Abstract
Purpose of review
The COVID-19 pandemic has disrupted healthcare and has disproportionately affected the marginalized populations. Patients with cancer and cardiovascular disease (cardio-oncology population) are uniquely affected. In this review, we explore the current data on COVID-19 vulnerability and outcomes in these patients and discuss strategies for cardio-oncology care with a focus on healthcare innovation, health equity, and inclusion.
Recent findings
The growing evidence suggest increased morbidity and mortality from COVID-19 in patients with comorbid cancer and cardiovascular disease. Additionally, de novo cardiovascular complications such as myocarditis, myocardial infarction, arrhythmia, heart failure, and thromboembolic events have increasingly emerged, possibly due to an accentuated host immune response and cytokine release syndrome.
Summary
Patient-centric policies are helpful for cardio-oncology surveillance like remote monitoring, increased use of biomarker-based surveillance, imaging modalities like CT scan, and point-of-care ultrasound to minimize the exposure for high-risk patients. Abundant prior experience in cancer therapy scaffolded the repurposed use of corticosteroids, IL-6 inhibitors, and Janus kinase inhibitors in the treatment of COVID-19 infection. COVID-19 vaccine timing and dose frequency present a challenge due to overlapping toxicities and immune cell depletion in patients receiving cancer therapies. The SARS-CoV-2 pandemic laid bare social and ethnic disparities in healthcare but also steered in innovation to combat problems of patient outreach, particularly with virtual care. In the recovery phase, the backlog in cardio-oncology care, interplay of cancer therapy-related side effects, and long COVID-19 syndrome are crucial issues to address.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The COVID-19 pandemic brought forth unforeseen challenges in cancer and cardiovascular (CV) care. CV disease (CVD) and its risk factors like diabetes and hypertension are associated with a higher incidence of adverse outcomes, including mortality [1]. In addition, there is substantial evidence suggesting that patients with cancer on immunosuppressive therapy have an increased risk of acquiring COVID-19 infection and developing severe illness [2]. Cancer, unfortunately, became the “forgotten C” due to fear of presentation due to exposure risk, precipitous drop in routine screening, and delayed treatment for identified oncology patients. The field of cardio-oncology suffered setbacks from difficultly navigating surveillance for cardiotoxicity while attempting to minimize the COVID-19 exposure to both patients and healthcare workers. This review addresses the unique challenges for cardio-oncology patients and strategies to care for this highly vulnerable population during the ongoing COVID-19 pandemic.
Vulnerability and Outcomes
In early 2020, Liang et al. brought to light that ~1% of COVID-19 patients had a history of cancer which was higher than the total incidence of cancer (0.29%) in the overall Chinese population [2]. A meta-analysis estimated an increased risk of severe and death in the cancer population [3]. Another study showed that patients with COVID-19 and a history of cancer had a 3-fold higher risk of death, with cancer being an independent risk factor for severe disease [4••]. Complications like venous thromboembolism and arrhythmias were more commonly noted in COVID-19 with a history of cancer [4••]. Additionally, patients on active or recent chemotherapy have a higher incidence of severe COVID-19-related disease [4••].
On the other side, CV risk factors like older age, diabetes [5], hypertension, and dyslipidemia [6] are also associated with a higher risk of in-hospital mortality in the COVID-19 population. Preexisting CVD poses an even higher risk of mortality than CV risk factors alone [7].
A retrospective study demonstrated that patients with comorbid cancer and CVD (cardio-oncology patients) had a higher likelihood of developing complications such as arrhythmia and encephalopathy as compared with patients with cancer only [4••]. Similarly, COVID-19-associated severe disease and mortality were noted at a significantly higher rate in cardio-oncology patients as compared with those with either cancer or CVD alone [4••].
Strategies for Cardio-Oncology Care during the COVID-19 Pandemic
While there is no one specific way of taking care of this high-risk patient population, and recommendations are likely to change time to time as our knowledge evolves, we provide some suggestions based on the lessons learnt during the pandemic so far (Fig. 1).
Strategies for cardio-oncology care during the COVID-19 pandemic. This figure suggests various strategies and dimensions to effectively care for cardio-oncology patients during the COVID-19 pandemic. Abbreviations: AI, artificial intelligence; CT, computed tomography; CIEDs, cardiac implantable electronic devices; CVD, cardiovascular disease; HCPs: healthcare providers; ICU, intensive care unit; ILRs, implantable loop recorders; POCUS, point of care ultrasound.
Telemedicine
Impetus from the pandemic has brought innovation to healthcare delivery. For close to 2 years now, telemedicine spearheaded navigation around the need to abate unnecessary healthcare exposure while providing high-quality care. The cardio-oncology population, at the pinnacle of vulnerability, sought to gain the most from telehealth. It became imperative to reduce visits to the hospital for investigations, consultations, or treatments to mitigate their exposure risk. Careful stratification of patients, who do not necessarily need a physical examination, to a virtual visit alleviates the added stress of in-person visit [8]. Scrupulous care coordination by scheduling tests like cardiac biomarkers for cardiotoxicity vigilance during pre-booked oncology treatment sessions would forestall frequent hospital exposures. Collaboration with oncology colleagues as they navigate clinical conundrums like chemotherapy regimen modifications to reduce the risk of infection in their patients is crucial [9]. Tele-visits ensure equitable care by obviating transportation costs, loss of income, and the need to arrange for childcare.
