Abstract
Purpose of Review
Although rare, idiopathic inflammatory myopathies (IIM) comprise a heterogeneous group of autoimmune conditions in adults and children. Increasingly, vasculopathy is recognised to be key in the underlying pathophysiology and plays a crucial role in some of the more challenging complications including calcinosis, gastrointestinal involvement and interstitial lung disease. The exciting prospect of development of biomarkers of vasculopathy would enable earlier detection and monitoring of these complications and possible prevention of their potentially devastating consequences. The purpose was to review the current literature on biomarkers of vasculopathy in IIM and offer insight as to the biomarkers most likely to have an impact on clinical care.
Recent Findings
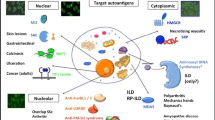
Multiple candidate biomarkers have been studied including circulating endothelial cells (CEC), microparticles (MP), soluble adhesion markers (ICAM-1, ICAM-3, VCAM-1), selectin proteins (E-, L-, P-selectin), coagulation factors, angiogenic factors, cytokines (including (IL-6, IL-10, TNF-α, IL-18) and interferon (IFN)-related biomarkers (including IFNα, IFN-β, IFNγ, galectin-9, interferon signature and interferon-related chemokines (MCP-1, IP-10 and MIG). There is a growing body of evidence of the potential role of biomarkers in detecting and monitoring the vasculopathy in IIM, detecting disease activity and predicting disease flares and overall prognosis.
Summary
Exciting progress has been made in the search for biomarkers of vasculopathy of IIM; however, none of the studies are validated and further research is required.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Meyer A, Meyer N, Schaeffer M, Gottenberg J-E, Geny B, Sibilia J. Incidence and prevalence of inflammatory myopathies: a systematic review. Rheumatology. 2015;54(1):50–63.
Lundberg IE, Miller FW, Tjärnlund A, Bottai M. Diagnosis and classification of idiopathic inflammatory myopathies. J Intern Med. 2016;280(1):39–51.
McCann LJ, Juggins AD, Maillard SM, Wedderburn LR, Davidson JE, Murray KJ, et al. The Juvenile Dermatomyositis National Registry and Repository (UK and Ireland)—clinical characteristics of children recruited within the first 5 yr. Rheumatology. 2006;45(10):1255–60.
Vincze M, Danko K. Idiopathic inflammatory myopathies. Best Pract Res Clin Rheumatol. 2012;26(1):25.
Kobayashi N, Takezaki S, Kobayashi I, Iwata N, Mori M, Nagai K, et al. Clinical and laboratory features of fatal rapidly progressive interstitial lung disease associated with juvenile dermatomyositis. Rheumatology (Oxford, England). 2015;54(5):784–91.
Gitiaux C, De Antonio M, Aouizerate J, Gherardi RK, Guilbert T, Barnerias C, et al. Vasculopathy-related clinical and pathological features are associated with severe juvenile dermatomyositis. Rheumatology. 2016;55(3):470–9.
Papadopoulou C, McCann LJ. The vasculopathy of juvenile dermatomyositis. Front Pediatr. 2018;6:284.
Miller FW, Oddis CV. Vasculitis in the idiopathic inflammatory myopathies. In: In: Inflammatory Diseases of Blood Vessels [Internet]. Hoboken: John Wiley & Sons, Ltd. p. 433–40. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781118355244.ch39.
Lilleker JB, Vencovsky J, Wang G, Wedderburn LR, Diederichsen LP, Schmidt J, et al. The EuroMyositis registry: an international collaborative tool to facilitate myositis research. Ann Rheum Dis 2017/08/30 ed. 2018;77(1):30–9.
Wedderburn LR, Rider LG. Juvenile dermatomyositis: new developments in pathogenesis, assessment and treatment. Best Pract Res Clin Rheumatol. 2009;23(5):665–78.
Wienke J, Deakin CT, Wedderburn LR, van Wijk F, van Royen-Kerkhof A. Systemic and tissue inflammation in juvenile dermatomyositis: from pathogenesis to the quest for monitoring tools. Front Immunol. 2018;9:2951.
