Abstract
Purpose of Review
Guidelines for the management of large vessel vasculitides have been recently updated by several scientific societies. We have evaluated the current recommendations for treatment of giant cell arteritis (GCA) and Takayasu arteritis (TA) and addressed potential future therapeutic strategies.
Recent Findings
While glucocorticoids (GCs) remain the gold standard for induction of remission, many patients relapse and acquire high cumulative GC exposure. Thus, GC-sparing therapies such as methotrexate are recommended for selected patients with GCA and all patients with TA. Recent high-quality evidence shows that tocilizumab is an effective GC-sparing agent in GCA. Non-biologic and biologic immunomodulators also appear to have GC-sparing properties in TA.
Summary
Tocilizumab is now considered to be part of the standard treatment for GCA, particularly with relapsing disease, but questions on its use such as length of treatment and monitoring of disease activity remain open. High-quality evidence to guide treatment of TA is still lacking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
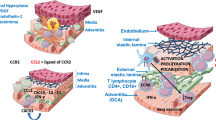
Giant cell arteritis (GCA) and Takayasu arteritis (TA) are the two major subtypes of large vessels vasculitis (LVV). Arterial inflammation can lead to vascular stenosis or aneurysm formation. Consequently, severe complications such as blindness and stroke or rupture of aortic aneurysms can occur. While some suggest that GCA reduces survival, at least in subsets of patients (e.g., those with aortic involvement), other studies have reported that the mortality was similar to that of the general population [1]. Mortality in TA is definitely increased, particularly in patients with rapidly progressive disease, thoracic aortic involvement, and/or retinopathy [2].
High-dose glucocorticoids effectively induce remission in LVV, but relapses are common, once the dose is reduced [3, 4]. Therefore, many patients need to be treated over an extended period of time resulting in high cumulative glucocorticoid (GC) doses [5]. As a result, patients are at risk of acquiring GC-related complications such as osteoporosis, infections, glaucoma, or diabetes mellitus [6, 7]. Conventional and biologic immunosuppressive drugs have been studied for their potential to reduce the GC exposure and maintain remission [8•, 9]. In view of these and other new data, the European League Against Rheumatism (EULAR) as well as the British, Swedish and French Societies of Rheumatology have recently updated their recommendations for the management of LVV [10,11,12,13]. In this review, we aim to summarize current guidelines for drug therapy of GCA and TA. Furthermore, we review the most recent developments including novel potential therapeutic targets.
Due to the paucity of data, no recommendations exist for treatment of other very rare forms of LVV such as isolated aortitis, infectious aortitis, or LVV in the context of IgG4-related disease.
Giant Cell Arteritis
General Approach
Patients with untreated active GCA are at immediate risk of permanent loss of vision or other ischemic complications. The current EULAR recommendations propose that patients with a clinical presentation suggestive of GCA and increased C-reactive protein (CRP) and/or erythrocyte sedimentation rate (ESR) should be referred immediately to a specialized team or center [10]. For this purpose, EULAR and other societies have defined key symptoms of GCA that should trigger specialist evaluation and treatment (Table 1). Of note, none of these “red flag signs” in isolation is highly specific and thus careful evaluation of differential diagnoses is crucial. Results from two retrospective cohort studies have shown that diagnostic work-up and treatment of patients with suspected GCA in a specialized center (so-called fast-track clinic) within 24 h significantly reduces the risk of permanent visual impairment [14, 15]. Therefore, recent guidelines of the British Society of Rheumatology (BSR) specify that patients should be evaluated by a specialist ideally on the same working day, but at least within 3 days from presentation to a general practitioner [11]. A vasculitis center should have experience in the diagnostic methods available to confirm the diagnosis (sonography, MRI, PET, temporal artery biopsy) and be able to offer these in a timely manner [10].
In patients with a high clinical suspicion of GCA, particularly in the presence of new visual impairment, high-dose GC should be given immediately. The sensitivity of positive diagnostic tests for GCA (including CRP and ESR, temporal artery biopsy, ultrasound, and large vessel imaging) decreases rapidly once high-dose GC therapy is commenced [16,17,18]. Pre-emptive therapy should (a) not delay early referral, should (b) be limited to a few days duration at the most, and should (c) be stopped once GCA is ruled out [10]. Blood should be taken for full blood cell count, CRP, and ESR (preferably) before or immediately after starting high-dose GCs [11].
