating more informed treatment decisions. In the realm of surgical planning, 3D printed heart models offer unparalleled insights for complex procedures, such as valve replacements or ventricular reconstructions. Surgeons can utilize these lifelike replicas to simulate surgical scenarios, optimize surgical approaches, and anticipate potential complications, ultimately enhancing procedural outcomes and minimizing risks for patients. Moreover, 3DP technology empowers medical researchers to develop and refine innovative therapies for heart failure. By leveraging these customizable models, scientists can conduct preclinical studies, test novel devices or interventions, and tailor treatment strategies to individual patient characteristics, paving the way for personalized medicine in cardiology. However, despite its immense potential, the widespread adoption of 3DP in heart failure management faces certain challenges, including cost constraints, regulatory hurdles, and the need for standardized protocols. Addressing these barriers will be crucial to realizing the full benefits of this transformative technology and ensuring its accessibility to patients worldwide.
Introduction
Cardiovascular disease is the leading cause of mortality worldwide, resulting in the death of over 19 million people in 2022 [1]. Among the spectrum of cardiovascular diseases, heart failure (HF) is one of the most common syndromes impacting the functionality of the heart. HF is characterized by impaired ventricular filling and ejection, and affects over 56 million individuals globally as of 2019 [2]. This condition poses a major public burden, accounting for 1–2% of the total healthcare expenditure in the United States [3, 4]. In clinical settings, imaging modalities such as computed tomography (CT), echocardiography, and magnetic resonance imaging (MRI) are the standard of care for disease diagnosis and management. The patient-to-patient variability in complex anatomical structures and pathologies often necessitates exploring personalized treatment strategies, especially when involving surgical or interventional techniques. Three-dimensional printing (3DP) has emerged as a valuable tool for surgeons and cardiovascular interventionalists, facilitating pre-operative planning by providing tangible, physical models that enhance visuospatial understanding of anatomical structures [5].
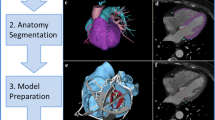
3DP, an additive manufacturing technique, allows for creating patient-specific physical models from digital imaging modalities such as MRI, CT, and 3D echocardiography. This process enhances visualization and conceptualization of anatomy and pathologies by transforming 2D digital image stacks into 3D physical models [6]. Among these modalities, CT imaging is most commonly used for 3DP due to its faster image acquisition and superior spatial resolution, although MRI techniques are preferred when ionizing radiation cannot be used. Following image acquisition, the volumetric data is stored in DICOM (Digital Imaging and Communications in Medicine) format [7]. Subsequently, the DICOM data undergoes segmentation in image processing software, isolating regions of interest through manual anatomical shape recognition or intensity-based segmentation techniques. The segmented digital file is then converted into a standard tessellation language (STL) format and uploaded to modeling software for potential design modifications. Next, the STL file is transferred to 3DP software to configure printing parameters and add support to overhanging structures. Following printing, the model undergoes post-processing to remove supports, and polishing and finishing techniques are applied to maintain its quality [8]. A step-by-step overview of this process is depicted in Fig. 1A. The selection of a suitable 3D printer depends on the specific application, with various commercially available options offering distinct properties. Notably, Fused Deposition Modeling (FDM) [9], Stereolithography (SLA) [10], and PolyJet [11] printers are frequently cited in the literature. The versatility of 3DP offers promising avenues for innovation in cardiovascular care, enabling the development of novel procedural approaches across various cardiac conditions.
A Overview of the 3DP process, from clinical imaging to manufacture of a 3D printed model. B Applications of 3DP technology in diagnosis and management of heart failure. Pictures of left ventricular assist devices (LVAD) in the presurgical planning quadrant reproduced with permission from refs. [11] and [12]. (Left): From Tadokoro et al. [12], with permission from Springer Nature; (Right): From Giorgi et al. [11], with permission from Elsevier. Figures were generated, in part, using BioRender.com
Prior literature has demonstrated that 3DP has aided clinicians in planning cardiovascular interventional and surgical procedures, as well as in education and patient communication [13,14,15]. For example, in aortic stenosis patients, 3DP assists in guiding transcatheter aortic valve implantation [16, 17]. 3D printed models may also help in determining the size of closure devices in the left atrial appendage [18], guiding septal myectomy for hypertrophic cardiomyopathy [5], and facilitating heart failure management through left ventricular assist devices (LVADs) [11, 12, 19]. An overview of applications of 3DP in diagnosis and management of heart failure and related conditions is depicted in Fig. 1B. In the subsequent sections, recent advances in specific applications are discussed.
