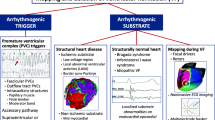
Abstract
Purpose of review
During challenging scenarios of ventricular arrhythmia (VA) ablation, novel strategies to target the arrhythmogenic substrate are sometimes essential for clinical success. While catheter ablation (CA) can offer relatively high efficacy, it may not always lead to complete VA eradication in certain individuals. This article provides a brief overview of difficult substrate ablation strategies in patients with premature ventricular contractions (PVC) or ventricular tachycardia (VT) and explores methods to improve outcomes for cases that do not respond to typical ablation techniques.
Recent findings
Recent developments highlight the crucial role of advanced imaging and mapping techniques in the visualization, characterization, and localization of challenging ventricular substrates. Additionally, some novel and advanced ablation strategies may be useful when PVC/VT is refractory to conventional ablation treatment.
Summary
An expanding spectrum of techniques which can optimize the precision and effectiveness of catheter ablation procedures may improve PVC/VT ablation outcomes. Novel ablation therapies such as alcohol ablation, optimization of dispersive patch positions, or bipolar ablation, can overcome obstacles associated with the challenging anatomy of arrhythmia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Opinion statement
Catheter ablation is currently the most effective treatment option for ventricular arrhythmias. However, in some circumstances, classic radiofrequency ablation cannot effectively eliminate some deeply located intramural arrhythmias. Thus, novel mapping approaches can be supported by such advanced ablation techniques as enhanced unipolar radiofrequency ablation, bipolar radiofrequency ablation, alcohol ablation, stereotactic body radio-ablation, or pulsed-field ablation.
Introduction
Catheter ablation (CA) is a widely recognized therapy for ventricular arrhythmias (VAs), demonstrating success rates ranging from 47 to 90% [1]. The effectiveness varies based on factors such as the presence of ventricular tachycardia (VT) substrate and its etiology, the location of the arrhythmia’s origin, and whether multisite ablation is needed for intramural sources [2,3,4, 5•], and if the substrate progression is likely to occur [6]. Despite the relatively high effectiveness, CA may encounter limitations, often associated with anatomical challenges such as deep intramural arrhythmogenic foci or proximity to the coronary arteries and conduction system. In this article, we provide a brief overview addressing challenging substrates in ventricular arrhythmias (VAs) and explore techniques to enhance outcomes in cases resistant to conventional ablation methods.
Imaging
At the stage of pre-procedural planning, an accurate determination of the anatomical substrates for VA occurrence is important for successful ablation procedures. Recent findings in the integration of advanced imaging technologies have impacted an approach to mapping substrates located in the ventricular myocardium. Especially, the dedicated scar segmentation software has emerged as a promising option. Automatic Detection of Arrhythmogenic Substrate (ADAS) was created to outline the structure of the ventricles and incorporate this information into electroanatomical mapping systems [7]. Based on computed tomography (CT) or cardiac magnetic resonance (CMR), it allows the generation of ventricular scar maps. Another software—In-HEART—evaluates CMR or CT to imaging data for the development of three-dimensional cardiac models. The software conducts detailed segmentation of scars and anatomical structures such as wall thinning, epicardial fat, coronary arteries, or phrenic nerves which can provide additional benefit, not only during intraprocedural design of the ablation lesion set [8] but also can be helpful for appropriate determination of ablation target at the stage of pre-procedural planning. Intracardiac echocardiography (ICE) can be helpful for intraprocedural visualization of the left ventricular scar [9] but also can help to navigate the ablation catheter in real-time and can track any possible occurrence of CA-related complications at their early stage [10].
