Abstract
Purpose of Review
Pathological roles of macrophage migration inhibitory factor (MIF) have recently been demonstrated in spondyloarthritis (SpA) preclinical models, identifying MIF as a new treatment target for SpA. However, the specific contribution of MIF and therapeutic potential of MIF-targeted therapies to various tissue types affected by SpA are not well delineated.
Recent Findings
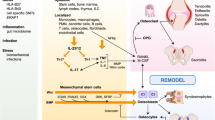
MIF and its cognate receptor CD74 are extensively involved in the pathogenesis of SpA including inflammation in the spine, joint, eyes, skin, and gut. The majority of the current evidence has consistently shown that MIF drives the inflammation in these distinct anatomical sites. In preclinical models, genetic deletion or blockade of MIF reduces the severity of inflammation. Although MIF is generally an upstream cytokine which regulates downstream effector cytokines, MIF also intensifies type 3 immunity by promoting helper T 17 (Th17) plasticity. MIF- or CD74-targeted therapies have also reported to be well tolerated in clinical trials for other diseases.
Summary
Recent findings suggest that MIF-CD74 axis is a new therapeutic target for SpA to improve various clinical features. Clinical trials for MIF- or CD74-targeted therapies for SpA patients are warranted.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Kusuda M, Haroon N, Nakamura A. Complexity of enthesitis and new bone formation in ankylosing spondylitis: current understanding of the immunopathology and therapeutic approaches. Mod Rheumatol. England; 2022;32(3):484-492.
Ranganathan V, Gracey E, Brown MA, Inman RD, Haroon N. Pathogenesis of ankylosing spondylitis — recent advances and future directions. Nat Rev Rheumatol United States. 2017;13:359–67.
Dougados M, Baeten D. Spondyloarthritis Lancet England. 2011;377:2127–37.
Nakamura A, Haroon N. Recent updates in the immunopathology of type 3 immunity-mediated enthesitis. CurrRheumatol Rep. United States; 2021;23:31.
Nakamura A, Talukdar A, Nakamura S, Pathan E, Haroon N. Bone formation in axial spondyloarthritis: is disease modification possible? Best Pract Res Clin Rheumatol. Netherlands. 2019;33: 101491.
Haroon NN, Paterson JM, Li P, Inman RD, Haroon N. Patients with ankylosing spondylitis have increased cardiovascular and cerebrovascular mortality: a population-based study. Ann Intern Med United States. 2015;163:409–16.
Kuriya B, Tia V, Luo J, Widdifield J, Vigod S, Haroon N. Acute mental health service use is increased in rheumatoid arthritis and ankylosing spondylitis: a population-based cohort study. Therapeutic Advances in Musculoskeletal Disease [Internet]. SAGE Publications; 2020;12:1759720X20921710. Available from: https://doi.org/10.1177/1759720X20921710
Deodhar A, Sliwinska-Stanczyk P, Xu H, Baraliakos X, Gensler LS, Fleishaker D, et al. Tofacitinib for the treatment of ankylosing spondylitis: a phase III, randomised, double-blind, placebo-controlled study. Annals of the Rheumatic Diseases [Internet]. 2021;80:1004. Available from: http://ard.bmj.com/content/80/8/1004.abstract
Genovese MC, Fleischmann R, Combe B, Hall S, Rubbert-Roth A, Zhang Y, et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. The Lancet [Internet]. Elsevier; 2018;391:2513–24. Available from: https://doi.org/10.1016/S0140-6736(18)31116-4
Wei JC-C, Kim T-H, Kishimoto M, Ogusu N, Jeong H, Kobayashi S, et al. Efficacy and safety of brodalumab, an anti-IL17RA monoclonal antibody, in patients with axial spondyloarthritis: 16-week results from a randomised, placebo-controlled, phase 3 trial. Ann Rheum Dis. 2021;
ClinicalTrials.gov. A study to evaluate the efficacy and safety of bimekizumab in subjects with active nonradiographic axial spondyloarthritis (BE MOBILE 1). 2021.
Macfarlane GJ, Pathan E, Jones GT, Dean LE. Predicting response to anti-TNFα therapy among patients with axial spondyloarthritis (axSpA): results from BSRBR-AS. Rheumatology (Oxford). 2020;59:2481–90.
Danve A, Deodhar A. Treatment of axial spondyloarthritis: an update. Nat Rev Rheumatol. 2022;18:205–16.
Kang I, Bucala R. The immunobiology of MIF: function, genetics and prospects for precision medicine. Nat Rev Rheumatol United States. 2019;15:427–37.