Pre-Operative Planning and Management
Patients with a history of cardiac surgery are at risk of encountering unexpected surgical challenges due to their complex morphological characteristics and associated risk factors [20, 21]. In such cases, surgeons often perform simulated surgical operations to deepen their understanding of the case and acquire new skills [21]. Bernhard et al. [15] conducted meta-analysis to assess the effectiveness of 3DP in guiding cardiovascular interventions. They analyzed 107 cases spanning from 2005 to 2021, involving 814 3D patient-specific phantoms (3DPSP). Despite variations in the efficacy of 3DPSP as a preassessment tool across studies, congenital heart disease demonstrated the widest range of applications in cardiovascular interventions (329 3DPSP), followed by left atrial appendage (LAA) occlusion (182 3DPSP). Overall, 3DPSP helped reduce operating time and informed management decisions.
In cases involving left ventricular aneurysm (LVA), characterized by advanced ventricular dysfunction and dilation, surgeons may reconstruct the left ventricle (LV) geometry to preserve its systolic function. Carrabba et al. [9] presented a case study involving a 59-year-old woman with heart failure (ejection fraction of 20%) and a prominent LVA. Utilizing contrast-enhanced CT, they obtained high-resolution 3D volumetric slices (0.4 mm thick), which were subsequently imported into an image processing software, Mimics v22.0 (Materialise Mimics Medical, Leuven, Belgium), for segmentation of the LV, mitral valve, annulus, and its leaflets. The resulting 3D model was fabricated using a rigid polylactic acid (PLA) filament material with a layer height of 0.1 mm to ensure adequate resolution on a Makerbot Replicator + printer based on fused filament fabrication technology. These 3D prints were pivotal in visualizing the patterns of LVA and the volume of LV and predicting their repair outcomes. The surgeons successfully optimized their surgical planning and enhanced the LV geometry, improving overall systolic function to an ejection fraction of 54%.
Ventricular Assistive Device (VAD) Placement
Ventricular assist devices (VADs) are essential in treating advanced heart failure by maintaining blood circulation and saving lives. National guidelines suggest that approximately 10–15% of the US heart failure population, totaling around 0.5 million individuals, may need LVAD implants [22]. In a case study presented by Giorgi et al. [11], a 55-year-old male patient with non-ischemic cardiomyopathy experienced sustained syncopal episodes and ventricular arrhythmia due to improper placement of the inflow cannula of HeartMate II (HMII). To address this issue, surgeons opted to replace the HMII with HMIII. They utilized a 3D model fabricated using a PolyJet printer to refine their surgical approach and determine the optimal graft length for aortic anastomosis. This reduced the operating time and contributed to successfully resolving the patient's symptoms.
LVAD is also used in Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC), a condition characterized by enlargement of the right ventricle leading to right heart failure, in order to mitigate the risk of sudden deaths or LV failure. Tadokoro et al. [12] reported a case involving a 43-year-old patient with a medical treatment history of over a decade for ARVC. HF with reduced ejection fraction in both ventricles necessitated the installation of an HMIII LVAD to prevent deterioration of right ventricle function by mitigating future left ventricular dysfunction. Polyurethane resin (CrossMedical, Tokyo, Japan) was utilized to fabricate a patient-specific 3D model, determining an optimal placement for the inflow cuff within the LV apex. The 3D printed models facilitated the mitigation of potential surgical challenges and acquired new suturing techniques. Ultimately, this approach contributed to maintaining good hemodynamics in the mitral valve.