Mapping
Appropriate mapping techniques are particularly important for the assessment of the substrate’s electrophysiological characteristics. Such evaluation of the ventricular myocardial substrate is significantly influenced by the dimensions of the mapping electrodes. Microelectrode mapping can overcome some limitations associated with standard bipolar mapping performed using classic diagnostic or ablation catheters. Utilizing electrophysiological signals recorded using catheters equipped with microelectrodes enables the recording of electrograms of higher specificity in regions characterized by low bipolar voltage during sinus rhythm [11]. Enhancing sampling density through the use of microelectrodes improves resolution and increases the probability of capturing near-field electrical information [12]. Due to such features, microelectrode mapping surpasses standard bipolar mapping in its sensitivity to identify viable myocytes during sinus rhythm, potentially facilitating the recognition of proper targets for CA [11]. Nevertheless, classic endocardial mapping can be still limited if the substrate is located in the intramural area [13]. In such circumstances, direct mapping of the septal perforator veins can be useful, and this can be performed using small-diameter catheters equipped with microelectrodes [12] or insulated angioplasty guidewires [14]. If the appropriate early activation is determined this way, the ablation can be performed from the anatomically adjacent chambers.
Enhancing the efficacy of VT ablation strategies requires the identification of crucial ablation targets. Such presumed areas of scar frequently have their border zones close to relatively healthy myocardial tissue. Collision of multiple wavefronts arising from scar and nearby myocardium can have an influence on the ultimate signal formation [15], and this can impair the accuracy of mapping performed during sinus rhythm or regular ventricular pacing. Previous studies have shown that ventricular electrograms, which demonstrated decremental conduction using decrement evoked potentials (DEEP) during right ventricular apical pacing with an extra-stimulus, are more likely to participate in reentrant VT circuits than conventional substrate ablation targets [16]. This mapping technique enables the identification of the functional substrate critical to the VT circuit with high specificity [17]. Moreover, it has been shown to be more specific than late potential mapping for identifying the critical targets of VT ablation [16] and should be considered in cases with difficult substrates of VT.
Additionally, high-density (HD) mapping emerges as a promising approach for accurate detailing of substrates for VT. HD mapping catheters provide detailed and comprehensive electroanatomical information across a number of points with superior resolution, enabling more accurate discrimination of local abnormal electrograms [18]. This high-resolution mapping enhances the precision of substrate delineation and characterization of scar substrates [19] [20] frequently leading to more accurate ablation target delineation.
Alternative mapping sites in ventricular arrhythmias
Various mapping sites beyond the conventional right and left ventricular endocardial surface locations have been investigated for the identification of recurrent PVC/VT true origin, expanding the range of possibly successful ablation targets. For classic outflow tract arrhythmias, an important area of interest are cusps of the pulmonic valve. Recent studies suggest that occasionally a subset of arrhythmias with morphology suggestive of the right ventricular outflow tract (RVOT) originates from these cusps [21,22,23]. The efficacy of the “reversed U-curve” technique has been established for mapping and radiofrequency ablation of these arrhythmias, providing improved catheter stability and contact. Sporadically, this method is also advantageous for some arrhythmias arising from the left ventricular summit (LVS), particularly its more septal aspect, which is in close proximity to the RVOT and left pulmonic cusp [24, 25]. Additionally, more classic but detailed mapping of pulmonic cusp junctions may sometimes eliminate the necessity for using the reversed U-curve technique [26].
Coronary veins can appear as another key mapping site, particularly for OT VAs refractory to endocardial ablation. Mapping using ablation or diagnostic catheter can reveal the earliest activation in the great coronary vein (GCV) or anterior interventricular vein (AIV), which is suggestive of some LVS arrhythmias. While ablation within GCV/AIV is feasible, obstacles such as high impedance [27] and proximity to major coronary arteries [28] necessitate caution. The telescopic approach, involving stepwise catheter [29], guidewire advancement [30], and microcatheters in GCV/AIV and their branches [31], can be helpful if catheter positioning in the coronary veins is challenging. In cases of recurrence post-ablation within GCV/AIV, the use of low- or non-ionic coolant for irrigation is safe and may enhance radiofrequency power delivery both from the coronary vein and opposite endocardium [32,33,34].
Although infrequently required, epicardial access has been considered for specific LVS arrhythmias [35]. Assessments of percutaneous epicardial mapping indicate limited success, with only a small subset of patients benefiting from direct epicardial ablation due to challenges like proximity to major coronary vessels or the presence of epicardial fat [35]. These findings may suggest that the direct epicardial ablation of LVS arrhythmias has a limited role in this application; however, epicardial access is frequently necessary for the complete elimination of arrhythmogenic tissue in a spectrum of non-ischemic cardiomyopathies [36].