On the flip side, to avoid complacency, it is vital to be cognizant of the limitations of telehealth like loss of subconscious visual cues, inability to perform a physical exam, need for interstate licensing when caring for patients across state lines, disparities in access to technology and high-speed Internet at home [10], and also the inability to use technology, particularly, in the elderly who may not be ready [11] for the telehealth revolution.
An unprecedented escalation in demand for intensivists due to the “surge” in COVID-19 admissions has also led to the adoption of tele-ICU care. Particularly in the rural parts of the USA, it has improved mortality outcomes [12]. In addition, virtual rehab programs utilizing digital health monitoring can facilitate controlled indoor rehabilitation allowing both cancer patients and COVID-19 survivors to remain physically active while at home [13].
Surveillance Strategies
Imaging
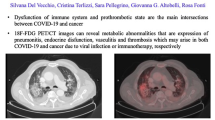
Use of CV imaging in the context of COVID-19 is complex especially when intertwined with its implications in cardio-oncology patients [14, 15]. This is particularly nuanced in patients requiring cardiovascular surveillance during and after cancer treatments with known cardiotoxicity, such as trastuzumab and anthracyclines [16, 17]. Strategies such as utilizing point of care ultrasound to answer specific questions like left ventricular function, strain or presence of a pericardial effusion, minimizing acquisition time to reduce the duration of interaction with healthcare professionals, and increasing the interval between surveillance echocardiograms in low-risk patients and those on drugs with lesser cardiotoxic effects have been suggested [18]. Temporarily deferring routine cardiovascular imaging in asymptomatic long-term cancer survivors is practical during the peak of the pandemic. Post-COVID infection cancer patients with either overt or subclinical myocardial injury should undergo repeat imaging before initiating the next treatment cycle. Atrial fibrillation is common in the cardio-oncology population, and drugs like ibrutinib and tyrosine kinase inhibitors are known precipitators [19]. Reckoning with the risk of aerosolization that transesophageal echocardiogram (TEE) would bring, cardiac computed tomography (CCT) might serve as an acceptable alternative to rule out intracardiac thrombus before cardioversion [20]. Its accuracy and quick acquisition time, ensuring reduced exposure, have brought CCT to the forefront during the pandemic. Coronary CT angiography is utilized in ruling out coronary artery disease (CAD) in those with no prior CAD presenting with acute chest pain by excluding high-risk anatomy with or without the CT-fractional flow reserve [21–23]. Measures to protect technicians and patients include appropriate PPE, surveillance COVID-19 testing, frequent disinfection of echo machines and environment, and using preassigned machines for COVID-19 patients [23].
Remote monitoring
Leveraging digital health technology to monitor heart rate, blood pressure (BP), and weight expands our reach when caring for patients at home. For example, patients on VEGF inhibitors [9] can digitally transmit their BP recordings to their providers. Those diagnosed with anthracycline-induced heart failure can have their weights monitored remotely, preemptively avoiding hospital admission. Patients with cardiac implantable electrical devices (CIEDs) [24, 25], CardioMEMs [26], and implantable loop recorders can be alerted by their cardiologists with clinical status changes with remote monitoring. These devices can serve as proxies for physical activity [27]. Data from commercially available wearable devices like smartwatches can also be linked to the patient’s electronic medical records for review.
Biomarkers
While imaging-based surveillance is considered superior, cardiac troponin I for anthracycline-based therapy is a valid alternative. Other cardiac biomarkers—natriuretic peptides, such as B-type NP (BNP) and N-terminal pro-BNP (NT-proBNP)—have been successfully used in patients with multiple myeloma undergoing treatment with carfilzomib [28]. Serial biomarker-based cardiotoxicity surveillance can be particularly helpful in minimizing exposure to SARS-CoV-2. Such a strategy can be used as a “gatekeeper” and reserve imaging surveillance to when only clinically needed. This approach was adapted by various centers and recommended in the consensus statement by the International Cardio-Oncology Society (ICOS) [29••]. Further efforts are needed to validate this approach in a broad range of patients receiving antineoplastic therapies. Cardiac troponin or BNP/NT-proBNP elevation may also occur due to COVID-19 infection, and hence, high index of suspicion is required for prompt diagnosis.
Hypercoagulability and Anticoagulation (AC) Strategies
The prevalence of venous thromboembolism (VTE) ranges between 8% in non-hospitalized COVID-19 patients and 22.7% in those admitted to the ICU [30]. The pooled prevalence of deep vein thrombosis and pulmonary embolism are 14.8% and 15.8% [31], respectively. Approximately 10% of hospitalized patients with COVID-19 develop atrial fibrillation [32]. Therapeutic anticoagulation is indicated in all these situations.
Increased risk of thrombogenic events in hospitalized patients with COVID-19 has sparked a significant interest in examining the role of therapeutic dose anticoagulation in this population. Sadeghipour et al. demonstrated that there was no difference in venous or arterial thrombosis, treatment with extracorporeal membrane oxygenation, or mortality within 30 days in COVID-19 patients who were treated with intermediate vs. standard-dose prophylactic AC [33]. The ACTION trial did not show any benefit of therapeutic AC over prophylactic AC in COVID-19 patients with an elevated D-dimer [34]. Three international trials (ATTACC, ACTIV-4, and REMAP-CAP) were prematurely terminated in the ICU arm due to the futility of therapeutic AC and a signal towards harm [35].