Stenzel W, Goebel H-H, Bader-Meunier B, Gitiaux C. Inflammatory myopathies in childhood. Neuromusc Disorders : NMD. 2021;31(10):1051–61.
Wedderburn LR, Varsani H, Li CKC, Newton KR, Amato AA, Banwell B, et al. International consensus on a proposed score system for muscle biopsy evaluation in patients with juvenile dermatomyositis: a tool for potential use in clinical trials. Arthritis Care Res. 2007;57(7):1192–201.
Smith RL, Sundberg J, Shamiyah E, Dyer A, Pachman LM. Skin involvement in juvenile dermatomyositis is associated with loss of end row nailfold capillary loops. J Rheumatol. 2004;31(8):1644–9.
• Papadopoulou C, Hong Y, Krol P, Obaidi M, Pilkington C, Wedderburn L, et al. The vasculopathy of juvenile dermatomyositis: endothelial injury, hypercoagulability, and increased arterial stiffness. Arthritis Rheum. 2021;73:1253–66 CEC, MP, cytokines and chemokines assessed in children with JDM and control populations. CEC and MP levels were elevated in JDM patients compared to healthy controls; CEC levels were increased in patients with active compared to inactive JDM. Galectin-9 was elevated in patients with active disease compared to inactive and this correlated with CEC level.
• Kishi T, Chipman J, Evereklian M, Nghiem K, Stetler-Stevenson M, Rick ME, et al. Endothelial activation markers as disease activity and damage measures in juvenile dermatomyositis. J Rheumatol. 2020;47. https://doi.org/10.3899/jrheum.181275. Various important biomarkers analysed including CEC, vWF, P-selectin and thrombomodulin in children with JDM. These markers were correlated with disease activity. Key findings showed that CEC, vWF were increased in peripheral blood of JDM patients; vWF and P-selectin were not. CEC levels correlated with pulmonary disease activity.
Baka Z, Senolt L, Vencovsky J, Mann H, Simon PS, Kittel Á, et al. Increased serum concentration of immune cell derived microparticles in polymyositis/dermatomyositis. Immunol Lett. 2010;128(2):124–30.
Shirafuji T, Hamaguchi H, Higuchi M, Kanda F. Measurement of platelet-derived microparticle levels using an enzyme-linked immunosorbent assay in polymyositis and dermatomyositis patients. Muscle Nerve. 2009;39(5):586–90.
Oyabu C, Morinobu A, Sugiyama D, Saegusa J, Tanaka S, Morinobu S, et al. Plasma platelet-derived microparticles in patients with connective tissue diseases. J Rheumatol. 2011;38(4):680.
Notarnicola A, Barsotti S, Näsman L, Tang Q, Holmqvist M, Lundberg I, et al. Evaluation of risk factors and biomarkers related to arterial and venous thrombotic events in idiopathic inflammatory myopathies. Scand J Rheumatol. 2021;50(5):390–7. https://doi.org/10.1080/03009742.2020.1861647.
Kumamoto T, Abe T, Ueyama H, Sugihara R, Shigenaga T, Tsuda T. Elevated soluble intercellular adhesion molecules-1 in inflammatory myopathy. Acta Neurol Scand. 1997;95(1):34–7.
Figarella-Branger D, Schleinitz N, Boutière-Albanèse B, Camoin L, Bardin N, Guis S, et al. Platelet-endothelial cell adhesion molecule-1 and CD146: soluble levels and in situ expression of cellular adhesion molecules implicated in the cohesion of endothelial cells in idiopathic inflammatory myopathies. J Rheumatol. 2006;33(8):1623.
Wienke J, Pachman LM, Morgan GA, Yeo JG, Amoruso MC, Hans V, et al. Endothelial and inflammation biomarker profiles at diagnosis reflecting clinical heterogeneity and serving as a prognostic tool for treatment response in two independent cohorts of patients with juvenile dermatomyositis. Arthritis Rheum. 2020;72(7):1214–26.
