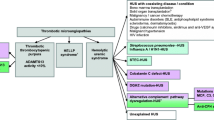
Opinion statement
Panel reactive antibody (PRA) testing has become standard in the evaluation of patients prior to cardiac transplant. Sensitizing events such as blood transfusions, which result in the accumulation of pre-transplant antibodies, should be avoided as clinically feasible. Desensitization therapy might be considered in sensitized patients with cPRA > 50 % although distinct cutoff PRA values for initiating therapy pre-transplant are patient and transplant program dependent. Post-cardiac transplant, quantitative antibodies should also be periodically analyzed, at intervals individualized to the patient. Donor-specific antibodies (DSA) after cardiac transplantation have been shown to be associated with worsened survival. It appears that complement fixing DSA confer the greatest risk for antibody-mediated rejection post-transplant. Desensitization strategies aim to reduce the number of clinically important antibodies prior to and after transplant, both by removal of antibodies and cessation of further production. Current desensitization regimens include pharmacologic, procedural, and surgical modalities, and must be individualized to the patient. Currently, most cardiac transplant programs tailor the post-transplant immunosuppressive regimen based on clinical factors and immunologic assays and may include the use of cytolytic induction and/or intravenous immune gammaglobulin in higher risk patients.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Kidambi S, Mohamedali B, Bhat G. Clinical outcomes in sensitized heart transplant patients bridged with ventricular assist devices. Clin Transplant. 2015;29(6):499–505.
Nwakanma LU et al. Annals Thoracic Surg. 2007;84:1556–63.
Montgomery RA, Lonze BE, King KE, et al. Desensitization in HLA incompatible kidney recipients and survival. N Engl J Med. 2011;365:318.
Kobashigawa J, Mehra M, West L, et al. Report from a consensus conference on the sensitized patient awaiting heart transplantation. J Heart Lung Transplant. 2009;28:213–25. This paper summarizes a consensus conference focused on the evaluation and management of circulating antibodies and sensitization in the cardiac transplant patient.
Smith JD, Banner NR, Hamour IM, Ozawa M, Goh A, Robinson D, et al. De novo donor HLA-specific antibodies after heart transplantation are an independent predictor of poor patient survival. Am J Transplant. 2011;11(2):312–9.
O'Connor MJ, Keeshan BC, Lin KY, Monos D, Lind C, Paridon SM, Mascio CE, Shaddy RE, Rossano JW. Changes in the methodology of pre-heart transplant human leukocyte antibody assessment: an analysis of the United Network for Organ Sharing database. Clin Transplant. 2015.
Altermann WW, Seliger B, Sel S, et al. Comparison of the established standard complement-dependent cytotoxicity and flow cytometric crossmatch assays with a novel ELISA-based HLA crossmatch procedure. Histol Histopathol. 2006;10:1115–24.
Smith JD, Hamour IM, Banner NR, et al. C4d fixing, Luminex binding antibodies—a new tool for prediction of graft failure after heart transplantation. Am J Transplant. 2007;7:2809–15.
Mahesh B, Leong HS, McCormack A, et al. Autoantibodies to vimentin cause accelerated rejection of cardiac allografts. Am J Pathol. 2007;170:1415–27.
Mizutani K, Terasaki P, Bignon JD, et al. Association of kidney transplant failure and antibodies against MICA. Hum Immunol. 2006;67:683–91.
Zou Y, Stastny P, Caner S, et al. Antibodies against MICA antigens and kidney-transplant rejection. N Engl J Med. 2007;357:1293–300.
Duquesnoy RJ, Kamoun M, Baxter-Lowe LA, Woodle ES, Bray RA, Claas FH, et al. Should HLA mismatch acceptability for sensitized transplant candidates be determined at the high-resolution rather than the antigen level? Am J Transplant. 2015;15:923–30.
Costanzo MR, Dichad A, Starling R, et al. The international society of heart and lung transplant guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29(8):914–56.