Congenital Heart Disease (CHD)
As of 2017, Congenital Heart Disease (CHD) impacts approximately 18 out of every 1000 individuals worldwide [23]. In the United States, around 20% of people diagnosed with CHD are at risk of developing heart failure [24]. Given the variability in presurgical assessment across diverse CHDs, the Radiological Society of North America's (RSNA) 3D Printing Special Interest Group (3DPSIG) adopted the American College of Radiology Appropriateness Criteria to devise a scoring mechanism for evaluating the suitability of 3DP as a tool in clinical scenarios using literature till July 2022. This scoring system encompasses three ranges: scores of 1–3 indicating an absence of clear benefits, scores of 4–6 suggest potential advantages in select cases but with limited available data for validation, and scores of 7–9 denote situations wherein 3D printing contributes value to the diagnostic process. Among the 28 surgical CHD cases analyzed, eight did not demonstrate any benefits from using 3D printing, whereas the remaining 20 cases, particularly those with higher complexity, showed direct benefits in patient care using 3D printing [25].
Liang et al. [26] conducted a study to evaluate the efficacy of SLA 3DP in diagnosing CHD. Established physicians and students were tasked with diagnosing 45 cases of CHD across eight categories using echocardiography and CT imaging, as well as blood pool and myocardial models. The 3D models were created using DICOM data obtained from CT scans and processed to generate blood pool and myocardial models, which were subsequently sectioned for detailed comparisons as needed. The physical models were produced using SLA technology (Shaanxi Hengtong Intelligent Machine Co., Ltd., Shannxi, China) with rigid resin. The average time for printing and post-processing the 3D models was around 7 h. Established physicians demonstrated significantly higher diagnostic accuracy for complex CHD cases using CT and echocardiography than students. However, the average accuracy across all eight cases increased after using 3D models for diagnosis, rising from 88.75% to 95.9% in the expert group and from 60% to 91.6% in the student group. This increase in accuracy was attributed to improved understanding of the spatial relationships of complex anatomy facilitated using 3D models. Similar to the findings of Ryan et al. [25], Liang et al. [26] also underscores the benefits of utilizing 3D models in diagnosing complex CHD cases.
Cardiomyopathy
Hypertrophic cardiomyopathy (HCM) is a genetic condition characterized by left ventricular hypertrophy unexplained by cardiac or systemic disease [27]. Globally, approximately 1 in every 200 to 500 individuals are affected by HCM, with an estimated prevalence of heart failure associated with HCM ranging from 0.7 to 1 million people in the United States [28]. Surgical myectomy is a common treatment for left ventricular outflow tract (LVOT) obstruction in patients HCM. Despite its conceptual simplicity, the procedure is complex due to the need for precise tissue resection tailored to the individual's disease spectrum [29]. 3DP technology offers a valuable tool to simulate the surgery, allowing surgeons to anticipate and mitigate potential challenges before they arise, thereby enhancing surgical outcomes and patient safety. Case reports have demonstrated the feasibility of 3DP in guiding septal myectomy [30,31,32].
Wang et al. [30] conducted a randomized trial with 46 patients having LVOT obstruction, where 22 patients formed the experimental group and 24 the control group. The experimental group underwent a simulated Morrow operation using 3D models prior to the procedure, while the control group did not utilize 3D models. Cardiac CT scans were obtained using a General Electric Revolution scanner, and the DICOM files were processed using Mimics Innovation Suite 19.0 for segmentation to create blood pool and myocardial models. These models were then exported to STL format and 3D printed using an Objet Eden 260VS 3D printer with Tango (rigid material) and Vero (soft and rubbery) series materials. Before the simulated surgery, hypertrophic tissues were identified, and the first assistant determined resection parameters through virtual resection. The chief surgeon then performed tissue resection on a solid model based on these parameters, refining as necessary. This approach reduced intraoperative time and blood loss by predetermining and practicing surgical details. Although postoperative ejection fraction did not significantly differ between groups, the experimental group exhibited lower interventricular septal thickness, LVOT pressure difference, and flow velocity. A similar study by Zhang et al. [33] involved using DaVinci robotic surgery combined with 3DP. The use of 3DP (printer model Stratasys J850) aided in the preoperative evaluation of the LVOT parameters in a 50-year-old male patient with hypertrophic obstructive cardiomyopathy. It helped reduce the LVOT septal thickness from 22.9 mm to 16.7 mm and resolve the mitral leaflet's regurgitation and systolic anterior motion. These studies indicate that 3D printing can assist in preoperative planning for HCM by visualizing different phenotypes [34] and determining resection parameters to achieve the desired septal thickness post-surgery.