Advanced ablation strategies
It has been demonstrated that in cases when scar substrate is located septally or is located within the LVS area, acute ablation success may be more difficult to achieve [3] and sometimes can be even associated with increased long-term cardiovascular mortality [37•]. With a better understanding of the limitations of intramural substrate ablation in such areas, several newer ablative techniques are still being implemented, such as alcohol ablation [38]. Injecting ethanol through coronary vessels has been effective in immediate cell destruction and the elimination of clinical PVC/VT [39]. While initially delivered through coronary arteries [40], recent improvements have revitalized the method by introducing ethanol injection through coronary veins [41]. The Valderrabano group has innovated with a double-balloon technique to reduce ethanol dispersion, making ablation more focused on the targeted ablation area [42]. While the results are encouraging, the exact shape of the anticipated lesions created by injecting ethanol in the LVS area is still not completely determined.
Another advanced ablation technique involves delivering radiofrequency current using two ablation catheters in a bipolar fashion, where one catheter connects to the ground port instead of the dispersive patch [43]. This approach appeared effective for treating PVC/VTs from challenging locations such as the interventricular septum [44], LVS [45,46,47], or RVOT diverticulum [48]. Some obstacles may include non-uniform energy transfer due to impedance mismatch, which can be attenuated using a large tip ablation catheter in the GCV [49, 50]. The absence of intramural capture, facilitated by a microcatheter or wire, seems encouraging for assessing the completeness of lesions in real-time during bipolar ablation procedures [51]. Precautions are necessary for procedures closer to the His bundle [52] and coronary arteries [53, 54], especially when the target is more anteriorly located [55].
The conventional radiofrequency (RF) ablation system uses a unipolar current between the catheter tip and a dispersive patch on the patient’s skin. Relocating the dispersive patch, especially to the front of the chest, has been suggested to improve the creation of lesions in PVC/VT from the anterior aspect of the OT [56]. Studies indicate that changing the dispersive patch location and using additional dispersive electrodes may result in larger RF lesions and deeper lesion formation [57].
Radiotherapy
Recent advancements in non-invasive VT therapy options have introduced the use of stereotactic body radiation therapy (SBRT) as a novel ablation approach. SBRT, originally developed for oncological purposes, has been adapted to target arrhythmogenic substrates within the heart [58]. The precision of SBRT allows for the focused delivery of high-dose radiation targeted to a specific area of tissue [59], potentially abolishing the arrhythmogenic focus without the need for invasive catheterization. Early clinical trials have shown promising results in reducing the burden of refractory VT, particularly in patients where conventional catheter ablation techniques have previously failed [60]. The mechanism of action is believed to involve the induction of fibrosis within the targeted tissue, leading to the electrical elimination of the arrhythmogenic substrate [61•]. While the initial outcomes are encouraging, and the method seems to be safe, potential complications of cardiac radiotherapy, such as radio-induced coronary lesions [62], are still not completely investigated. Future promising and unique features of SBRT may include possible acceleration of conduction within slow-propagating zones of scar [63].
Pulsed-field ablation
Another novel technique in the spectrum of VA therapies is pulsed-field ablation (PFA), which offers a potentially safer and more precise alternative to traditional thermal ablation methods [64]. PFA uses ultra-short electrical pulses to induce irreversible electroporation, selectively targeting cardiac tissues responsible for arrhythmias while minimizing collateral damage to adjacent structures [65]. This specificity can be particularly advantageous in ventricular arrhythmia ablation, where the proximity to vital cardiac structures like the coronary arteries and the conduction system poses significant risks with thermal ablation techniques. Recent studies have demonstrated PFA’s efficacy in effectively eliminating arrhythmogenic foci with a substantially lower risk of complications such as steam pops or collateral tissue damage [66]. Furthermore, PFA’s ability to create more homogenous and transmural lesions [67] could potentially lead to improved long-term success rates in treating ventricular tachycardias, especially in substrates where conventional ablation has some limitations [68]. Pivotal VT ablation cases with PFA appeared successful [69, 70]; however, the durability of the lesions and long-term effectiveness still remain unknown. Given a possible risk of coronary arterial spasm, precautions such as systemic or intracoronary nitroglycerin administration should be performed preceding PFA applications in order to minimize such risk of high-voltage delivery into ventricles [71].