Pak SW, Uriel N, Burke E, Cappleman SA, Sutton EM, Takayama H, et al. High incidence of elevated panel reactive antibodies in patients supported with HeartMate II left ventricular assist devices sang. Circulation. 2010;122:A20395.
George I, Colley P, Russo MJ, Martens TP, Burke E, Oz MC, et al. Association of device surface and biomaterials with immunologic sensitization after mechanical support. J Thorac Cardiovasc Surg. 2008;135(6):1372–9.
Urban M, Gazdic T, Slimackova E, et al. Alloimmunosensitization in left ventricular assist device recipients and impact on posttransplantation outcome. ASAIO J. 2012;58:554–61.
Grinda JM, Bricourt MO, Amrein C, et al. Human leukocyte antigen sensitization in ventricular assist device recipients: a lesser risk with the DeBakey axial pump. Ann Thorac Surg. 2005;80:945–8.
Kwon MH, Zhang JQ, Schaenman JM, Cadeiras M, Gjertson DW, Krystal CA, et al. Characterization of ventricular assist device-mediated sensitization in the bridge-to-heart-transplantation patient. J Thorac Cardiovasc Surg. 2015;149(4):1161–6.
John R, Pagani FD, Naka Y, et al. Post-cardiac transplant survival after support with a continuous-flow left ventricular assist device: impact of duration of left ventricular assist device support and other variables. J Thorac Cardiovasc Surg. 2010;140:174–81.
Pagani FD, Dyke DB, Wright S, Cody R, Aaronson KD. Development of anti-major histocompatibility complex class I or II antibodies following left ventricular assist device implantation: effects on subsequent allograft rejection and survival. J Heart Lung Transplant. 2001;20:646–53.
Pamboukian SV, Costanzo MR, Dunlap S, et al. Relationship between bridging with ventricular assist device on rejection after heart transplantation. J Heart Lung Transplant. 2005;24:310–5.
Joyce DL, Southard RE, Torre-Amione G, et al. Impact of left ventricular assist device (LVAD)-mediated humoral sensitization on post-transplant outcomes. J Heart Lung Transplant. 2005;24:2054–9.
Gonzalez-Stawinski GV, Cook DJ, Chang AS, et al. Early and midterm risk of coronary allograft vasculopathy in patients bridged to orthotopic heart transplantation with ventricular assist devices. Transplantation. 2005;79:1175–9.
Ballow M. The IgG molecule as a biological immune response modifier: mechanisms of action of intravenous immune serum globulin in autoimmune and inflammatory disorders. J Allergy Clin Immunol. 2011;127(2):315–23. quiz 24–5.
John R, Lietz K, Burke E, Ankersmit J, Mancini D, Suciu-Foca N, et al. Intravenous immunoglobulin reduces anti-HLA alloreactivity and shortens waiting time to cardiac transplantation in highly sensitized left ventricular assist device recipients. Circulation. 1999;100(19 Suppl):II229–35.
Micromedex® 2.0, (electronic version). Truven Health Analytics, Greenwood Village, Colorado, USA. Available at: http://www.micromedexsolutions.com/ (cited:09/18/2015), IVIg
Jordan SC, Reinsmoen N, Lai CH, Vo A. Novel immunotherapeutic approaches to improve rates and outcomes of transplantation in sensitized renal allograft recipients. Discov Med. 2012;13:235–45.
Vo AA, Lukovsky M, Toyoda M, et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med. 2008;359:242–51. This randomized clinical trial was one of the first to highlight the efficacy of desensitization therapy prior to transplantation.
Reinsmoen NL, Lai CH, Vo A, Jordan SC. Evolving paradigms for desensitization in managing broadly HLA sensitized transplant candidates. Discov Med. 2012;13:267–73.
Jordan SC, Tyan D, Stablein D, McIntosh M, Rose S, Vo A, et al. Evaluation of intravenous immunoglobulin as an agent to lower allosensitization and improve transplantation in highly sensitized adult patients with end-stage renal disease: report of the NIH IG02 trial. J Am Soc Nephrol. 2004;15:3256–62.