In contrast, in the moderately ill group of patients, therapeutic heparin or low molecular weight heparin (LMWH) increased survival until hospital discharge, however with an increased risk of major bleeding [35]. Therefore, the CDC currently only recommends prophylactic AC in hospitalized SARS-CoV-2 patients in absence of thromboembolic event. While active or recent cancer and many antineoplastic therapies increase the risk of thromboembolic complications, there is also an increased risk of bleeding complications in these patients. Severe COVID-19 infection in this subset of patients adds complexity in determining the role of therapeutic AC in the absence of an established indication. Currently, most experts recommend only prophylactic dose anticoagulation in this high-risk population [36]. However, an individualized approach is warranted.
Overlaps between Cardio-Oncology and COVID-19
The presenting symptoms of COVID-19 infection, cancer, de novo cardiovascular disease, and adverse effects of antineoplastic therapy have considerable overlap which brings on several diagnostic conundrums (Fig. 2). Patients on immune checkpoint inhibitors presenting with troponinemia may have ICI-associated myocarditis. However, due consideration for other diagnoses such as COVID-19 infection–related myocardial injury, COVID-19 vaccine–related myocarditis, or even type 2 myocardial infarction from the underlying cancer physiology itself may need to be entertained. A known catastrophic adverse effect of CAR-T cell therapy is the development of cytokine release syndrome (CRS). If these patients contract COVID-19, severe infection may mimic CRS which can pose a diagnostic dilemma. Similar situations may arise in patients presenting with venous thromboembolism or acute coronary syndrome where it may be muddling to determine the primary cause as cancer-related, antineoplastic therapy–related or COVID-19-related. Detailed clinical history with emphasis on the chronology of events along with biomarker testing and imaging may help delineate these diagnoses. The upmost care should be taken to avoid false diagnoses to ensure appropriate cancer therapy and a robust vaccination coverage in this population.
Overlap in cardiovascular manifestations between cancer, cardiovascular disease, adverse effects of anti-neoplastic therapy, COVID-19 infection, and COVID-19 vaccine–related side effects. This figure demonstrates the potential overlapping cardiovascular manifestations in cardio-oncology population (from cancer, de-novo cardiovascular disease, adverse effects of antineoplastic therapies, COVID-19 infection, and COVID-19 vaccine adverse effects) along with strategies for diagnosis.
Repurposing Cancer and Cardio-Oncology Therapies for COVID-19
Research exploring the links between the pathophysiology of cancer, mechanism of cytokine storm in CAR-T therapy, and severe COVID-19 infection is breaking new ground with several clinical trials testing the benefit of repurposed cancer drugs in SARS-CoV-2 infection [37].
Severe COVID-19 Infection Pathophysiology
The hyperinflammatory stage of COVID-19 leads to multiorgan dysfunction and poses a poor prognosis. Mechanistically, SARS-CoV-2 enters the cell via the angiotensin-converting enzyme-related carboxypeptidase 2 (ACE2), which are present on macrophages and alveolar type 2 pneumocytes [38]. The activation of NF-κB [39, 40] and JAK/STAT pathways [41] elicit the production of pro-inflammatory cytokines leading to CRS. IL-1β, IL-6, IL-7, IL-2, and TNF-α are upregulated in patients with severe infection and contribute to the perturbed immune response seen in severe COVID-19 infection. Elevated IL-6 levels are considered a marker of an overzealous immune response and indicate grave outcomes. Similar mechanism has been noted in cytokine storm related to variety of cancers and therapies, particularly with CAR T-cell therapy [42, 43]. Several anti-inflammatory and anti-cytokine agents such as corticosteroids, anti-IL-6 agents, and JAK1/2 kinase inhibitor [36] have been explored in COVID-19 management and the currently used drugs are listed in Table 1.
COVID-19 Vaccination in Patients With Cancer
In a remarkable feat in the history of medical science, the COVID-19 vaccines represented the fastest vaccine rollout. The response to the available COVID-19 vaccines is less robust in cancer patients than the general population [58, 59]. The poor efficacy of one dose of the BNT162b2 (Pfizer-BioNTech) vaccine in patients with solid tumors and hematological malignancies [59] improves significantly within 2 weeks of the second dose at day 21, which supported the prioritization of these patients for an early second dose. Increased prevalence of breakthrough infections in the immunocompromised prompted the CDC to approve the third dose of an mRNA vaccine at least 28 days after the second dose of either the Pfizer-BioNTech or Moderna COVID-19 vaccine [60].
The timing of vaccines is critical since overlapping toxicities/adverse effects, particularly hypersensitivity reactions, can confound the tolerability of either the vaccine or anticancer therapy. A gap of > 3 months is endorsed for those post-transplant or on adoptive cell therapies (e.g., CAR-T cell therapy) to avoid graft-vs-host reaction and the effects of immunosuppressive therapy [61]. Astute navigation of vaccine timing around chemotherapy infusions causing thrombocytopenia, lymphopenia, and neutropenia is essential. Anti-CD 20 antibodies like rituximab and ocrelizumab induce B cell depletion and have the potential to annul the efficacy of vaccination against SARS CoV-2 [62]. In one study, patients with at least partially repopulated B cells mounted a measurable antibody response. All patients had a robust T cell response irrespective of the presence of B cells [63]. It may be reasonable to await ample repopulation of B cells in clinically stable patients [64]. Prioritization of non-professional caregiver vaccination is another strategy to extend immunity coverage in their families.