The current COVID-19 pandemic highlights the importance of an accurate diagnosis of GCA, both to avoid the complications of the disease in those with GCA, but even more importantly, to avoid the unnecessary use of high doses of glucocorticoids (with their attendant risk of infection) in patients with diagnostic uncertainty in whom a definitive imaging test or biopsy has not been performed in a timely fashion [19].
The goals of treatment in GCA are to achieve remission and prevent acute ischemic complications and long-term damage. Evidence-based criteria for remission or response to therapy do not yet exist. Recently, EULAR has defined activity stages, based on an expert consensus (Table 2).
Glucocorticoids
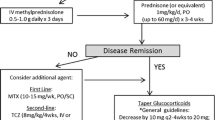
EULAR and BSR recommend starting treatment of GCA with GC at a dose of 40–60 mg prednisolone-equivalent per day [10, 11]. The initial dose is continued until GCA-related symptoms resolve and CRP and ESR decrease, which is the case in the vast majority of patients after a few days of treatment. There is no evidence that dosing GC according to body weight leads to better clinical outcomes than administration of a fixed dose [4]. Also, current guidelines do not recommend the use of high-dose intravenous GC in patients without visual impairment. Two randomized controlled clinical trials (RCTs) on the use of intravenous GC in patients with new-onset GCA without ischemic complications reported contradictory results [4]. In the smaller of the two trials, 27 patients received either 15 mg/kg body weight/day intravenous methylprednisolone for 3 days or placebo plus 40 mg/day oral prednisone. At week 78, the cumulative GC dose in the methylprednisolone group (median cumulative glucocorticoid dose 5636 mg (interquartile range (IQR) 4050–6690)) was lower, compared to the control group (7860 mg (IQR 7373–9005), but the GC pulses (3 g) were not counted for calculation of the cumulative dose [20, 21]. No GC-sparing effect of intravenous GCs after 12 months was observed in a larger (N = 164) but lower quality open RCT, where patients received either intravenous methylprednisolone at a single dose of 240 mg followed by 0.7 mg/kg oral prednisone or oral prednisone at 0.7 mg/kg alone, or a single dose of 240 mg intravenous methylprednisolone followed by 0.5 mg/kg oral prednisone. Current guidelines do not recommend alternate day administration of oral GC, splitting the GC dose in two or more daily doses or modified-release prednisone [10, 11], because there is no evidence of improved outcomes compared to using GCs once daily [4].
Although acute visual loss is the most common type of severe ischemic complication of GCA, there are no high-quality data on its management. Low-quality evidence from observational studies suggests that immediate initiation of GC therapy is the most important factor for visual recovery [22]. If the onset of treatment is delayed (> 24 h), vision improves only in a minority of patients [23]. Acknowledging the low level of evidence available, EULAR recommends considering the administration of 0.25–1 g intravenous methylprednisolone daily for up to 3 consecutive days in GCA patients with acute visual loss or amaurosis fugax [10].
Once the patient is in remission (Table 2), the GC dose is gradually tapered. There is no high-quality evidence to guide GC taper in GCA [4]. Although not specifically designed to compare different GC taper protocols, a RCT of tocilizumab for treatment of GCA provides information on GC dosing because it had two placebo arms with a different speed of GC taper [8•, 24]. Prednisone doses were decreased at the same rate in both arms until a dose of 20 mg was reached [24]. Then, in one arm, the dose was tapered to 0 mg at week 26 and the other arm at week 52. Post hoc analysis of data from 101 patients revealed two important messages regarding GC monotherapy in GCA. First, the relapse rate within the first year of treatment is higher with a 26-week taper (68%) compared to a 52-week taper (49%) [25•]. Relapse rates in this range are in line with data from observational studies (summarized in [4]) with different relapse definitions and taper protocols reporting relapse rates ranging from 34 to 75%. Second, a relapse rarely occurs at a prednisone dose of > 20 mg/day (only one of 101 patients). Therefore, these data support the recent EULAR recommendation to taper GC to 15–20 mg/day within 2–3 months and to ≤ 5 mg/day 1 year. Then, a further reduction at a rate of 1 mg every 1–2 months should be attempted. A more rapid dose reduction is appropriate for patients receiving concomitant GC-sparing therapy. However, the abovementioned 26-week taper protocol has been only been formally tested in patients receiving concomitant tocilizumab (TCZ); the GC taper needs to be individualized for patients receiving methotrexate or other GC-sparing agents.