Greven D, Leng L, Bucala R. Autoimmune diseases: MIF as a therapeutic target. Expert Opin Ther Targets England. 2010;14:253–64.
Bae S-C, Lee YH. Associations between circulating macrophage migration inhibitory factor (MIF) levels and rheumatoid arthritis, and between MIF gene polymorphisms and disease susceptibility: a meta-analysis. Postgrad Med J. 2018;94:109–15.
Sreih A, Ezzeddine R, Leng L, LaChance A, Yu G, Mizue Y, et al. Dual effect of the macrophage migration inhibitory factor gene on the development and severity of human systemic lupus erythematosus. Arthritis Rheum. 2011;63:3942–51.
Kim H-R, Park M-K, Cho M-L, Yoon C-H, Lee S-H, Park S-H, et al. Macrophage migration inhibitory factor upregulates angiogenic factors and correlates with clinical measures in rheumatoid arthritis. J Rheumatol. 2007;34:927–36.
Leech M, Lacey D, Xue JR, Santos L, Hutchinson P, Wolvetang E, et al. Regulation of p53 by macrophage migration inhibitory factor in inflammatory arthritis. Arthritis & Rheumatism [Internet]. John Wiley & Sons, Ltd; 2003;48:1881–9. Available from: https://doi.org/10.1002/art.11165
Onodera S, Nishihira J, Iwabuchi K, Koyama Y, Yoshida K, Tanaka S, et al. Macrophage migration inhibitory factor up-regulates matrix metalloproteinase-9 and -13 in rat osteoblasts. Relevance to intracellular signaling pathways. J Biol Chem. 2002;277:7865–74.
Hoi AY, Hickey MJ, Hall P, Yamana J, O’Sullivan KM, Santos LL, et al. Macrophage migration inhibitory factor deficiency attenuates macrophage recruitment, glomerulonephritis, and lethality in MRL/lpr mice. J Immunol. 2006;177:5687–96.
Gürel Ç, İnanır A, Nursal AF, Tekcan A, Rüstemoğlu A, Yigit S. Evaluation of MIF -173 G/C polymorphism in Turkish patients with ankylosing spondylitis. Balkan Med J. 2016;33:614–9.
Kozaci LD, Sari I, Alacacioglu A, Akar S, Akkoc N. Evaluation of inflammation and oxidative stress in ankylosing spondylitis: a role for macrophage migration inhibitory factor. Mod Rheumatol. 2010;20:34–9.
Ranganathan V, Ciccia F, Zeng F, Sari I, Guggino G, Muralitharan J, et al. Macrophage migration inhibitory factor induces inflammation and predicts spinal progression in ankylosing spondylitis. Arthritis and Rheumatology. 2017;69(9):1796–806.
Nakamura A, Zeng F, Nakamura S, Reid KT, Gracey E, Lim M, et al. Macrophage migration inhibitory factor drives pathology in a mouse model of spondyloarthritis and is associated with human disease. Sci Transl Med. 2021;13:eabg1210.
Rahman MA, Thomas R. The SKG model of spondyloarthritis. Best Pract Res Clin Rheumatol Netherlands. 2017;31:895–909.
Rich AR, Lewis MR. The nature of allergy in tuberculosis at revealed by tissue culture studies. Bull Johns Hopkins Hosp. 1932;50:115–31.
Goldberg LS, Louie JS, Baker MH. Inhibition of macrophage migration: a test system using human monocytes. J Immunol. 1971;107:906–9.
Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791–800.
Merk M, Zierow S, Leng L, Das R, Du X, Schulte W, et al. The D-dopachrome tautomerase (DDT) gene product is a cytokine and functional homolog of macrophage migration inhibitory factor (MIF). Proc Natl Acad Sci U S A. 2011;108:E577–85.
Tilstam PV, Schulte W, Holowka T, Kim B-S, Nouws J, Sauler M, et al. MIF but not MIF-2 recruits inflammatory macrophages in an experimental polymicrobial sepsis model. J Clin Invest. 2021;131(23):e127171. https://doi.org/10.1172/JCI127171.
Donn R, Alourfi Z, de Benedetti F, Meazza C, Zeggini E, Lunt M, et al. Mutation screening of the macrophage migration inhibitory factor gene: positive association of a functional polymorphism of macrophage migration inhibitory factor with juvenile idiopathic arthritis. Arthritis Rheum. 2002;46:2402–9.