Education
Multiple studies have found 3DP to be an effective tool for educating and training students and staff about heart failure phenotypes [35,36,37,38]. Its effectiveness is on par with cadaveric materials [39], traditionally utilized for such purposes, which are often expensive and come with ethical constraints. Additionally, integrating 3D models during consultation has enhanced doctor-patient communication, rendering conversations more interactive and enhancing the patient’s comprehension of the disease [40, 41].
Patient-Specific Mock Circulatory Loops
Mock circulatory loops (MCLs) provide a controlled environment to simulate patient-specific cardiovascular responses to diverse disease conditions and cardiac assistive devices on the benchtop, aiding in comprehension of pressure and flow hemodynamics and pathophysiology [42,43,44,45]. With the rising need for VAD in advanced HF patients [46], there is a growing need to simulate flow patterns due to the variability in patients and heart failure etiologies. Kizilski et al. [47] created an MCL using a 3D printed pediatric anatomical model of the right ventricular outflow tract. The model, made with TPU 95A Shore hardness material from Ultimaker B.V., was designed to simulate and measure the flow dynamics of pulmonary expanded polytetrafluoroethylene (ePTFE) mono-cusp leaflets post-intervention. While the study achieved repeatable measurements of pressure gradient and flow regurgitation, variability arose due to differences in how the leaflets were sewn into the models. MCLs have also been used to assess the hemodynamics of LVAD devices. Khienwad et al. [48] developed an MCL using a 3D printed mold to manufacture a simple silicone LV model and investigated the ventricular flow patterns during the Lavare cycle, an algorithm that modulates the VAD pump speed, in the HeartWare VAD (Medtronic, USA).
Conclusions
The integration of 3DP technology into the diagnosis and management of heart failure holds significant promise for revolutionizing patient care. From enhancing preoperative planning to facilitating the creation of patient-specific cardiac models and implants, 3D printing offers unprecedented opportunities for personalized medicine in cardiovascular healthcare. While 3DP has significantly progressed and aids in clinical decision-making, its current constraints within cardiac medicine must be acknowledged. Firstly, commercially available platforms for 3DP fail to replicate the complex native mechanical properties of tissues accurately. Second, 3D models only represent data from a single instant of the cardiac cycle, restricting the portrayal of the dynamic motion of the heart. Finally, depending on the choice of printer, the entire 3DP process can be time-consuming and expensive, and only feasible in practice for one-off complex cases.
The emerging area of 3D bioprinting holds promise as a new therapeutic avenue for treating advanced heart failure patients through the creation of 3D volumetric myocardial tissues [49]. 3D bioprinting has advanced by creating living tissues from scaffolds, incorporating cells to produce personalized patches, grafts, and even constructing organs from scratch for potential implantation into animal models [50]. A recently funded moonshot project at Stanford University aims to implant a bioprinted human heart into a pig within the next five years [51]. To fabricate the heart, the team of scientists and clinicians intend to utilize an automated bank of bioreactors, facilitating the production of cardiac cells with distinct functions which will act as the bioink. Upon scaling, these cells will be assembled into a fully functioning heart using 3D bioprinting [52].
As this field continues to advance, it is imperative for clinicians, basic science researchers, and engineers to collaborate closely to optimize the utilization of 3DP technology, ultimately leading to improved outcomes and quality of life for individuals affected by heart failure.
Data Availability
No datasets were generated or analysed during the current study.
References
Mensah GA, Fuster V, Murray CJL, et al. Global burden of cardiovascular diseases and risks, 1990–2022. J Am Coll Cardiol. 2023;82:2350–473.
Yan T, Zhu S, Yin X, et al. Burden, trends, and inequalities of heart failure globally, 1990 to 2019: A secondary analysis based on the global burden of disease 2019 study. J Am Heart Assoc. 2023;12:e027852.
Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States. Circ Heart Fail. 2013;6:606–19.
Hollenberg SM, Warner Stevenson L, Ahmad T, et al. 2019 ACC expert consensus decision pathway on risk assessment, management, and clinical trajectory of patients hospitalized with heart failure. J Am Coll Cardiol. 2019;74:1966–2011.
Farooqi KM, Sengupta PP. Echocardiography and three-dimensional printing: Sound ideas to touch a heart. J Am Soc Echocardiogr. 2015;28:398–403.