Reinsmoen NL, Lai CH, Vo A, Cao K, Ong G, Naim M, et al. Acceptable donor-specific antibody levels allowing for successful deceased and living donor kidney transplantation after desensitization therapy. Transplantation. 2008;86(6):820–5.
Jordan SC, Toyoda M, Kahwaji J, Vo A. Clinical Aspects of intravenous immunoglobulin use in solid organ transplant recipients. Am J Transplant. 2011;11(2):196–202.
Kobashigawa JA, Patel JK, Kittleson MM, Kawano MA, Kiyosaki KK, Davis SN, et al. The long-term outcome of treated sensitized patients who undergo heart transplantation. Clin Transpl. 2011;25(1):E61–7. This was the first long term study of desensitization therapy in cardiac transplantation, and revealed acceptable long term outcomes in treated sensitized patients after transplant.
Bearden CM, Agarwal A, Book BK, Sidner RA, Gebel HM, Bray RA. Pronase treatment facilitates alloantibody flow cytometric and cytotoxic crossmatching in the presence of rituximab. Hum Immunol. 2004;65:803–9.
Book BK, Agarwal A, Milgrom AB, Bearden CM, Sidner RA, Higgins NG, et al. New crossmatch technique eliminates interference by humanized and chimeric monoclonal antibodies. Transplant Proc. 2005;37(2):640–2.
Micromedex® 2.0, (electronic version). Truven Health Analytics, Greenwood Village, Colorado, USA. Available at: http://www.micromedexsolutions.com/ (cited:09/18/2015), Rituximab.
Itescu S, Burke E, Lietz K, John R, Mancini D, Michler R, et al. Intravenous pulse administration of cyclophosphamide is an effective and safe treatment for sensitized cardiac allograft recipients. Circulation. 2002;105(10):1214–9.
Micromedex® 2.0, (electronic version). Truven Health Analytics, Greenwood Village, Colorado, USA. Available at: http://www.micromedexsolutions.com/ (cited:09/18/2015), Cyclophosphamide.
Higgins R, Kirklin JK, Brown RN, et al. To induce or not to induce: do patients at greatest risk for fatal rejection benefit from cytolytic induction therapy? J Heart Lung Transplant. 2005;24:392.
Penninga L, Moller CH, Gustafsson F, Gluud C, Steinbruchel DA. Immunosuppressive T-cell antibody induction for heart transplant recipients. Cochrane Database Syst Rev. 2013;12, CD008842.
Kho MM, Bouvy AP, Cadogan M, Kraaijeveld R, Baan CC, Weimar W. The effect of low and ultra-low dosages Thymoglobulin on peripheral T, B and NK cells in kidney transplant recipients. Transpl Immunol. 2012;26:186.
Lund LH, Edwards LB, Kucheryavaya AY, et al. The registry of the international society for heart and lung transplantation: thirtieth official adult heart transplant report–2013; focus theme: age. J Heart Lung Transplant. 2013;32:951.
Zuckermann A, Schulz U, Deuse T, Ruhpawar A, Schmitto JD, Beiras-Fernandez A, et al. Thymoglobulin induction in heart transplantation: patient selection and implications for maintenance immunosuppression. Transpl Int. 2015;28(3):259–69.
Brennan DC, Flavin K, Lowell JA, Howard TK, Shenoy S, Burgess S, et al. A randomized, double-blinded comparison of Thymoglobulin versus Atgam for induction immunosuppressive therapy in adult renal transplant recipients. Transplantation. 1999;67(7):1011–8.
Hardinger KL, Schnitzler MA, Miller B, Lowell JA, Shenoy S, Koch MJ, et al. Five-year follow up of thymoglobulin versus ATGAM induction in adult renal transplantation. Transplantation. 2004;78(1):136–41.
Rafiei M, Kittleson M, Patel J, Osborne A, Chang D, Czer L, et al. Anti-thymocyte globulin may prevent antibody production after heart transplantation. Transplant Proc. 2014;46(10):3570–4. This retrospective study suggested the benefit of anti-thymocyte globulin in suppressing antibody production after cardiac transplant, supporting its utility in induction therapy.