Bloom BJ, Miller LC, Blier PR. Soluble adhesion molecules in pediatric rheumatic diseases. J Rheumatol. 2002;29(4):832.
Limaye VS, Bonder CS, Sun WY, Lester S, Roberts-Thomson PJ, Blumbergs P. Levels of soluble adhesion molecules and their associations in inflammatory myositis. Int J Rheum Dis. 2013;16(1):99–101.
Kubo M, Ihn H, Yamane K, Yazawa N, Kikuchi K, Soma Y, et al. Increased serum levels of soluble vascular cell adhesion molecule-1 and soluble E-selectin in patients with polymyositis/dermatomyositis. Br J Dermatol. 2000;143(2):392–8.
Kim E, Cook-Mills J, Morgan G, Sredni ST, Pachman LM. Increased expression of vascular cell adhesion molecule 1 in muscle biopsy samples from juvenile dermatomyositis patients with short duration of untreated disease is regulated by miR-126. Arthritis Rheum. 2012;64(11):3809–17.
Komiya T, Negoro N, Kondo K, Miura K, Hirota Y, Yoshikawa J. Clinical significance of von Willebrand factor in patients with adult dermatomyositis. Clin Rheumatol. 2005;24(4):352–7.
Funauchi M, Shimadsu H, Tamaki C, Yamagata T, Nozaki Y, Sugiyama M, et al. Role of endothelial damage in the pathogenesis of interstitial pneumonitis in patients with polymyositis and dermatomyositis. J Rheumatol. 2006;33(5):903.
Scott JP, Arroyave C. Activation of complement and coagulation in juvenile dermatomyositis. Arthritis Rheum. 1987;30(5):572–6.
Chai K-X, Chen Y-Q, Fan P-L, Yang J, Yuan X. STROBE: The correlation of Cyr61, CTGF, and VEGF with polymyositis/dermatomyositis. Medicine (Baltimore). 2018;97(34):e11775.
Silva T, Silva M, Shinjo S. Relevance of serum angiogenic cytokines in adult patients with dermatomyositis. Adv Rheumatol. 2018;58:17.
Szodoray P, Alex P, Knowlton N, Centola M, Dozmorov I, Csipo I, et al. Idiopathic inflammatory myopathies, signified by distinctive peripheral cytokines, chemokines and the TNF family members B-cell activating factor and a proliferation inducing ligand. Rheumatology (Oxford). 2010/06/29 ed. 2010;49(10)):1867–77.
Tawalbeh SM, Marin W, Morgan GA, Dang UJ, Hathout Y, Pachman LM. Serum protein biomarkers for juvenile dermatomyositis: a pilot study. BMC Rheumatol. 2020;4:52.
Liao AP, Salajegheh M, Nazareno R, Kagan JC, Jubin RG, Greenberg SA. Interferon β is associated with type 1 interferon-inducible gene expression in dermatomyositis. Ann Rheum Dis. 2011;70(5):831.
Chen M, Quan C, Diao L, Xue F, Xue K, Wang B, et al. Measurement of cytokines and chemokines and association with clinical severity of dermatomyositis and clinically amyopathic dermatomyositis. Br J Dermatol. 2018;179(6):1334–41.
Gono T, Kaneko H, Kawaguchi Y, Hanaoka M, Kataoka S, Kuwana M, et al. Cytokine profiles in polymyositis and dermatomyositis complicated by rapidly progressive or chronic interstitial lung disease. Rheumatology. 2014;53(12):2196–203.
Melki I, Devilliers H, Gitiaux C, Bondet V, Belot A, Bodemer C, et al. Circulating Interferon-α measured with a highly sensitive assay as a biomarker for juvenile inflammatory myositis activity: comment on the article by Mathian et al. Arthritis Rrheumatol (Hoboken, NJ). 2020;72(1):195–7.
Rodero MP, Decalf J, Bondet V, Hunt D, Rice GI, Werneke S, et al. Detection of interferon alpha protein reveals differential levels and cellular sources in disease. J Exp Med 2017/04/18 ed. 2017;214(5):1547–55.