Aimar A, Palermo A, Innocenti B. The role of 3D printing in medical applications: A state of the art. J Healthc Eng. 2019;2019:1–10.
Rengier F, Mehndiratta A, von Tengg-Kobligk H, Zechmann CM, Unterhinninghofen R, Kauczor H-U, Giesel FL. 3D printing based on imaging data: Review of medical applications. Int J Comput Assist Radiol Surg. 2010;5:335–41.
Harb SC, Rodriguez LL, Vukicevic M, Kapadia SR, Little SH. Three-dimensional printing applications in percutaneous structural heart interventions. Circ Cardiovasc Imaging. 2019;12:1–11.
Carrabba N, Buonamici F, Furferi R, Carfagni M, Vannini M, Valenti R, Cerillo AG, Marchionni N, Stefàno P. Case report: Three-dimensional printing model for surgical planning of left ventricular aneurysm: evolution toward tailoring surgery. Front Cardiovasc Med. 2022;9:1–6.
Sodian R, Weber S, Markert M, Rassoulian D, Kaczmarek I, Lueth TC, Reichart B, Daebritz S. Stereolithographic models for surgical planning in congenital heart surgery. Ann Thorac Surg. 2007;83:1854–7.
Giorgi J, Barbagelata A, Luoma-Overstreet G, Kaplinsky E, Jorge SDC, Scanavacca M, Scordamaglio PR, Mentz RJ. 3D printing for left ventricular assist device exchange. JACC Case Rep. 2024;29:102194. 3D printing technology reduced operating time and contributed to the successful surgery, which resolved the ventricular arrhythmias and syncope caused by improper positioning of the inflow cannula. The patient recovered without complication and was discharged on postoperative day 22, with complete resolution of symptoms.
Tadokoro N, Tonai K, Kainuma S, et al. A unique cuff-sewing method to implant heartmate3 LVAD for arrhythmogenic right ventricular cardiomyopathy, facilitated by 3D-printing heart model. J Artif Organs. 2023;26:151–5.
Diment LE, Thompson MS, Bergmann JHM. Clinical efficacy and effectiveness of 3D printing: A systematic review. BMJ Open. 2017;7:e016891.
Huang H, Tayyab H, Khan A, et al. Three-dimensional printing and modeling in interventional cardiology: A comprehensive review. Ann Clin Cardiol. 2023;5:53–62.
Bernhard B, Illi J, Gloeckler M, Pilgrim T, Praz F, Windecker S, Haeberlin A, Gräni C. Imaging-based, patient-specific three-dimensional printing to plan, train, and guide cardiovascular interventions: A systematic review and meta-analysis. Heart Lung Circ. 2022;31:1203–18. This meta-analysis covers 107 studies that prospectively investigated a total of 814 3D printed patient-specific models used in cardiovascular interventions. Benefits for patients including reduced intervention time, potential for lower radiation exposure, and shorter mechanical ventilation times are reported.
Gallo M, D’Onofrio A, Tarantini G, Nocerino E, Remondino F, Gerosa G. 3D-printing model for complex aortic transcatheter valve treatment. Int J Cardiol. 2016;210:139–40.
Fujita T, Saito N, Minakata K, Imai M, Yamazaki K, Kimura T. Transfemoral transcatheter aortic valve implantation in the presence of a mechanical mitral valve prosthesis using a dedicated TAVI guidewire: Utility of a patient-specific three-dimensional heart model. Cardiovasc Interv Ther. 2017;32:308–11.
Hell M, Achenbach S, Yoo I, Franke J, Blachutzik F, Roether J, Graf V, Raaz-Schrauder D, Marwan M, Schlundt C. 3D printing for sizing left atrial appendage closure device: Head-to-head comparison with computed tomography and transoesophageal echocardiography. EuroIntervention. 2017;13:1234–41.
Miller J, Billadello J, Simon-Lee R, Sharma M, Singh G, Woodard P, Eghtesady P, Anwar S. 3D printing for preoperative planning and surgical simulation of VAD implantation in a failing right systemic ventricle. J Am Coll Cardiol. 2018;71:A545.