Oncology and Cardio-Oncology Clinical Trials in the Pandemic Times
There was a rapid disruption in oncology clinical trial accrual with the COVID-19 outbreak [65]. The need to combat the SARS-CoV-2 virus without trepidation redirected investigators towards COVID-19-related research. Most of the trials on COVID-19 vaccinations excluded patients with malignancies, thus limiting the data on the safety and efficacy of the vaccines in patients with cancer. Oncology patients were posed with a “catch-22” of choosing between investigational oncology drugs and being vaccinated. For many patients, trial drugs may be the best or only available treatment option. Receiving the COVID-19 vaccine under EUA does not constitute treatment with an investigational agent. Therefore, enrollment in an oncology clinical trial should not prohibit one from the vaccination as it can be recorded as concomitant therapy [66]. Conversely, if eligible, simultaneous admission to a vaccine trial should not prevent acceptance in an oncology trial.
Special considerations during screening for trials may be required. For instance, in breast cancer trials, screening exams should be done before the first dose or 4–6 weeks after the second dose of the COVID-19 vaccine because of its propensity to cause axillary lymphadenopathy [67]. In general, deferral of vaccination during the first cycle of most novel anticancer agents, including immunotherapy, is pragmatic. An interlude of 72 h to 2 weeks after vaccine administration is recommended to avoid intersection of adverse effects [66].
In patients that receive whole-body radiation, vaccination may be delayed to allow for immune reconstitution58. In addition, a few cases of radiation recall phenomenon [68–70] (an acute inflammatory skin reaction localized to an area of skin previously irradiated) have been described after inoculation against COVID-19.
Variants of Concerns (VOC)
The current COVID-19 variants of concern (VOC) in the USA are Alpha - B.1.1.7, Beta - B.1.351, Gamma - P.1, and Delta - B.1.617.2, with Delta being highly contagious causing rapid spread. The Omicron variant is the most heavily mutated variant so far, with enhanced transmissibility and partial resistance to vaccine induced immunity [71]. More data is awaited regarding this variant. mRNA vaccine efficacy against new variants such as Delta is around 79–88% in the average population after the second dose, but lower in solid organ transplant and immunocompromised patients [72]. In addition, approximately half of cardiac transplant recipients do not generate IgG antibodies following two doses of SARS-CoV-2 mRNA vaccine [73].
Hence, CDC currently recommends that moderately to severely immunocompromised people (active cancer treatment, solid organ transplant recipients including cardiac transplant and stem cell transplant, on immunosuppressive medications including steroids) receive an additional dose at least 28 days after completing the initial mRNA COVID-19 vaccine series. In a recent RCT [74], only 55% of the transplant recipients had an antibody level above the protective threshold of 100 U per milliliter after the third dose. Therefore, social distancing, protective measures like wearing a mask, and vaccination of relatives of these patients are strongly warranted.
Social and Ethnic Disparities
Disproportionate mortality and morbidity in the African American (AA) and Latino community with COVID-19 infection has exposed the preexisting equity chasm in healthcare [75]. A report released by the CDC showed that counties with the highest social vulnerability index, particularly those with a higher percentage of ethnic minority patients, crowded housing, and high-density housing structures, were at higher risk of becoming a COVID-19 hotspot [76].
There has been a precipitous decline in cancer screening with the pandemic, including colon, cervical, and breast cancer screening. This could overturn the gains on early cancer detection in the most vulnerable population. The impact is significantly higher in socially and economically marginalized population [77]. To catch up with the swift decline in newly identified cancers, measures like home-based self-sampling (HPV DNA sampling in cervical cancer screening and fecal DNA sampling for colon cancer screening) should be adopted along with efforts to increase awareness regarding the importance of timely preventative care.
AAs experience higher rates of cardiotoxicity from anthracyclines and trastuzumab than Caucasians [78] due to increased CV risk factors and likely due to suboptimal surveillance as well as management of such risk factors, driven by th e social determinants of health (SDOH). There is also insufficient representation in research, including medications that prevent cardiotoxicity like statins and dexrazoxane [79]. Environmental racism encompassing housing, working conditions, food, air, water, and soil can be the root cause of cancer and infectious diseases like COVID-19 (e.g., living in places with air pollution near chemical plants).
While there has been a significant gap in healthcare accessibility and outcomes, it has exponentially increased in this challenging time of the pandemic. Healthcare policy changes to help bring the marginalized population to the benefits of advanced healthcare infrastructure enjoyed by the mainstream and targeted resource allocation are required to minimize such inequities. Recognition of SDOH is crucial but we need to go a step forward and integrate them in the real-time clinical healthcare delivery system.
Other Aspects
Precision and innovation
While healthcare has adopted digital health technology, we have been relatively cautious and slow in using them to its maximum potential. The pandemic accelerated the adaption of existing digital technology and innovation to meet the compelling need and provide care. Using artificial intelligence and machine learning, novel and individualized risk stratification tools to identify cancer patients at high risk for complications with COVID-19 such as CORONET has been developed [80]. Molecular docking techniques using computational software (in silico medicine) have been utilized to identify potential antiviral agents for COVID-19. One such example is baricitinib, a JAK1/JAK2 inhibitor currently approved for COVID-19 treatment with remdesivir [57]. Genomic sequencing and -omics technology enables identification of genetic variants with increased susceptibility to drug-specific cardiotoxicity helping to minimize in-person interactions [81].