Niewold TB, Kariuki SN, Morgan GA, Shrestha S, Pachman LM. Elevated serum interferon-alpha activity in juvenile dermatomyositis: associations with disease activity at diagnosis and after thirty-six months of therapy. Arthritis Rheum. 2009;60(6):1815–24.
Greenberg SA, Higgs BW, Morehouse C, Walsh RJ, Won Kong S, Brohawn P, et al. Relationship between disease activity and type 1 interferon- and other cytokine-inducible gene expression in blood in dermatomyositis and polymyositis. Genes Immun. 2012;13(3):207–13.
Walsh RJ, Kong SW, Yao Y, Jallal B, Kiener PA, Pinkus JL, et al. Type I interferon-inducible gene expression in blood is present and reflects disease activity in dermatomyositis and polymyositis. Arthritis Rheum. 2007;56(11):3784–92.
Rigolet M, Hou C, Amer Y, Aouizerate J, Periou B, Gherardi R, et al. Distinct interferon signatures stratify inflammatory and dysimmune myopathies. RMD Open. 2019;5:e000811.
Baechler EC, Bauer JW, Slattery CA, Ortmann WA, Espe KJ, Novitzke J, et al. An interferon signature in the peripheral blood of dermatomyositis patients is associated with disease activity. Mol Med. 2007;13(1–2):59–68.
Reed AM, Peterson E, Bilgic H, Ytterberg SR, Amin S, Hein MS, et al. Changes in novel biomarkers of disease activity in juvenile and adult dermatomyositis are sensitive biomarkers of disease course. Arthritis Rheum. 2012;64(12):4078–86.
Bilgic H, Ytterberg SR, Amin S, McNallan KT, Wilson JC, Koeuth T, et al. Interleukin-6 and type I interferon–regulated genes and chemokines mark disease activity in dermatomyositis. Arthritis Rheum. 2009;60(11):3436–46.
Piper CJM, Wilkinson MGL, Deakin CT, Otto GW, Dowle S, Duurland CL, et al. CD19(+)CD24(hi)CD38(hi) B cells are expanded in juvenile dermatomyositis and exhibit a pro-inflammatory phenotype after activation through toll-like receptor 7 and interferon-α. Front Immunol. 2018;9:1372.
• Wienke J, Bellutti Enders F, Lim J, Mertens JS, van den Hoogen LL, Wijngaarde CA, et al. Galectin-9 and CXCL10 as biomarkers for disease activity in juvenile dermatomyositis: a Longitudinal Cohort Study and Multicohort Validation. Arthritis Rrheumatol (Hoboken, NJ). 2019;71(8):1377–90 Elevated galectin-9 and CXCL-10 in patients with active JDM compared to patients with inactive disease, levels of these biomarkers rose prior to a flare of the JDM. These biomarkers were also elevated in adult patients with active IIM compared to inactive disease.
Bellutti Enders F, van Wijk F, Scholman R, Hofer M, Prakken BJ, van Royen-Kerkhof A, et al. Correlation of CXCL10, tumor necrosis factor receptor type II, and Galectin 9 with disease activity in juvenile dermatomyositis. Arthritis Rheum. 2014;66(8):2281–9.
Matsuda S, Kotani T, Ishida T, Fukui K, Fujiki Y, Suzuka T, et al. Exploration of pathomechanism using comprehensive analysis of serum cytokines in polymyositis/dermatomyositis-interstitial lung disease. Rheumatology. 2020;59(2):310–8.
Bai J, Wu C, Zhong D, Xu D, Wang Q, Zeng X. Hierarchical cluster analysis of cytokine profiles reveals a cutaneous vasculitis-associated subgroup in dermatomyositis. Clin Rheumatol. 2021;40(3):999–1008.
Sanner H, Schwartz T, Flatø B, Vistnes M, Christensen G, Sjaastad I. Increased levels of eotaxin and MCP-1 in juvenile dermatomyositis median 16.8 years after disease onset; associations with disease activity, duration and organ damage. PLoS One. 2014;9(3):e92171.