Conclusions
Catheter ablation of ventricular arrhythmias is highly successful, but failure may occur in case of anatomical obstacles such as intramural arrhythmia origin or proximity to the coronary arteries. In situations where standard ablation proves unsuccessful, innovative ablation techniques can serve as a backup strategy and may attain success, especially in cases with a difficult substrate of arrhythmia.
References and Recommended Readings
Papers of particular interest, published recently, have been highlighted as: • Of importance
Vaseghi M, Hu TY, Tung R, Vergara P, Frankel DS, Di Biase L, et al. Outcomes of catheter ablation of ventricular tachycardia based on etiology in nonischemic heart disease: an international ventricular tachycardia ablation center collaborative study. JACC Clin Electrophysiol. 2018;4(9):1141–50.
Chung FP, Lin YJ, Chang SL, Lo LW, Hu YF, Chen YY, et al. Long-term follow-up of catheter ablation of ventricular arrhythmias: experiences from a tertiary referral center in Taiwan. Acta Cardiol Sin. 2015;31(1):8–17.
Chung FP, Lin CY, Shirai Y, Futyma P, Santangeli P, Lin YJ, et al. Outcomes of catheter ablation of ventricular arrhythmia originating from the left ventricular summit: a multicenter study. Heart Rhythm. 2020;17(7):1077–83.
Huizar JF, Ellenbogen KA, Tan AY, Kaszala K. Arrhythmia-induced cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(18):2328–44.
• Hanson M, Futyma P, Bode W, Liang JJ, Tapia C, Adams C, et al. Catheter ablation of intramural outflow tract premature ventricular complexes: a multicentre study. Europace. 2023;25(5):euad100. Findings from this study suggest that ablation of intramural PVCs is challenging and can sometimes require advanced ablation strategies to achieve acute success.
Berte B, Sacher F, Venlet J, Andreu D, Mahida S, Aldhoon B, et al. VT recurrence after ablation: incomplete ablation or disease progression? A multicentric European study. J Cardiovasc Electrophysiol. 2016;27(1):80–7.
Roca-Luque I, Van Breukelen A, Alarcon F, Garre P, Tolosana JM, Borras R, et al. Ventricular scar channel entrances identified by new wideband cardiac magnetic resonance sequence to guide ventricular tachycardia ablation in patients with cardiac defibrillators. EP Europace. 2020;22(4):598–606.
John LA, Tomashitis B, Gowani Z, Levin D, Vo C, John I, et al. inHEART Models software - novel 3D cardiac modeling solution. Expert Rev Med Devices. 2023;20(10):797–803.
Kanawati J, De Silva K, Bhaskaran A, Turnbull S, Zhou J, Kotake Y, et al. Intracardiac echocardiography techniques to identify ventricular arrhythmia substrate. Heart Rhythm O2. 2022;3(5):602–12.
Kuwahara T. Intracardiac echocardiography in catheter ablation for atrial fibrillation: it is better to see what you are doing? J Atr Fibrillation. 2015;7(6):1215.
Dello Russo A, Compagnucci P, Bergonti M, Cipolletta L, Parisi Q, Volpato G, et al. Microelectrode voltage mapping for substrate assessment in catheter ablation of ventricular tachycardia: a dual-center experience. J Cardiovasc Electrophysiol. 2023;34(5):1216–27.
Berte B, Zeppenfeld K, Tung R. Impact of micro-, mini- and multi-electrode mapping on ventricular substrate characterisation. Arrhythm Electrophysiol Rev. 2020;9(3):128–35.