Indications for GC-Sparing Therapies
Available evidence suggests that around 30–60% of patients do not relapse and are able to taper GC to ≤ 5 mg/day at 1 year [5, 25•, 26,27,28]. As a EULAR task force considered this dose to be acceptably safe [29], EULAR and BSR recommend that patients without risk factors for GC AEs and/or prolonged course of GCA may be treated with GC only [11]. However, patients with GCA have an increased risk of developing osteoporosis, diabetes, glaucoma, infections, and other complications attributable to GC therapy at a higher rate than the general population [6, 7]. One study calculated an increase of the hazard ratio for adverse events by 3% for each 1000 mg increase in cumulative glucocorticoid exposure [30]. Cumulative GC dose increases with each relapse of GCA [3, 26]. Therefore, EULAR and national guidelines recommend adjunctive GC-sparing therapies for relapsing GCA patients and/or patients who have developed or have an increased risk of developing such GC-related comorbidities and complications. As only few patients in the elderly population affected by GCA have no comorbidities and up to 90% of patients treated with GC experience at least one adverse event [31], the vast majority of patients are potential candidates for adjunctive GC-sparing therapies in clinical practice. In the individual patient, the decision to use adjunctive immunosuppressive therapies must be balanced against potential treatment-related complications based on individual risk profiles and comorbidities.
In some studies, a higher relapse rate and prolonged treatment was associated with scalp tenderness at diagnosis, PMR, and a composite of features of inflammation (defined as three or four of the following: fever, weight loss, ESR ≥ 85 mm/h, and hemoglobin < 11 g/dl), but data on clinical and laboratory features predicting relapses are not consistent across all studies [4]. Imaging evidence of extracranial involvement has been associated with prolonged glucocorticoid treatment and disease progression such as aortic dilatation compared with patients with cranial GCA who did not have imaging evidence of LV-GCA [32, 33]. In a recent prospective study, high 18-FDG-uptake on PET-CT was associated with an increased relapse risk [34] and PET activity increased after the intensity of treatment was reduced [35]. In a retrospective analysis of 326 patients, LVV was an independent predictive factor of relapse (HR 1.49, 95% CI 1.002–2.12; p = 0.04) and GC dependence (OR 2.19, 95% CI 1.19–4.05; p = 0.01) in multivariable analyses [36]. In that study, patients with a GC-dependent disease who received a GC-sparing agent had a shorter GC treatment duration than those who did not receive GC-sparing therapy (p = 0.008). In summary, there is growing evidence that high disease activity and extent at disease onset appear to be associated with a more severe and more prolonged disease course. Therefore, patients with high disease activity, particularly active extracranial disease, might benefit from early adjunctive GC-sparing treatment. Prospective studies that include LV imaging are needed to confirm this hypothesis.