Social media
With 61% of healthcare workers (HCW) using social media (SoMe), it helped to continue networking opportunities, distribute educational materials, and raise public awareness during the pandemic [82]. SoMe platforms, particularly Twitter, have helped in real-time navigation of novel research findings at a time that in person conferences were cancelled [82]. SoMe will continue to play an important role not just in connecting people, but also in shaping the narrative. HCWs are usually seen as a reliable source of unbiased information, and hence, it is important that we leverage our societal influence responsibly for scientific information dissemination, vaccination and preventative care campaigns, research study recruitments and equally importantly to combat the misinformation.
Education and training
There has been a dramatic paradigm in inpatient training for fellows with a large number of trainees being re-deployed to care for non-cardiac intensive care unit and for COVID-19 patients. Although this translated to less dedicated time for trainees to pursue cardio-oncology electives or see cardio-oncology inpatient consults, commonalities between COVID-19 and cardio-oncology care can strengthen overall clinical decision-making skills. Exposure to telehealth and virtual classrooms opened new realms of learning. Access to experts in the field has surpassed geographic boundaries leading to a global classroom and thus democratizing cardio-oncology education and that should continue.
Recovery Phase
The consequences of staggered surveillance for cardiotoxicity, delayed cancer screening, delay in preventative CV care, and necessary procedures like TAVR and atrial fibrillation ablation in this vulnerable population are profound. There is growing evidence that cancer patients are lost to follow-up, and new cancers are being missed [83]. Vigilance and active scrutiny of patients at risk of cancer and cardiovascular disease are necessary to avoid delays in diagnosis. Continued utilization of telehealth services may partially assist in overcoming this backlog in cardio-oncology care. However, with the provision of adequate personal protective equipment and widespread vaccination coverage, transitioning to in-person visits in those who need more closer follow-up should no longer be deferred as delay in such care may have detrimental effects.
The most frequent symptoms of long COVID (Post-Acute Sequelae of SARS-CoV-2 infection (PASC)) are fatigue and dyspnea, which overlap with cancer symptoms posing a risk of under-recognition of early cancer [84]. In addition, physicians face the challenges of further delaying cancer treatment due to debilitating symptoms like extreme fatigue, cough, dyspnea, chest pain, and palpitations.
There have been theoretical considerations for cancer as a sequela of COVID-19 infection. Major signaling pathways implicated in aberrant cellular growth are activated; the ensuing cytokine storm weakens the immune system’s response to tumors, and patients may develop cancer as a result of superimposed mutagenic and/or carcinogenic events [85]. COVID-19 and cancer intersect through 4 common signaling pathways: cytokine, IFN-I, androgen receptor (AR), and immune checkpoint signaling [85]. COVID-19-associated T cell depletion and activation of oncogenic pathways like JAK-STAT, MAPK and NF-kB [86] potentially increase the risk of cancer in this population. Due to inflammation and virus-induced ACE-2 depletion, hypoxia can lead to oxidative stress resulting in DNA damage and subsequent carcinogenesis. The SARS-CoV virus nonstructural protein 3 is implicated in degradation of p53 (a tumor suppressor protein) [87]. Multiorgan dysfunction with extensive tissue damage from COVID-19 is also a likely oncogenic driver.
Conclusion
Patients with comorbid cancer and CVD are at higher risk for adverse outcomes when affected with COVID-19 as compared to patients with cancer or CVD alone. Additionally, even those who have not acquired the infection themselves, face enormous challenges due to the pandemic-related strain on healthcare infrastructure leading to suboptimal or delayed care. As the pandemic waxes and wanes, patient-centric recovery policies are required in all realms of healthcare, prioritizing high-risk populations such as those afflicted with a dual diagnosis of cancer and CVD (cardio-oncology population) with the focus on minimizing the interruptions in routine disease specific and preventative care while avoiding exposure to COVID-19. Widespread education and awareness among stakeholders and inclusion of cardio-oncology in our training curricula at the outset would help lay the foundation. Policy measures are needed to improve equitable access drawing attention to the social disparities in cardio-oncology care delivery. Though this is a moving target, disruptive innovation like digital health technology, telemedicine, genomics, and artificial intelligence will serve as driving forces towards health equilibrium for these patients.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nature Reviews Cardiology. 2020;17:543–58.
Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–7.
Tian Y, Qiu X, Wang C, et al. Cancer associates with risk and severe events of COVID-19: a systematic review and meta-analysis. International Journal of Cancer. 2021;148:363–74.
•• Ganatra S, Dani SS, Redd R, et al. Outcomes of COVID-19 in patients with a history of cancer and comorbid cardiovascular disease. J Natl Compr Canc Netw. 2020;1:1–10. This study demonstrates the patients with a history of both cancer and CVD are at significantly higher risk of experiencing COVID-19-associated adverse outcomes including mortality.
Silverio A, Di Maio M, Citro R, et al. Cardiovascular risk factors and mortality in hospitalized patients with COVID-19: systematic review and meta-analysis of 45 studies and 18,300 patients. BMC Cardiovascular Disorders. 2021;21:23.