Anderson RD, Rodriguez Padilla J, Joens C, Masse S, Bhaskaran A, Magtibay K, et al. On the electrophysiology and mapping of intramural arrhythmic focus. Circ Arrhythm Electrophysiol. 2022;15(5):e010384.
Segal OR, Wong T, Chow AWC, Jarman JWE, Schilling RJ, Markides V, et al. Intra-coronary guidewire mapping-a novel technique to guide ablation of human ventricular tachycardia. J Interv Card Electrophysiol. 2007;18(2):143–54.
Vlachos K, Letsas KP, Srinivasan NT, Frontera A, Efremidis M, Dragasis S, et al. The value of functional substrate mapping in ventricular tachycardia ablation. Heart rhythm O2. 2023;4(2):134–46.
Jackson N, Gizurarson S, Viswanathan K, King B, Massé S, Kusha M, et al. Decrement evoked potential mapping. Circ Arrhythm Electrophysiol. 2015;8(6):1433–42.
Porta-Sánchez A, Jackson N, Lukac P, Kristiansen SB, Nielsen JM, Gizurarson S, et al. Multicenter study of ischemic ventricular tachycardia ablation with decrement-evoked potential (DEEP) mapping with extra stimulus. JACC Clin Electrophysiol. 2018;4(3):307–15.
Vázquez-Calvo S, Garre P, Sanchez-Somonte P, Borras R, Quinto L, Caixal G, et al. Orthogonal high-density mapping with ventricular tachycardia isthmus analysis vs. pure substrate ventricular tachycardia ablation: a case–control study. Front Cardiovasc Med. 2022;9:912335.
Jiang R, Beaser AD, Aziz Z, Upadhyay GA, Nayak HM, Tung R. High-density grid catheter for detailed mapping of sinus rhythm and scar-related ventricular tachycardia: comparison with a linear duodecapolar catheter. JACC Clini Electrophysiol. 2020;6(3):311–23.
Denham N, Ding WY, Campbell T, Modi S, Luther V, Todd D, et al. UltraSOUND-based characterization of ventricular tachycardia SCAR and arrhythmogenic substrate: THE SOUNDSCAR study. Heart Rhythm. 2024;21(1):45–53.
Liao Z, Zhan X, Wu S, Xue Y, Fang X, Liao H, et al. Idiopathic ventricular arrhythmias originating from the pulmonary sinus cusp: prevalence, electrocardiographic/electrophysiological characteristics, and catheter ablation. J Am Coll Cardiol. 2015;66(23):2633–44.
Zhang J, Tang C, Zhang Y, Su X. Pulmonary sinus cusp mapping and ablation: a new concept and approach for idiopathic right ventricular outflow tract arrhythmias. Heart Rhythm. 2018;15(1):38–45.
Heeger CH, Kuck KH, Ouyang F. Catheter ablation of pulmonary sinus cusp-derived ventricular arrhythmias by the reversed U-curve technique. J Cardiovasc Electrophysiol. 2017;28(7):776–7.
Futyma P, Moroka K, Derndorfer M, Kollias G, Martinek M, Pürerfellner H. Left pulmonary cusp ablation of refractory ventricular arrhythmia originating from the inaccessible summit. Europace. 2019;21(8):1253.
Liang Z, Wang Y, Zhang T, Han Z, Dong J, Ren X. Catheter ablation of ventricular arrhythmias with QRS morphology resembling that of aortic sinus cusp arrhythmias: significance of mapping the left pulmonary sinus cusp. J Cardiovasc Electrophysiol. 2018;29(4):591–9.
Dong X, Sun Q, Tang M, Zhang S. Catheter ablation of ventricular arrhythmias originating from the junction of the pulmonary sinus cusp via a nonreversed U curve approach. Heart Rhythm. 2019;16(10):1513–20.
Nagashima K, Choi EK, Lin KY, Kumar S, Tedrow UB, Koplan BA, et al. Ventricular arrhythmias near the distal great cardiac vein: challenging arrhythmia for ablation. Circ Arrhythm Electrophysiol. 2014;7(5):906–12.