Crowson CS, Hein MS, Pendegraft RS, Strausbauch MA, Niewold TB, Ernste FC, et al. Interferon Chemokine score and other cytokine measures track with changes in disease activity in patients with juvenile and adult dermatomyositis. ACR Open Rheumatol. 2019;1(2):83–9.
Marstein H, Schwartz T, Aaløkken M, Lund M, Flatø B, Sjaastad I, et al. Novel associations between cytokines and pulmonary involvement in juvenile dermatomyositis - a cross-sectional study of long-term disease. Rheumatology (Oxford, England). 2019;18:59.
Xu D, Huang C-C, Kachaochana A, Morgan GA, Bonaldo MF, Soares MB, et al. MicroRNA-10a regulation of proinflammatory mediators: an important component of untreated juvenile dermatomyositis. J Rheumatol. 2016;43(1):161.
Wu H, Chen H, Hu P. Circulating endothelial cells and endothelial progenitors as surrogate biomarkers in vascular dysfunction. Clin Lab. 2007;53:285–95.
Blann A, Woywodt A, Bertolini F, Bull T, Buyon J, Clancy R, et al. Circulating endothelial cells. Biomarker of vascular disease. Thromb Haemost. 2005;93:228–35.
Sabatier F, Camoin-Jau L, Anfosso F, Sampol J, Dignat-George F. Circulating endothelial cells, microparticles and progenitors: key players towards the definition of vascular competence. J Cell Mol Med. 2009;13(3):454–71.
Clarke LA, Hong Y, Eleftheriou D, Shah V, Arrigoni F, Klein NJ, et al. Endothelial injury and repair in systemic vasculitis of the young. Arthritis Rheum. 2010;62(6):1770–80.
Clancy RM. Circulating endothelial cells and vascular injury in systemic lupus erythematosus. Curr Rheumatol Rep. 2000;2(1):39–43.
Woywodt A, Streiber F, de Groot K, Regelsberger H, Haller H, Haubitz M. Circulating endothelial cells as markers for ANCA-associated small-vessel vasculitis. Lancet (British edition). 2003;361(9353):206–10.
Zhang M, Malik AB, Rehman J. Endothelial progenitor cells and vascular repair. Curr Opin Hematol. 2014;21(3):224–8.
Morel O, Jesel L, Freyssinet J-M, Toti F. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler Thromb Vasc Biol. 2011;31(1):15–26.
Curtis AM, Edelberg J, Jonas R, Rogers WT, Moore JS, Syed W, et al. Endothelial microparticles: sophisticated vesicles modulating vascular function. Vasc Med. 2013;18(4):204–14.
Hugel B, Martínez MC, Kunzelmann C, Freyssinet J-M. Membrane microparticles: two sides of the coin. Physiology. 2005;20(1):22–7.
Falati S, Liu Q, Gross P, Merrill-Skoloff G, Chou J, Vandendries E, et al. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med. 2003;197(11):1585–98.
Katopodis JN, Kolodny L, Jy W, Horstman LL, De Marchena EJ, Tao JG, et al. Platelet microparticles and calcium homeostasis in acute coronary ischemias. Am J Hematol. 1997;54(2):95–101.
Aird WC. Phenotypic heterogeneity of the endothelium. Circ Res. 2007;100(2):158–73.
Sallum AME, Marie SKN, Wakamatsu A, Sachetti S, Vianna MAAG, Silva CAA, et al. Immunohistochemical analysis of adhesion molecule expression on muscle biopsy specimens from patients with juvenile dermatomyositis. J Rheumatol. 2004;31(4):801.
Bartoccioni E, Gallucci S, Scuderi F, Ricci E, Servidei S, Broccolini A, et al. MHC class I, MHC class II and intercellular adhesion molecule-1 (ICAM-1) expression in inflammatory myopathies. Clin Exp Immunol. 1994;95(1):166–72.
Tews DS, Goebel HH. Expression of cell adhesion molecules in inflammatory myopathies. J Neuroimmunol. 1995;59(1):185–94.
Jain A, Sharma CM, Sarkar C, Singh S, Handa R. Increased expression of cell adhesion molecules in inflammatory myopathies: diagnostic utility and pathogenetic insights. Folia Neuropathol. 2009;47(1):33–42.