Collard D, Nurmohamed NS, Kaiser Y, et al. Cardiovascular risk factors and COVID-19 outcomes in hospitalised patients: a prospective cohort study. BMJ Open. 2021;11: e045482.
O’Gallagher K, Shek A, Bean DM, et al. Pre-existing cardiovascular disease rather than cardiovascular risk factors drives mortality in COVID-19. BMC Cardiovascular Disorders. 2021;21:327.
Ganatra S, Hammond SP, Nohria A. The novel coronavirus disease (COVID-19) threat for patients with cardiovascular disease and cancer. JACC CardioOncol. 2020;2:350–5.
Parikh A, Kumar AA, Jahangir E. Cardio-oncology care in the time of COVID-19 and the role of telehealth. JACC CardioOncol. 2020;2:356–8.
Roberts ET, Mehrotra A. Assessment of disparities in digital access among medicare beneficiaries and implications for telemedicine. JAMA Internal Medicine. 2020;180:1386–9.
Lam K, Lu AD, Shi Y, Covinsky KE. Assessing telemedicine unreadiness among older adults in the United States during the COVID-19 pandemic. JAMA Internal Medicine. 2020;180:1389–91.
Fusaro MV, Becker C, Miller D, Hassan IF, Scurlock C. ICU telemedicine implementation and risk-adjusted mortality differences between daytime and nighttime coverage. Chest. 2021;159:1445–51.
Brown S-A, Rhee J-W, Guha A, Rao VU. Innovation in precision cardio-oncology during the coronavirus pandemic and into a post-pandemic world. Front Cardiovasc Med. 2020;7.
Kicska G, Litmanovich DE, Ordovas KG, et al. Statement from the North American Society for Cardiovascular Imaging on imaging strategies to reduce the scarcity of healthcare resources during the COVID-19 outbreak. The international journal of cardiovascular imaging. 2020;36:1387–93.
Beitzke D, Salgado R, Francone M, et al. Cardiac imaging procedures and the COVID-19 pandemic: recommendations of the European Society of Cardiovascular Radiology (ESCR). The international journal of cardiovascular imaging. 2020;36:1801–10.
Abdel-Qadir H, Nolan MT, Thavendiranathan P. Routine prophylactic cardioprotective therapy should be given to all recipients at risk of cardiotoxicity from cancer chemotherapy. Can J Cardiol. 2016;32:921–5.
Dani SS, Bagga S, Ganatra S. Editorial commentary: cardiovascular imaging in COVID-19: focus on safety, value, and clinical relevance. Trends Cardiovasc Med. 2021;31:17–9.
Baldassarre LA, Yang EH, Cheng RK, et al. Cardiovascular care of the oncology patient during COVID-19: an expert consensus document from the ACC cardio-oncology and imaging councils. J Natl Cancer Inst. 2021;113:513–22.
Yang X, Li X, Yuan M, et al. Anticancer therapy-induced atrial fibrillation: electrophysiology and related mechanisms. Front Pharmacol. 2018;9:1058.
Singh V, Choi AD, Leipsic J, et al. Use of cardiac CT amidst the COVID-19 pandemic and beyond: North American perspective. J Cardiovasc Comput Tomogr. 2021;15:16–26.
Choi AD, Abbara S, Branch KR, et al. Society of cardiovascular computed tomography guidance for use of cardiac computed tomography amidst the COVID-19 pandemic endorsed by the American College of Cardiology. J Cardiovasc Comput Tomogr. 2020;14:101–4.
Zoghbi WA, DiCarli MF, Blankstein R, et al. Multimodality cardiovascular imaging in the midst of the COVID-19 pandemic: ramping up safely to a new normal. JACC Cardiovasc Imaging. 2020;13:1615–26.
Mahmud E, Dauerman HL, Welt FGP, et al. Management of acute myocardial infarction during the COVID-19 pandemic: a position statement from the society for cardiovascular angiography and interventions (SCAI), the American College of Cardiology (ACC), and the American College of Emergency Physicians (ACEP). J Am Coll Cardiol. 2020;76:1375–84.
Kurek A, Tajstra M, Gadula-Gacek E, et al. Impact of remote monitoring on long-term prognosis in heart failure patients in a real-world cohort: results from all-comers COMMIT-HF trial. J Cardiovasc Electrophysiol. 2017;28:425–31.
Lakkireddy DR, Chung MK, Gopinathannair R, et al. Guidance for cardiac electrophysiology during the COVID-19 pandemic from the Heart Rhythm Society COVID-19 Task Force; Electrophysiology Section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology. American Heart Association Heart Rhythm. 2020;17:e233–41.
Bayes-Genis A, Codina P, Abdul-Jawad Altisent O, et al. Advanced remote care for heart failure in times of COVID-19 using an implantable pulmonary artery pressure sensor: the new normal. European Heart Journal Supplements. 2020;22:P29–32.
Al Fagih A, Al Onazi M, Al Basiri S, et al. Remotely monitored inactivity due to COVID-19 lockdowns. Potential hazard for heart failure patients. Saudi Med J 2020;41:1211–16
Waxman AJ, Clasen S, Hwang W-T, et al. Carfilzomib-associated cardiovascular adverse events: a systematic review and meta-analysis. JAMA Oncology 2018;4:e174519-e.