Non-biologic Immunosuppressive Therapies
Methotrexate
A meta-analysis of 3 placebo-controlled studies revealed a reduced risk of first relapse (HR 0.65, 95% CI 0.44 to 0.98, p = 0.04) and second relapse (HR 0.49, 95% CI 0.27 to 0.89, p = 0.02), a higher probability of GC-free remission for ≥ 24 weeks (HR 2.84, 95% CI 1.52 to 5.28, p < 0.001) and a lower cumulative GC dose of − 842 mg at week 48 in new-onset GCA patients treated with methotrexate (MTX) versus controls [37]. Only one of the 3 studies included in the meta-analysis met its primary endpoint. However, at least one of the studies that enrolled only 21 patients and used a low methotrexate dose of 7.5 mg per week was underpowered to exclude or demonstrate a treatment effect of MTX [38]. A recent pooled analysis of the two larger studies [39, 40] revealed that MTX reduced the relapse rate at 12–24 months (RR 3.20 (95% CI 1.49, 6.87)) [11]. A recent large monocentric case-control study involving 166 patients showed that in real life, the use of MTX lead to a twofold stronger (P = 0.004) reduction of relapse rates compared to matched controls without GC-sparing therapy [41]. Overall, no evidence was found that the use of MTX in GCA is associated with an increased risk of adverse events [37], but comorbidities such as renal or liver disease can limit its use in this elderly population. Recent guidelines recommend that MTX might be considered as an adjunctive immunosuppressant in combination with GC (BSR), as an alternative to TCZ (EULAR) or in cases of late relapse without major inflammatory activity or with marked GC side effects, when the patient does not meet the criteria for TCZ treatment (Swedish Society of Rheumatology) [10, 11, 13]. In contrast to TCZ, there is no evidence-based standard GC taper scheme for MTX, and thus, GC dose has to be reduced on an individual basis.
Leflunomide
In an open-label single-center study, 76 patients were given leflunomide after 12 weeks of GC therapy. During a follow-up period of 48 weeks, relapses were lower in the group of patient receiving leflunomide (13.3%) compared to those patients who received GC only (39.1%; p = 0.02; NNT 3.9 (95% CI 2.2–17.4)). As the study is biased by the lack of randomization and a placebo group, data need to be interpreted with caution but raise interest in a prospective controlled trial.
Other Conventional Immunosuppressants
Only low-quality and/or negative data exist for the use of azathioprine, mycophenolate mofetil, cyclophosphamide, hydroxychloroquine, dapsone, and ciclosporin [4]. The use of these agents in GCA is currently not recommended [10, 11].
Biologic Therapies
Tocilizumab
Two double-blind RCTs have shown significant efficacy of the interleukin-6 (IL-6) receptor antagonist TCZ compared to GC monotherapy [8•, 9]. In the larger of the two trials (enrolling 251 patients with either new-onset or relapsing GCA), the rate of sustained GC-free remission at week 52 (primary endpoint) was achieved in 56%/53% of patients receiving 162 mg TCZ weekly/every other week plus a 26-week GC taper compared to only 14%/18% in patients receiving GC alone for 26/52 weeks [8•]. In addition, a significant GC-sparing effect was documented. There was no increase in adverse events during TCZ therapy. Based on these data, TCZ has been approved for the treatment of GCA in the EU, the USA, and other countries. EULAR and national scientific societies recommend the use of TCZ for patients with GCA in whom there is an indication for GC-sparing therapy [10,11,12,13].
TCZ therapy was discontinued in both studies after 1 year. Long-term data from both studies (published in abstract form only for the larger phase 3 trial) indicate that less than 50% of patients with GCA who discontinued TCZ after 1 year had a sustained remission for the next 2 years, while the rest of the patients relapsed [42, 43]. Analysis of flares after stopping 4-weekly intravenous TCZ in the phase 2 RCT showed that neither clinical features, serum biomarkers ,nor magnetic resonance imaging of large vessels were reliable predictors of future relapse [43]. Elevations of CRP and ESR were rarely observed at and before relapse in the phase 3 study while patients were still on TCZ [25•]. In the two GC-only groups of this trial, an elevation of CRP at the time of relapse was found in 65 and 68% of patients, respectively [25•]. While the lack of CRP elevations in patients receiving tocilizumab was expected based on the mechanism of action of TCZ, the poor correlation of acute phase reactant levels with relapse in patients treated with GC only in the phase 3 study is a bit surprising. Measurement of IL-6-serum levels appears to be of little value in clinical practice, as results from two recent studies [43, 44] did not confirm a previously reported moderate association of longitudinally decreasing IL-6 levels and the risk for a future relapses [45]. Elevations of matrix metalloproteinase-3 (MMP-3), pentraxin-3, soluble tumor necrosis factor receptor 2 (sTNFR2), or serum amyloid A have been reported in patients with active GCA. As these biomarkers are no easily available in routine and data showing an adequate diagnostic performance (sensitivity, specificity, positive/negative predictive value) or predict relapses are lacking, these markers are, at present, of no use in clinical practice. Increased serum concentrations of osteopontine have been found in relapsing versus non-relapsing patients in one study [46]. As osteopontine expression and protein production in cultured arteries were not significantly modified by TCZ, osteopontine may have potential value in TCZ-treated patients where CRP is usually not detectable. Further studies are needed to evaluate if increases in serum ostepontine concentrations are reliable in predicting current and/or future relapse. No other biomarkers have been identified to date, to predict future relapses in GCA with sufficient sensitivity and specificity to guide treatment decisions [4].