Sepehri A, Beggs T, Hassan A, Rigatto C, Shaw-Daigle C, Tangri N, Arora RC. The impact of frailty on outcomes after cardiac surgery: A systematic review. J Thorac Cardiovasc Surg. 2014;148:3110–7.
Ganguli A, Pagan-Diaz GJ, Grant L, Cvetkovic C, Bramlet M, Vozenilek J, Kesavadas T, Bashir R. 3D printing for preoperative planning and surgical training: A review. Biomed Microdevices. 2018;20:65.
Bowen RES, Graetz TJ, Emmert DA, Avidan MS. Statistics of heart failure and mechanical circulatory support in 2020. Ann Transl Med. 2020;8:827–827.
Wu W, He J, Shao X. Incidence and mortality trend of congenital heart disease at the global, regional, and national level, 1990–2017. Medicine. 2020;99:e20593.
Bozkurt B, Ahmad T, Alexander KM, et al. Heart failure epidemiology and outcomes statistics: A report of the heart failure society of America. J Card Fail. 2023;29:1412–51.
Ryan JR, Ghosh R, Sturgeon G, et al. Clinical situations for which 3D printing is considered an appropriate representation or extension of data contained in a medical imaging examination: Pediatric congenital heart disease conditions. 3D Print Med. 2024;10:3.
Liang J, Zhao X, Pan G, Zhang G, Zhao D, Xu J, Li D, Lu B. Comparison of blood pool and myocardial 3D printing in the diagnosis of types of congenital heart disease. Sci Rep. 2022;12:7136.
Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2011;58:e212–60.
Maron BJ, Rowin EJ, Udelson JE, Maron MS. Clinical spectrum and management of heart failure in hypertrophic cardiomyopathy. JACC Heart Fail. 2018;6:353–63.
Al Kindi HN, Yacoub MH. Surgical myectomy: Rationale and personalized technique. Glob Cardiol Sci Pract. 2018. https://doi.org/10.21542/gcsp.2018.35.
Wang X, Liang J, Mu C, Zhang W, Xue C, He Y, Zhang G, Li D. Effect of 3D-printed hearts used in left ventricular outflow tract obstruction: A multicenter study. BMC Cardiovasc Disord. 2022;22:200. This study shows that operation time, cardiopulmoary bypass time, intraoperative blood loss, and hospitalization time were all significantly lower in group where extracorporeal surgical simulation was performed on 3D printed patient-specific heart models, compared to a control group with no surgical simulation.
Wang Y, Guo H, Wang S, Lai Y. Effectiveness of a patient-specific 3-dimensional printed model in Septal Myectomy of hypertrophic cardiomyopathy. Pak J Med Sci. 2020;36:1678–82.
Farooqi KM, Smerling J, Jorde UP. Application of 3D printing technology in heart failure. Heart Fail Clin. 2022;18:325–33.
Zhang S, Liu Z, Liu H, Zhang C. Assisted Da Vinci robotic surgery combined with 3D printing technology applied in septal myectomy. Eur J Med Res. 2023;28:18.
Oberoi M, Foley TA, Schaff HV. Hypertrophic cardiomyopathy: Interactive 3-dimensional modeling of phenotypic variants. J Thorac Cardiovasc Surg. 2022;163:e243–5.
Costello JP, Olivieri LJ, Krieger A, Thabit O, Marshall MB, Yoo S-J, Kim PC, Jonas RA, Nath DS. Utilizing three-dimensional printing technology to assess the feasibility of high-fidelity synthetic ventricular septal defect models for simulation in medical education. World J Pediatr Congenit Heart Surg. 2014;5:421–6.
Luxford JC, Cheng TL, Mervis J, Anderson J, Clarke J, Croker S, Nusem E, Bray L, Gunasekera H, Scott KM. An opportunity to see the heart defect physically: Medical student experiences of technology-enhanced learning with 3D printed models of congenital heart disease. Med Sci Educ. 2023;33:1095–107.
Smerling J, Marboe CC, Lefkowitch JH, Pavlicova M, Bacha E, Einstein AJ, Naka Y, Glickstein J, Farooqi KM. Utility of 3D printed cardiac models for medical student education in congenital heart disease: Across a spectrum of disease severity. Pediatr Cardiol. 2019;40:1258–65.