Carruthers EC, Choi HK, Sayre EC, Aviña-Zubieta JA. Risk of deep venous thrombosis and pulmonary embolism in individuals with polymyositis and dermatomyositis: a general population-based study. Ann Rheum Dis 2014/09/05 ed. 2016;75(1):110–6.
Wahezi D, Arena V, Choi J, Gao Q. The role of Von Willebrand factor as a disease biomarker in the clinical assessment of children with juvenile dermatomyositis [abstract]. Arthritis Rheumatol. 2016;68 (supple 10).
Barnes TC, Anderson ME, Moots RJ. The many faces of interleukin-6: the role of IL-6 in inflammation, vasculopathy, and fibrosis in systemic sclerosis. Int J Rheumatol. 2011;2011:721608–6.
Wakiguchi H, Hasegawa S, Hirano R, Kaneyasu H, Wakabayashi-Takahara M, Ohga S. Successful control of juvenile dermatomyositis-associated macrophage activation syndrome and interstitial pneumonia: distinct kinetics of interleukin-6 and -18 levels. Pediatr Rheumatol Online J. 2015;13:49.
Gitiaux C, Latroche C, Weiss-Gayet M, Rodero MP, Duffy D, Bader-Meunier B, et al. Myogenic progenitor cells exhibit type I interferon–driven proangiogenic properties and molecular signature during juvenile dermatomyositis. Arthritis Rheum. 2018;70(1):134–45.
Ladislau L, Suárez-Calvet X, Toquet S, Landon-Cardinal O, Amelin D, Depp M, et al. JAK inhibitor improves type I interferon induced damage: proof of concept in dermatomyositis. Brain (London, England : 1878). 2018;141(6):1609–21.
Kim H. Updates on interferon in juvenile dermatomyositis: pathogenesis and therapy. Curr Opin Rheumatol. 2021;33(5):371–7.
Barrat FJ, Crow MK, Ivashkiv LB. Interferon target-gene expression and epigenomic signatures in health and disease. Nat Immunol. 2019;20(12):1574–83.
Kim H, Gunter-Rahman F, McGrath JA, Lee E, de Jesus AA, Targoff IN, et al. Expression of interferon-regulated genes in juvenile dermatomyositis versus Mendelian autoinflammatory interferonopathies. Arthritis Res Ther. 2020;22(1):69–12.
Schwartz T, Sjaastad I, Flatø B, Vistnes M, Christensen G, Sanner H. In active juvenile dermatomyositis, elevated eotaxin and MCP-1 and cholesterol levels in the upper normal range are associated with cardiac dysfunction. Rheumatology. 2014;53(12):2214–22.
Wienke J, Mertens JS, Garcia S, Lim J, Wijngaarde CA, Yeo JG, et al. Biomarker profiles of endothelial activation and dysfunction in rare systemic autoimmune diseases: implications for cardiovascular risk. Rheumatology. 2021;60(2):785–801.
Lutz J, Huwiler KG, Fedczyna T, Lechman TS, Crawford S, Kinsella TR, et al. Increased plasma thrombospondin-1 (TSP-1) levels are associated with the TNFα-308A allele in children with juvenile dermatomyositis. Clin Immunol. 2002;103(3):260–3.
Pachman LM, Liotta-Davis MR, Hong DK, Kinsella TR, Mendez EP, Kinder JM, et al. TNFα-308A allele in juvenile dermatomyositis: association with increased production of tumor necrosis factor α, disease duration, and pathologic calcifications. Arthritis Rheum. 2000;43(10):2368–77.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
ChP has received speaker fees from Sobi and Consultancy fees from Novartis.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topical Collection on Inflammatory Muscle Disease
Rights and permissions
About this article
Cite this article
McLellan, K., Papadopoulou, C. Update on Biomarkers of Vasculopathy in Juvenile and Adult Myositis. Curr Rheumatol Rep 24, 227–237 (2022). https://doi.org/10.1007/s11926-022-01076-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11926-022-01076-4