•• Lenihan D, Carver J, Porter C, et al. Cardio-oncology care in the era of the coronavirus disease 2019 (COVID-19) pandemic: an International Cardio-Oncology Society (ICOS) statement. CA Cancer J Clin. 2020;70(6):480–504. https://doi.org/10.3322/caac.21635. This consensus document by the International Cardio-Oncology Society (ICOS) provides in depth guidance about how to provide cardio-oncology care during the COVID-19 pandemic.
Nopp S, Moik F, Jilma B, Pabinger I, Ay C. Risk of venous thromboembolism in patients with COVID-19: a systematic review and meta-analysis. Research and Practice in Thrombosis and Haemostasis. 2020;4:1178–91.
Suh YJ, Hong H, Ohana M, et al. Pulmonary embolism and deep vein thrombosis in COVID-19: a systematic review and meta-analysis. Radiology. 2021;298:E70–80.
Musikantow, Daniel R et al. “Atrial fibrillation in patients hospitalized with COVID-19: incidence, predictors, outcomes, and comparison to influenza.” JACC. Clinical electrophysiology vol. 7,9 (2021): 1120-1130. https://doi.org/10.1016/j.jacep.2021.02.009
Investigators I. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325:1620–30.
Lopes RD, de Barros ESPGM, Furtado RHM, et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet (London, England) 2021;397:2253-63.
Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with Covid-19. New England Journal of Medicine 2021;385:790-802.
Ganatra S, Dani SS, Shah S, et al. Management of cardiovascular disease during coronavirus disease (COVID-19) pandemic. Trends Cardiovasc Med. 2020;30(6):315–25. https://doi.org/10.1016/j.tcm.2020.05.004.
Borcherding N, Jethava Y, Vikas P. Repurposing anti-cancer drugs for COVID-19 treatment. Drug Des Devel Ther. 2020;14:5045–58.
Turnquist C, Ryan BM, Horikawa I, Harris BT, Harris CC. Cytokine storms in cancer and COVID-19. Cancer Cell. 2020;38:598–601.
Hariharan A, Hakeem AR, Radhakrishnan S, Reddy MS, Rela M. The role and therapeutic potential of NF-kappa-B pathway in severe COVID-19 patients. Inflammopharmacology. 2021;29:91–100.
Kircheis R, Haasbach E, Lueftenegger D, Heyken WT, Ocker M, Planz O. NF-κB pathway as a potential target for treatment of critical stage COVID-19 patients. Frontiers in Immunology. 2020;11:3446.
Amini-Farsani Z, Farsani MY, Arab S, Forouzanfar F, Yadollahi M, Asgharzade S. Prediction and analysis of microRNAs involved in COVID-19 inflammatory processes associated with the NF-kB and JAK/STAT signaling pathways. Int Immunopharmacol 2021:108071.
Ganatra S, Carver JR, Hayek SS, et al. Chimeric antigen receptor T-cell therapy for cancer and heart: JACC Council Perspectives. J Am Coll Cardiol. 2019;74(25):3153–63. https://doi.org/10.1016/j.jacc.2019.10.049.
Ganatra S, Redd R, Hayek SS, et al. Chimeric antigen receptor T-cell therapy-associated cardiomyopathy in patients with refractory or relapsed non-hodgkin lymphoma. Circulation. 2020;142(17):1687–90. https://doi.org/10.1161/CIRCULATIONAHA.120.048100.
Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med 2020;384:693-704.
Pasin L, Navalesi P, Zangrillo A, et al. Corticosteroids for patients with coronavirus disease 2019 (COVID-19) with different disease severity: a meta-analysis of randomized clinical trials. J Cardiothorac Vasc Anesth. 2021;35:578–84.
Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324:1307–16.
Villar J, Belda J, Añón JM, et al. Evaluating the efficacy of dexamethasone in the treatment of patients with persistent acute respiratory distress syndrome: study protocol for a randomized controlled trial. Trials. 2016;17:342.
Dequin P-F, Heming N, Meziani F, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA. 2020;324:1298–306.
Ramakrishnan S, Nicolau DV Jr, Langford B, et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med. 2021;9:763–72.
Yu LM, Bafadhel M, Dorward J, et al. Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet (London, England) 2021.
Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med 2021;384:1491-502.
Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384:1503–16.
Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2020;384:20–30.
Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet (London, England) 2021;397:1637-45.
Lescure FX, Honda H, Fowler RA, et al. Sarilumab in patients admitted to hospital with severe or critical COVID-19: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021;9:522–32.
Gritti G, Raimondi F, Bottazzi B, et al. Siltuximab downregulates interleukin-8 and pentraxin 3 to improve ventilatory status and survival in severe COVID-19. Leukemia 2021.
Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2020;384:795–807.
Monin L, Laing AG, Muñoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–78.
Palich R, Veyri M, Marot S, et al. Weak immunogenicity after a single dose of SARS-CoV-2 mRNA vaccine in treated cancer patients. Ann Oncol. 2021;32:1051–3.
Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021.
Di Giacomo AM, Giacobini G, Gandolfo C, Lofiego MF, Cusi MG, Maio M. Severe acute respiratory syndrome coronavirus 2 vaccination and cancer therapy: a successful but mindful mix. Eur J Cancer. 2021;156:119–21.
Houot R, Levy R, Cartron G, Armand P. Could anti-CD20 therapy jeopardise the efficacy of a SARS-CoV-2 vaccine? Eur J Cancer. 2020;136:4–6.