Czer LS, Phan A, Ruzza A, Rafiei M, Setareh-Shenas S, Caceres M, et al. Antithymocyte globulin induction therapy adjusted for immunologic risk after heart transplantation. Transplant Proc. 2013;45(6):2393–8.
Brennan DC, Daller JA, Lake KD, et al. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006;355:1967.
Wang R, Moura LA, Lopes SV, Costa FD, Souza Filho NF, Fernandes TL, et al. Reduced progression of cardiac allograft vasculopathy with routine use of induction therapy with basiliximab. Arq Bras Cardiol. 2015;105(2):176–83.
Ansari D, Lund LH, Stehlik J, Andersson B, Höglund P, Edwards L, Nilsson J; Induction with anti-thymocyte globulin in heart transplantation is associated with better long-term survival compared with basiliximab. J Heart Lung Transplant. 2015.
Pisani BA, Mullen GM, Malinowska K, Lawless CE, Mendez J, Silver MA, et al. Plasmapheresis with intravenous immunoglobulin G is effective in patients with elevated panel reactive antibody prior to cardiac transplantation. J Heart Lung Transplant: Off Public Int Soc Heart Transplant. 1999;18(7):701–6.
Rummler S, Barz D. Plasma exchange and immunoadsorption of patients with thoracic organ transplantation. Transfus Med Hemother: Offizielles Organ Der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie. 2012;39(4):234–40.
Sicard A, Phares TW, Yu H, Fan R, Baldwin 3rd WM, Fairchild RL, et al. The spleen is the major source of antidonor antibody-secreting cells in murine heart allograft recipients. Am J Transplant. 2012;12(7):1708–19.
Warren DS, Zachary AA, Sonnenday CJ, King KE, Cooper M, Ratner LE, et al. Successful renal transplantation across simultaneous ABO incompatible and positive crossmatch barriers. Am J Transplant: Off J Am Soc Transplant Am Soc Transplant Surg. 2004;4(4):561–8.
Perry DK, Burns JM, Pollinger HS, Amiot BP, Gloor JM, Gores GJ, et al. Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production. Am J Transplant: Off J Am Soc Transplant Am Soc Transplant Surg. 2009;9(1):201–9.
Patel J, Everly M, Chang D, Kittleson M, Reed E, Kobashigawa J. Reduction of alloantibodies via proteasome inhibition in cardiac transplantation. J Heart Lung Transplant. 2011;30(12):1320–6.
Sacha L, et al. Carfilzomib for Refractory Antibody Mediated Rejection and Allosensitization in Heart Transplantation. The Journal of Heart and Lung Transplantation 33(Issue 4):S31.
Stegall MD, Diwan T, Raghavaiah S, Cornell LD, Burns J, Dean PG, et al. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant: Off J Am Soc Transplant Am Soc Transplant Surg. 2011;11(11):2405–13. This is the first clinical study demonstrating the efficacy of eculizumab in sensitized transplant patients.
Patel J. Cedars-Sinai Medical Center. The De-novo Use of Eculizumab in Presensitized Patients Receiving Cardiac Transplantation (DUET) In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [cited 2015 Sep 28]. Available from: http://clinicaltrials.gov/ct2/show/NCT02013037 NLM Identifier: NCT02013037
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Robert M. Cole declares no potential conflicts of interest.
Jon A. Kobashigawa reports grants and personal fees from Novartis Pharmaceuticals, grants and personal fees from CareDx, Inc., grants and personal fees from TransMedics Inc.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Heart Failure
Rights and permissions
About this article
Cite this article
Cole, R.M., Kobashigawa, J.A. Desensitization Strategies Pre- and Post-Cardiac Transplantation. Curr Treat Options Cardio Med 18, 8 (2016). https://doi.org/10.1007/s11936-015-0431-9
Published:
DOI: https://doi.org/10.1007/s11936-015-0431-9