Suresh A, Chang SL, Lin YJ, Lo LW, Chung FP, Chen SA. Ablation of ventricular tachycardia arising from the great cardiac vein – a rare cause of coronary artery injury. Acta Cardiol Sin. 2017;33(5):553–5.
Baszko A, Kałmucki P, Siminiak T, Szyszka A. Telescopic coronary sinus cannulation for mapping and ethanol ablation of arrhythmia originating from left ventricular summit. Cardiol J. 2020;27(3):312–5.
Enriquez A, Malavassi F, Saenz LC, Supple G, Santangeli P, Marchlinski FE, et al. How to map and ablate left ventricular summit arrhythmias. Heart Rhythm. 2017;14(1):141–8.
Guandalini GS, Santangeli P, Schaller R, Pothineni NVK, Briceño DF, Enriquez A, et al. Intramyocardial mapping of ventricular premature depolarizations via septal venous perforators: differentiating the superior intraseptal region from left ventricular summit origins. Heart Rhythm. 2022;19(9):1475–83.
Tung R, Liu Q, Jiang R, Jiang C. Nonionic irrigated radiofrequency ablation of refractory incessant ventricular tachycardia via great cardiac vein. Heart Rhythm Case Rep. 2018;4(12):572–5.
Nguyen DT, Tzou WS, Sandhu A, Gianni C, Anter E, Tung R, et al. Prospective multicenter experience with cooled radiofrequency ablation using high impedance irrigant to target deep myocardial substrate refractory to standard ablation. JACC Clin Electrophysiol. 2018;4(9):1176–85.
Hasegawa K, Yoneda ZT, Powers EM, Tokutake K, Kurata M, Richardson TD, et al. Safety of ventricular arrhythmia radiofrequency ablation with half normal saline irrigation. EP Europace. 2024;26(2):euae018.
Santangeli P, Marchlinski FE, Zado ES, Benhayon D, Hutchinson MD, Lin D, et al. Percutaneous epicardial ablation of ventricular arrhythmias arising from the left ventricular summit: outcomes and electrocardiogram correlates of success. Circ Arrhythm Electrophysiol. 2015;8(2):337–43.
Okubo K, Gigli L, Della BP. Catheter ablation of ventricular tachycardia in nonischemic cardiomyopathy. J Arrhythm. 2018;34(4):347–55.
• Mueller J, Chakarov I, Halbfass P, Nentwich K, Ene E, Berkovitz A, et al. Adverse Prognosis of Patients With Septal Substrate After VT ablation due to electrical storm. JACC Clin Electrophysiol. 2023;9(6):790–804. In this study patients with septal substrate for VT were compared with patients without septal substrate. The first group had significantly decreased acute ablation successes and increased long-term cardiovascular mortality. VT recurrence rates were similar in both groups during follow-up.
Sultan A, Futyma P, Metzner A, Anic A, Richter S, Roten L, et al. Management of ventricular tachycardias: insights on centre settings, procedural workflow, endpoints, and implementation of guidelines-results from an EHRA survey. Europace. 2024;26(2):euae030.
Neira V, Santangeli P, Futyma P, Sapp J, Valderrabano M, Garcia F, et al. Ablation strategies for intramural ventricular arrhythmias. Heart Rhythm. 2020;17(7):1176–84.
Kay GN, Epstein AE, Bubien RS, Anderson PG, Dailey SM, Plumb VJ. Intracoronary ethanol ablation for the treatment of recurrent sustained ventricular tachycardia. J Am Coll Cardiol. 1992;19(1):159–68.
Tavares L, Lador A, Fuentes S, Da-Wariboko A, Blaszyk K, Malaczynska-Rajpold K, et al. Intramural venous ethanol infusion for refractory ventricular arrhythmias: outcomes of a multicenter experience. JACC Clin Electrophysiol. 2020;6(11):1420–31.
Da-Wariboko A, Lador A, Tavares L, Dave AS, Schurmann PA, Peichl P, et al. Double-balloon technique for retrograde venous ethanol ablation of ventricular arrhythmias in the absence of suitable intramural veins. Heart Rhythm. 2020;17(12):2126–34.