Wu S-P, Leng L, Feng Z, Liu N, Zhao H, McDonald C, et al. Macrophage migration inhibitory factor promoter polymorphisms and the clinical expression of scleroderma. Arthritis Rheum. 2006;54:3661–9.
Llamas-Covarrubias MA, Valle Y, Bucala R, Navarro-Hernández RE, Palafox-Sánchez CA, Padilla-Gutiérrez JR, et al. Macrophage migration inhibitory factor (MIF): genetic evidence for participation in early onset and early stage rheumatoid arthritis. Cytokine. 2013;61:759–65.
Baugh JA, Chitnis S, Donnelly SC, Monteiro J, Lin X, Plant BJ, et al. A functional promoter polymorphism in the macrophage migration inhibitory factor (MIF) gene associated with disease severity in rheumatoid arthritis. Genes Immun. 2002;3:170–6.
Wang F-F, Zhu L-A, Zou Y-Q, Zheng H, Wilson A, Yang C-D, et al. New insights into the role and mechanism of macrophage migration inhibitory factor in steroid-resistant patients with systemic lupus erythematosus. Arthritis Res Ther. 2012;14:R103.
Eder L, Chandran V, Ueng J, Bhella S, Lee K-A, Rahman P, et al. Predictors of response to intra-articular steroid injection in psoriatic arthritis. Rheumatology (Oxford). 2010;49:1367–73.
Jankauskas SS, Wong DWL, Bucala R, Djudjaj S, Boor P. Evolving complexity of MIF signaling. Cell Signal England. 2019;57:76–88.
Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med. 2007;13:587–96.
De R, Sarkar S, Mazumder S, Debsharma S, Siddiqui AA, Saha SJ, et al. Macrophage migration inhibitory factor regulates mitochondrial dynamics and cell growth of human cancer cell lines through CD74-NF-κB signaling. J Biol Chem. 2018;293:19740–60.
Onodera S, Tanji H, Suzuki K, Kaneda K, Mizue Y, Sagawa A, et al. High expression of macrophage migration inhibitory factor in the synovial tissues of rheumatoid joints. Cytokine. 1999;11:163–7.
Meazza C, Travaglino P, Pignatti P, Magni-Manzoni S, Ravelli A, Martini A, et al. Macrophage migration inhibitory factor in patients with juvenile idiopathic arthritis. Arthritis Rheum. 2002;46:232–7.
de Jong YP, Abadia-Molina AC, Satoskar AR, Clarke K, Rietdijk ST, Faubion WA, et al. Development of chronic colitis is dependent on the cytokine MIF. Nat Immunol. 2001;2:1061–6.
Kozaci LD, Sari I, Alacacioglu A, Akar S, Akkoc N. Evaluation of inflammation and oxidative stress in ankylosing spondylitis: a role for macrophage migration inhibitory factor. Mod Rheumatol. 2010;20:34–9.
Park M-C, Kwon OC, Lee S-W, Song JJ, Park Y-B. MiR-451 suppresses inflammatory responses in ankylosing spondylitis by targeting macrophage migration inhibitory factor. Clin Exp Rheumatol. 2020;38(2):275–81.
Baerlecken NT, Nothdorft S, Stummvoll GH, Sieper J, Rudwaleit M, Reuter S, et al. Autoantibodies against CD74 in spondyloarthritis. Ann Rheum Dis England. 2014;73:1211–4.
Baraliakos X, Baerlecken N, Witte T, Heldmann F, Braun J. High prevalence of anti-CD74 antibodies specific for the HLA class II-associated invariant chain peptide (CLIP) in patients with axial spondyloarthritis. Ann Rheum Dis England. 2014;73:1079–82.
Riechers E, Baerlecken N, Baraliakos X, Achilles-Mehr Bakhsh K, Aries P, Bannert B, et al. Sensitivity and specificity of autoantibodies against CD74 in nonradiographic axial spondyloarthritis. Arthritis Rheumatol United States. 2019;71:729–35.
Hu C-J, Li M-T, Li X, Peng L-Y, Zhang S-Z, Leng X-M, et al. CD74 auto-antibodies display little clinical value in Chinese Han population with axial spondyloarthritis. Medicine. 2020;99: e23433.
Liu M, Xie Z, Sun G, Chen L, Qi D, Zhang H, et al. Macrophage migration inhibitory factor may play a protective role in osteoarthritis. Arthritis Res Ther. 2021;23:59.