Osakwe O, Moore R, Divanovic A, Del GE, Tegtmeyer K, Madsen N, Taylor M. Improving patient experience and education on congenital heart defects: The evolving role of digital heart models, 3D-printing and mobile application. Pediatrics. 2019;144:340–340.
Lim KHA, Loo ZY, Goldie SJ, Adams JW, McMenamin PG. Use of 3D printed models in medical education: a randomized control trial comparing 3D prints versus cadaveric materials for learning external cardiac anatomy. Anat Sci Educ. 2016;9:213–21.
Guo H-C, Wang Y, Dai J, Ren C-W, Li J-H, Lai Y-Q. Application of 3D printing in the surgical planning of hypertrophic obstructive cardiomyopathy and physician-patient communication: A preliminary study. J Thorac Dis. 2018;10:867–73.
Biglino G, Koniordou D, Gasparini M, Capelli C, Leaver L-K, Khambadkone S, Schievano S, Taylor AM, Wray J. Piloting the use of patient-specific cardiac models as a novel tool to facilitate communication during cinical consultations. Pediatr Cardiol. 2017;38:813–8.
D’Souza GA, Rinaldi JE, Meki M, Crusan A, Richardson E, Shinnar M, Herbertson LH. Using a mock circulatory loop as a regulatory science tool to simulate different heart failure conditions. J Biomech Eng. 2024;146:1–11.
Xu K-W, Gao Q, Wan M, Zhang K. Mock circulatory loop applications for testing cardiovascular assist devices and in vitro studies. Front Physiol. 2023;14:1–17.
Rosalia L, Ozturk C, Goswami D, et al. Soft robotic patient-specific hydrodynamic model of aortic stenosis and ventricular remodeling. Sci Robot. 2023;8:eade2184.
Rosalia L, Ozturk C, Coll-Font J, et al. A soft robotic sleeve mimicking the haemodynamics and biomechanics of left ventricular pressure overload and aortic stenosis. Nat Biomed Eng. 2022;6:1134–47.
Han JJ, Acker MA, Atluri P. Left ventricular assist devices. Circulation. 2018;138:2841–51.
Kizilski SB, Zhang X, Kneier NE, Chaillo Lizarraga MD, Schulz NE, Hammer PE, Hoganson DM. An in vitro circulatory loop model of the pediatric right ventricular outflow tract as a platform for valve evaluation. Cardiovasc Eng Technol. 2023;14:217–29.
Khienwad T, Maurer A, Ghodrati M, Schlöglhofer T, Moscato F, Stoiber M, Schima H, Aigner P. Effect of timings of the lavare cycle on the ventricular washout in an in vitro flow visualization setup. ASAIO J. 2021;67:517–28.
Yadid M, Oved H, Silberman E, Dvir T. Bioengineering approaches to treat the failing heart: from cell biology to 3D printing. Nat Rev Cardiol. 2022;19:83–99.
Matthews N, Pandolfo B, Moses D, Gentile C. Taking it personally: 3D bioprinting a patient-specific cardiac patch for the treatment of heart failure. Bioengineering. 2022;9:93.
Theis L. Scientists work to 3D bioprint a human heart in 5 years. In: Scripps News. 2023. https://www.scrippsnews.com/science-and-tech/scientists-work-to-3d-bioprint-a-human-heart-in-5-years. Accessed May 15, 2024.
Sexton ZA, Hudson AR, Herrmann JE, Shiwarski DJ, Pham J, Szafron JM, Wu SM, Skylar-Scott M, Feinberg AW, Marsden A. Rapid model-guided design of organ-scale synthetic vasculature for biomanufacturing. 2023. arXiv:2308.07586v1.
Funding
Dr. Nguyen was supported by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH) under award number R01HL151704.
Author information
Authors and Affiliations
Contributions
D.G. and C.T.N conceived the review. D.G. and M.K. wrote the manuscript text and prepared the figures. All authors reviewed and edited the manuscript and approved the final version. C.T.N. provided supervision and funding support.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Goswami, D., Kazim, M. & Nguyen, C.T. Applications of 3D Printing Technology in Diagnosis and Management of Heart Failure. Curr Treat Options Cardio Med (2024). https://doi.org/10.1007/s11936-024-01045-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s11936-024-01045-3