Futyma P, Chen S, Enriquez A, Pürerfellner H, Santangeli P. Bipolar ablation of ventricular arrhythmias: step-by-step. J Cardiovasc Electrophysiol. 2023;34(12):2599–606.
Teh AW, Reddy VY, Koruth JS, Miller MA, Choudry S, D’Avila A, et al. Bipolar radiofrequency catheter ablation for refractory ventricular outflow tract arrhythmias. J Cardiovasc Electrophysiol. 2014;25(10):1093–9.
Futyma P, Santangeli P, Pürerfellner H, Pothineni NV, Głuszczyk R, Ciąpała K, et al. Anatomic approach with bipolar ablation between the left pulmonic cusp and left ventricular outflow tract for left ventricular summit arrhythmias. Heart Rhythm. 2020;17(9):1519–27.
Enriquez A, Hanson M, Nazer B, Gibson DN, Cano O, Tokioka S, et al. Bipolar ablation involving coronary venous system for refractory left ventricular summit arrhythmias. Heart Rhythm O2. 2023;5(1):24–33.
Futyma P, Sauer WH. Bipolar radiofrequency catheter ablation of left ventricular summit arrhythmias. Card Electrophysiol Clin. 2023;15(1):57–62.
Zhou B, Yu J, Ju W, Li X, Zhang F, Chen H, et al. Bipolar catheter ablation strategies for outflow tract ventricular arrhythmias refractory to unipolar ablation. J Cardiovasc Electrophysiol. 2022;33(8):1769–78.
Futyma P, Sander J, Ciąpała K, Głuszczyk R, Wysokińska A, Futyma M, et al. Bipolar radiofrequency ablation delivered from coronary veins and adjacent endocardium for treatment of refractory left ventricular summit arrhythmias. J Interv Card Electrophysiol. 2020;58(3):307–13.
Tokioka S, Fukamizu S, Kawamura I, Kitamura T, Hojo R. Bipolar radiofrequency catheter ablation between the left ventricular endocardium and great cardiac vein for refractory ventricular premature complexes originating from the left ventricular summit. J Arrhythm. 2020;36(2):363–6.
Waight MC, Wiles BM, Li AC, Saba MM. Bipolar radiofrequency ablation of septal ventricular tachycardia facilitated by an intramural catheter. JACC Case Rep. 2021;3(8):1119–24.
Futyma P, Ciąpała K, Sander J, Głuszczyk R, Futyma M, Kułakowski P. Bipolar radiofrequency ablation of ventricular arrhythmias originating in the vicinity of his bundle. Circ Arrhythm Electrophysiol. 2020;13(3):e008165.
Futyma P, Kułakowski P. Bipolar ablation delivered between the pulmonary and aortic valve cusps. Rev Esp Cardiol (Engl Ed). 2019;72(12):1078.
Futyma P, Wysokińska A, Sander J, Futyma M, Kułakowski P. Bipolar endo-epicardial radiofrequency ablation of arrhythmia originating from the left ventricular summit. Circ J. 2018;82(6):1721–2.
Igarashi M, Nogami A, Fukamizu S, Sekiguchi Y, Nitta J, Sakamoto N, et al. Acute and long-term results of bipolar radiofrequency catheter ablation of refractory ventricular arrhythmias of deep intramural origin. Heart Rhythm. 2020;17(9):1500–7.
Futyma P, Kułakowski P. Frontal placement of dispersive patch for effective ablation of arrhythmia originating from the anterior right ventricular outflow tract. J Interv Card Electrophysiol. 2017;49(3):327.
Barkagan M, Rottmann M, Leshem E, Shen C, Buxton AE, Anter E. Effect of baseline impedance on ablation lesion dimensions. Circ Arrhythm Electrophysiol. 2018;11(10):e006690.
Wei C, Qian P, Tedrow U, Mak R, Zei PC. Non-invasive stereotactic radioablation: a new option for the treatment of ventricular arrhythmias. Arrhythm Electrophysiol Rev. 2019;8(4):285–93.