Stoppe C, Averdunk L, Goetzenich A, Soppert J, Marlier A, Kraemer S, et al. The protective role of macrophage migration inhibitory factor in acute kidney injury after cardiac surgery. Sci Transl Med. 2018; 10.aan4886.
Kitayama S, Onodera S, Kondo E, Kobayashi T, Miyatake S, Kitamura N, et al. Deficiency of macrophage migration inhibitory factor gene delays healing of the medial collateral ligament: a biomechanical and biological study. J Biomech. 2011;44:494–500.
Schett G, Lories RJ, D’Agostino M-A, Elewaut D, Kirkham B, Soriano ER, et al. Enthesitis: from pathophysiology to treatment. Nat Rev Rheumatol United States. 2017;13:731–41.
Sherlock JP, Joyce-Shaikh B, Turner SP, Chao C-C, Sathe M, Grein J, et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4-CD8- entheseal resident T cells. Nat Med United States. 2012;18:1069–76.
Kim D-H, Noh S-U, Chae S-W, Kim S-J, Lee Y-T. Altered differentiation of tendon-derived stem cells in diabetic conditions mediated by macrophage migration inhibitory factor. Int J Mol Sci. 2021;22(16):8983.
• Akbar M, MacDonald L, Crowe LAN, Carlberg K, Kurowska-Stolarska M, Ståhl PL, et al. Single cell and spatial transcriptomics in human tendon disease indicate dysregulated immune homeostasis. Ann Rheum Dis. 2021;80(11):1494–7. This study found tenocyte MIF upregulation in tendinopathy, as well as increase CD74 in macrophages within damaged tendon tissue.
Kim SJ, Song D-H, Kim SJ. Characteristics of tendon derived stem cells according to different factors to induce the tendinopathy. J Cell Physiol United States. 2018;233:6196–206.
Stojanović I, Cvjetićanin T, Lazaroski S, Stosić-Grujicić S, Miljković D. Macrophage migration inhibitory factor stimulates interleukin-17 expression and production in lymph node cells. Immunology. 2009;126:74–83.
Berendsen AD, Olsen BR. Bone development. Bone. 2015;80:14–8.
Zheng Y, Cai B, Ren C, Xu H, Du W, Wu Y, et al. Identification of immune related cells and crucial genes in the peripheral blood of ankylosing spondylitis by integrated bioinformatics analysis. PeerJ. 2021;9: e12125.
•• Yu T, Zhang J, Zhu W, Wang X, Bai Y, Feng B, et al. Chondrogenesis mediates progression of ankylosing spondylitis through heterotopic ossification. Bone Res. 2021;9:19. This study firmly shows that endochondral ossification is one of the central process of new bone formation in axSpA.
Fujihara Y, Hikita A, Takato T, Hoshi K. Roles of macrophage migration inhibitory factor in cartilage tissue engineering. J Cell Physiol United States. 2018;233:1490–9.
• Deng M, Tan J, Dai Q, Luo F, Xu J. Macrophage-mediated bone formation in scaffolds modified with MSC-derived extracellular matrix is dependent on the migration inhibitory factor signaling pathway. Front Cell Dev Biol. 2021;9: 714011. This study identified macrophage-derived MIF as a regulatory cytokine in osteogenesis using cartilage implants.
Jacquin C, Koczon-Jaremko B, Aguila HL, Leng L, Bucala R, Kuchel GA, et al. Macrophage migration inhibitory factor inhibits osteoclastogenesis. Bone. 2009;45:640–9.
Mun SH, Won HY, Hernandez P, Aguila HL, Lee S-K. Deletion of CD74, a putative MIF receptor, in mice enhances osteoclastogenesis and decreases bone mass. J Bone Miner Res. 2013;28:948–59.
Onodera S, Sasaki S, Ohshima S, Amizuka N, Li M, Udagawa N, et al. Transgenic mice overexpressing macrophage migration inhibitory factor (MIF) exhibit high-turnover osteoporosis. J Bone Miner Res. 2006;21:876–85.
Zheng L, Gao J, Jin K, Chen Z, Yu W, Zhu K, et al. Macrophage migration inhibitory factor (MIF) inhibitor 4-IPP suppresses osteoclast formation and promotes osteoblast differentiation through the inhibition of the NF-κB signaling pathway. FASEB J. 2019;33:7667–83.
Christodoulou-Vafeiadou E, Geka C, Ntari L, Kranidioti K, Argyropoulou E, Meier F, et al. Ectopic bone formation and systemic bone loss in a transmembrane TNF-driven model of human spondyloarthritis. Arthritis Res Ther. 2020;22:232.