Mrak D, Tobudic S, Koblischke M, et al. SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity. Annals of the Rheumatic Diseases 2021:annrheumdis-2021-220781.
van Assen S, Agmon-Levin N, Elkayam O, et al. EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2011;70:414–22.
Unger JM, Blanke CD, LeBlanc M, Hershman DL. Association of the coronavirus disease 2019 (COVID-19) outbreak with enrollment in cancer clinical trials. JAMA Netw Open 2020;3:e2010651-e.
Desai A, Gainor JF, Hegde A, et al. COVID-19 vaccine guidance for patients with cancer participating in oncology clinical trials. Nat Rev Clin Oncol. 2021;18:313–9.
Özütemiz C, Krystosek LA, Church AL, et al. Lymphadenopathy in COVID-19 vaccine recipients: diagnostic dilemma in oncologic patients. Radiology. 2021;300:E296–300.
Soyfer V, Gutfeld O, Shamai S, Schlocker A, Merimsky O. COVID-19 vaccine-induced radiation recall phenomenon. International Journal of Radiation Oncology* Biology* Physics. 2021.
Afacan E, Öğüt B, Üstün P, Şentürk E, Yazıcı O, Adışen E. Radiation recall dermatitis triggered by inactivated COVID-19 vaccine. Clinical and Experimental Dermatology;n/a.
Stewart R, McDowell L. Radiation recall phenomenon following COVID-19 vaccination. Int J Radiat Oncol Biol Phys. 2021.
Torjesen I. Covid-19: Omicron may be more transmissible than other variants and partly resistant to existing vaccines, scientists fear.
Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385:585–94.
Itzhaki Ben Zadok O, Shaul AA, Ben-Avraham B, et al. Immunogenicity of the BNT162b2 mRNA vaccine in heart transplant recipients - a prospective cohort study. Eur J Heart Fail. 2021.
Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. New England Journal of Medicine. 2021;385:661–2.
Hooper MW, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020;323(24):2466–7.
CDC report: association between social vulnerability and a county’s risk for becoming a COVID-19 hotspot — United States, June 1–July 25, 2020. 2020. at https://www.cdc.gov/mmwr/volumes/69/wr/mm6942a3.htm.
Schmidt AL, Bakouny Z, Bhalla S, et al. Cancer care disparities during the COVID-19 pandemic: COVID-19 and cancer outcomes study. Cancer Cell. 2020;38:769–70.
Al-Sadawi M, Hussain Y, Copeland-Halperin RS, et al. Racial and socioeconomic disparities in cardiotoxicity among women with HER2-positive breast cancer. Am J Cardiol. 2021;147:116–21.
Prasad P, Branch M, Asemota D, Elsayed R, Addison D, Brown S-A. Cardio-oncology preventive care: racial and ethnic disparities. Current Cardiovascular Risk Reports. 2020;14:18.
Lee R, Wysocki O, Zhou C, Calles A, Eastlake L, Ganatra S, Harrison M, Horsley L, Huddar P, Khan K, Mckenzie H. CORONET; COVID-19 in Oncology evaluatiON Tool: Use of machine learning to inform management of COVID-19 in patients with cancer.
Moazeni S, Cadeiras M, Yang EH, Deng MC, Nguyen KL. Anthracycline induced cardiotoxicity: biomarkers and “Omics” technology in the era of patient specific care. Clin Transl Med. 2017;6:17.
Walsh MN. Social Media and Cardiology. J Am Coll Cardiol. 2018;71:1044–7.
Yekedüz E, Karcıoğlu AM, Utkan G, Ürün Y. A clinical dilemma amid COVID-19 pandemic: missed or encountered diagnosis of cancer? Future Oncology. 2020;16:1879–81.
Francis S. Collins, M.D., Ph.D. NIH launches new initiative to study “Long COVID”. 2021; Available from: https://www.nih.gov/about-nih/who-we-are/nih-director/statements/nih-launches-new-initiative-study-long-covid.
Zong Z, Wei Y, Ren J, Zhang L, Zhou F. The intersection of COVID-19 and cancer: signaling pathways and treatment implications. Molecular Cancer. 2021;20:76.
Satarker S, Tom AA, Shaji RA, Alosious A, Luvis M, Nampoothiri M. JAK-STAT Pathway Inhibition and their Implications in COVID-19 Therapy. Postgrad Med. 2021;133:489–507.
Saini G, Aneja R. Cancer as a prospective sequela of long COVID-19. Bioessays. 2021;43: e2000331.
Author information
Authors and Affiliations
Contributions
All authors contributed to the article including the literature search. Sonu Abraham, Sourbha S. Dani, and Sarju Ganatra also read and critically revised the work leading to the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
The article was an invited review addressed to the corresponding author.
Conflict of Interest
Sonu Abraham declares that she has no conflict of interest. Shamitha Alisa Manohar declares that she has no conflict of interest. Rushin Patel declares that he has no conflict of interest. Anu Mariam Saji declares that she has no conflict of interest. Sourbha S. Dani declares that he has no conflict of interest. Sarju Ganatra declares that he has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardio-Oncology
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abraham, S., Manohar, S.A., Patel, R. et al. Strategies for Cardio-Oncology Care During the COVID-19 Pandemic. Curr Treat Options Cardio Med 24, 137–153 (2022). https://doi.org/10.1007/s11936-022-00965-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11936-022-00965-2