Cozzi S, Bottoni N, Botti A, Trojani V, Alì E, Finocchi Ghersi S, et al. The use of cardiac stereotactic radiation therapy (SBRT) to manage ventricular tachycardia: a case report, review of the literature and technical noTes. J Pers Med. 2022;12(11):1783.
Cuculich PS, Schill MR, Kashani R, Mutic S, Lang A, Cooper D, et al. Noninvasive cardiac radiation for ablation of ventricular tachycardia. N Engl J Med. 2017;377(24):2325–36.
• Kautzner J, Jedlickova K, Sramko M, Peichl P, Cvek J, Ing LK, et al. Radiation-induced changes in ventricular myocardium after stereotactic body radiotherapy for recurrent ventricular tachycardia. JACC Clin Electrophysiol. 2021;7(12):1487–92. This study revealed that SBRT may be promising option for VT treatment especially in patients after previously failed catheter ablation.
Ninni S, Gallot-Lavallée T, Klein C, Longère B, Brigadeau F, Potelle C, et al. Stereotactic radioablation for ventricular tachycardia in the setting of electrical storm. Circ Arrhythm Electrophysiol. 2022;15(9):e010955.
Amino M, Yoshioka K, Tanabe T, Tanaka E, Mori H, Furusawa Y, et al. Heavy ion radiation up-regulates Cx43 and ameliorates arrhythmogenic substrates in hearts after myocardial infarction. Cardiovasc Res. 2006;72(3):412–21.
Schmidt B, Bordignon S, Neven K, Reichlin T, Blaauw Y, Hansen J, et al. EUropean real-world outcomes with pulsed field ablation in patients with symptomatic atRIAl fibrillation: lessons from the multi-centre EU-PORIA registry. Europace. 2023;25(7):euad185.
Koruth JS, Kuroki K, Iwasawa J, Viswanathan R, Brose R, Buck ED, et al. Endocardial ventricular pulsed field ablation: a proof-of-concept preclinical evaluation. EP Europace. 2020;22(3):434–9.
Im SI, Higuchi S, Lee A, Stillson C, Buck E, Morrow B, et al. Pulsed field ablation of left ventricular myocardium in a swine infarct model. JACC Clin Electrophysiol. 2022;8(6):722–31.
Stewart MT, Haines DE, Verma A, Kirchhof N, Barka N, Grassl E, et al. Intracardiac pulsed field ablation: Proof of feasibility in a chronic porcine model. Heart Rhythm. 2019;16(5):754–64.
Hartl S, Reinsch N, Füting A, Neven K. Pearls and pitfalls of pulsed field ablation. Korean Circ J. 2023;53(5):273–93.
Lozano-Granero C, Hirokami J, Franco E, Tohoku S, Matía-Francés R, Schmidt B, Hernández-Madrid A, Zamorano Gómez JL, Moreno J, Chun J. Case Series of Ventricular Tachycardia Ablation With Pulsed-Field Ablation: Pushing Technology Further (Into the Ventricle). JACC Clin Electrophysiol. 2023;9(9):1990–4.
Ouss A, van Stratum L, van der Voort P, Dekker L. First in human pulsed field ablation to treat scar-related ventricular tachycardia in ischemic heart disease: a case report. J Interv Card Electrophysiol. 2023;66(3):509–10.
Reddy VY, Petru J, Funasako M, Kopriva K, Hala P, Chovanec M, et al. Coronary arterial spasm during pulsed field ablation to treat atrial fibrillation. Circ. 2022;146(24):1808–19.
Author information
Authors and Affiliations
Contributions
Both authors (L.Z. and P.F.) contributed equally to the content of the manuscript and this included manuscript draft, careful revision of the manuscript and acceptance of the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
PF reports patent applications related with bipolar and high-voltage ablation and that he has equity in CorSystem. Łukasz Zarębski declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zarębski, Ł., Futyma, P. Challenges in Ventricular Arrhythmia Ablation: Difficult Substrates and Advanced Ablation Strategies. Curr Treat Options Cardio Med 26, 111–120 (2024). https://doi.org/10.1007/s11936-024-01036-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11936-024-01036-4