Kopylov U, Starr M, Watts C, Dionne S, Girardin M, Seidman EG. Detection of Crohn disease in patients with spondyloarthropathy: the SpACE capsule study. J Rheumatol. 2018;45:498–505.
Mielants H, Veys EM, Cuvelier C, de Vos M. Ileocolonoscopic findings in seronegative spondylarthropathies. Br J Rheumatol. 1988;27(Suppl 2):95–105.
van Praet L, van den Bosch FE, Jacques P, Carron P, Jans L, Colman R, et al. Microscopic gut inflammation in axial spondyloarthritis: a multiparametric predictive model. Ann Rheum Dis. 2013;72:414–7.
Oliver J, Márquez A, Gómez-Garcia M, Martinez A, Mendoza JL, Vilchez JR, et al. Association of the macrophage migration inhibitory factor gene polymorphisms with inflammatory bowel disease. Gut. 2007;56:150–1.
Shen Y, Guo S, Yang T, Jia L, Chen L, An J, et al. The -173 G/C polymorphism of the MIF gene and inflammatory bowel disease risk: a meta-analysis. Int J Mol Sci. 2013;14:11392–401.
Yang J, Li Y, Zhang X. Meta-analysis of macrophage migration inhibitory factor (MIF) gene -173G/C polymorphism and inflammatory bowel disease (IBD) risk. Int J Clin Exp Med. 2015;8:9570–4.
Singh UP, Singh NP, Murphy EA, Price RL, Fayad R, Nagarkatti M, et al. Chemokine and cytokine levels in inflammatory bowel disease patients. Cytokine. 2016;77:44–9.
Ohkawara T, Miyashita K, Nishihira J, Mitsuyama K, Takeda H, Kato M, et al. Transgenic over-expression of macrophage migration inhibitory factor renders mice markedly more susceptible to experimental colitis. Clin Exp Immunol. 2005;140:241–8.
Ohkawara T, Mitsuyama K, Takeda H, Asaka M, Fujiyama Y, Nishihira J. Lack of macrophage migration inhibitory factor suppresses innate immune response in murine dextran sulfate sodium-induced colitis. Scand J Gastroenterol. 2008;43:1497–504.
Ohkawara T, Nishihira J, Takeda H, Hige S, Kato M, Sugiyama T, et al. Amelioration of dextran sulfate sodium-induced colitis by anti-macrophage migration inhibitory factor antibody in mice. Gastroenterology. 2002;123:256–70.
Farr L, Ghosh S, Jiang N, Watanabe K, Parlak M, Bucala R, et al. CD74 Signaling links inflammation to intestinal epithelial cell regeneration and promotes mucosal healing. Cell Mol Gastroenterol Hepatol. 2020;10:101–12.
Vujicic M, Saksida T, Despotovic S, Bajic SS, Lalić I, Koprivica I, et al. The role of macrophage migration inhibitory factor in the function of intestinal barrier. Sci Rep. 2018;8:6337.
Stolwijk C, van Tubergen A, Castillo-Ortiz JD, Boonen A. Prevalence of extra-articular manifestations in patients with ankylosing spondylitis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74:65–73.
Gómez RS, Diepgen TL, Neumann C, Sorg C. Detection of migration inhibitory factor (MIF) by a monoclonal antibody in the microvasculature of inflamed skin. Arch Dermatol Res. 1990;282:374–8.
Shimizu T, Nishihira J, Mizue Y, Nakamura H, Abe R, Watanabe H, et al. High macrophage migration inhibitory factor (MIF) serum levels associated with extended psoriasis. J Invest Dermatol. 2001;116:989–90.
Steinhoff M, Meinhardt A, Steinhoff A, Gemsa D, Bucala R, Bacher M. Evidence for a role of macrophage migration inhibitory factor in psoriatic skin disease. Br J Dermatol. 1999;141:1061–6.
Donn RP, Plant D, Jury F, Richards HL, Worthington J, Ray DW, et al. Macrophage migration inhibitory factor gene polymorphism is associated with psoriasis. J Invest Dermatol. 2004;123:484–7.
Bezdek S, Leng L, Busch H, Mousavi S, Rades D, Dahlke M, et al. Macrophage migration inhibitory factor (MIF) drives murine Psoriasiform dermatitis. Front Immunol. 2018;9:2262.
Abe R, Shimizu T, Ohkawara A, Nishihira J. Enhancement of macrophage migration inhibitory factor (MIF) expression in injured epidermis and cultured fibroblasts. Biochim Biophys Acta. 2000;1500:1–9.
Hsieh C-Y, Chen C-L, Lin Y-S, Yeh T-M, Tsai T-T, Hong M-Y, et al. Macrophage migration inhibitory factor triggers chemotaxis of CD74+CXCR2+ NKT cells in chemically induced IFN-γ-mediated skin inflammation. J Immunol. 2014;193:3693–703.
Kitaichi N, Kotake S, Sasamoto Y, Namba K, Matsuda A, Ogasawara K, et al. Prominent increase of macrophage migration inhibitory factor in the sera of patients with uveitis. Invest Ophthalmol Vis Sci. 1999;40:247–50.
Taguchi C, Sugita S, Tagawa Y, Nishihira J, Mochizuki M. Macrophage migration inhibitory factor in ocular fluids of patients with uveitis. Br J Ophthalmol. 2001;85:1367–71.
Zhang C, Liu S, Hou S, Lei B, Zheng X, Xiao X, et al. MIF gene polymorphisms confer susceptibility to Vogt-Koyanagi-Harada syndrome in a Han Chinese population. Invest Ophthalmol Vis Sci. 2013;54:7734–8.
Nursal AF, Yigit S, Tural E, Kalkan G, Tumer MK, Tekcan A. Macrophage migration inhibitory factor −173GC variant might increase the risk of Behçet’s disease. Med Princ Pract. 2018;27:285–9.
Zheng X, Wang D, Hou S, Zhang C, Lei B, Xiao X, et al. Association of macrophage migration inhibitory factor gene polymorphisms with Behçet’s disease in a Han Chinese population. Ophthalmology. 2012;119:2514–8.
Yang H, Zheng S, Mao Y, Chen Z, Zheng C, Li H, et al. Modulating of ocular inflammation with macrophage migration inhibitory factor is associated with notch signalling in experimental autoimmune uveitis. Clin Exp Immunol. 2016;183:280–93.
Harrison DE, Strong R, Reifsnyder P, Kumar N, Fernandez E, Flurkey K, et al. 17-a-estradiol late in life extends lifespan in aging UM-HET3 male mice; nicotinamide riboside and three other drugs do not affect lifespan in either sex. Aging Cell England. 2021;20(5):e13328.
•• Mahalingam D, Patel MR, Sachdev JC, Hart LL, Halama N, Ramanathan RK, et al. Phase I study of imalumab (BAX69), a fully human recombinant antioxidized macrophage migration inhibitory factor antibody in advanced solid tumours. Br J Clin Pharmacol. 2020;86:1836–48. This trial shows that MIF antagonism yields a desirable safety profile in humans.
•• Wallace DJ, Figueras F, Wegener WA, Goldenberg DM. Experience with milatuzumab, an anti-CD74 antibody against immunomodulatory macrophage migration inhibitory factor (MIF) receptor, for systemic lupus erythematosus (SLE). Ann Rheum Dis. 2021;80:954–5. This trial shows that monoclonal antibody against CD74 is effective for a sizable proportion of SLE patients and well-tolerable.
Nauseef WM, Borregaard N. Neutrophils at work. Nat Immunol United States. 2014;15:602–11.
Burn GL, Foti A, Marsman G, Patel DF, Zychlinsky A. The neutrophil Immunity United States. 2021;54:1377–91.
Tabrizi ZA, Khosrojerdi A, Aslani S, Hemmatzadeh M, Babaie F, Bairami A, et al. Multi-facets of neutrophil extracellular trap in infectious diseases: moving beyond immunity. Microb Pathog, England. 2021;158:105066.
Freemont AJ, Denton J. Disease distribution of synovial fluid mast cells and cytophagocytic mononuclear cells in inflammatory arthritis. Ann Rheum Dis. 1985;44:312–5.
Xiao Z, Song S, Chen D, van Merkerk R, van der Wouden PE, Cool RH, et al. Proteolysis targeting chimera (PROTAC) for macrophage migration inhibitory factor (MIF) has anti-proliferative activity in lung cancer cells. Angew Chem Int Ed Engl. 2021;60:17514–21.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Spondyloarthritis.
Rights and permissions
About this article
Cite this article
Wu, B., Nakamura, A. Deep Insight into the Role of MIF in Spondyloarthritis. Curr Rheumatol Rep 24, 269–278 (2022). https://doi.org/10.1007/s11926-022-01081-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11926-022-01081-7