In summary, there are no valid data available on which the optimal duration of therapy with TCZ can be reliably based on. The decision to continue, extend the interval, or terminate therapy after 1 year must therefore be made individually and should be discussed with the patient. Possible criteria for decision making, which are not yet supported by evidence, may be a history of relapse, a previously high GC demand or the presence of vascular complications favoring a strategy not to stop TCZ.
In a recent open label trial in 8 patients with GCA and 3 patients with TA, TCZ monotherapy (8 mg/kg) was administered every 2 weeks for 2 months without glucocorticoids or other immunosuppressive agents, and then every 4 weeks for 10 months [47]. After 24 and 52 weeks, 75% of GCA patients were in complete remission and 25% in partial remission. Two out of 3 TA patients achieved full remission after 24 and 52 weeks, one patient did not respond. Since the administration of TCZ was the only intervention, these data support the hypothesis that TCZ therapy might be effective without additional administration of GC, at least in GCA. Obvious limitations of this study are the open study design without a control group and the small number of cases. A particular concern is the possibility that GCA/TA cannot be adequately controlled by TCZ monotherapy in the early phase of the disease. TCZ serum concentrations after 4 weeks of intravenous therapy are only about 10% of the steady-state level, which is finally reached after the 6th or 7th dose (after 4–6 months) [48]. This poses the potential risk of irreversible damage such as blindness due to insufficiently controlled vasculitis in early disease phase.
Is TCZ superior to MTX or other agents used for GC-sparing in GCA? While studies on TCZ are of superior quality and appear to show a stronger effect size in terms of GC-sparing [4], the very different study designs do not allow reliable conclusions in this regard. Results from a currently recruiting head-to-head study between TCZ and MTX (NCT03892785) will provide more robust information on the role of each agent in the treatment of GCA.
Abatacept
In a double-blind phase 2 RCT, patients receiving the CTLA-4 agonist abatacept (ABA) had higher relapse-free survival (48%) compared to patients treated with GC alone (31%, P = 0.049). The study was not designed to detect a difference in GC exposure. There were no safety concerns in the group treated with ABA. Despite the positive signals in the phase 2 study, no phase 3 studies are currently listed at clinicaltrials.gov.
Ustekinumab
Ustekinumab is a monoclonal antibody targeting the subunit common to IL-12 and IL-23 (p40) and inhibits both Th1 and Th-17 responses, which are major drivers of inflammation in the pathogenesis of GCA [49]. In one study, all 25 patients with refractory GCA who received ustekinumab in addition to GC stayed in remission while the median daily prednisolone dose was decreased from 20 to 5 mg over 1 year and improvement of vasculitis using CT imaging was reported [50]. The second study, which has yet been reported in abstract form, was prematurely terminated after 7 of the first 11 patients relapsed [51]. In contrast to the first study, GC was tapered to 0 at 6 months in the second study, which may account for the higher relapse rate. The conflicting results show the limitations of open-label studies in GCA related to concomitant GCs and highlight the need for a double-blind RCT.
TNF-α Inhibitors
RCTs on adalimumab, etanercept (ETA), and infliximab (IFX) did not provide evidence of efficacy in terms of GC-sparing effect, disease activity, and GC withdrawal or reduction of GC cumulative doses and adverse events [52,53,54].
Future Perspectives of Immunomodulating Therapy in GCA
Clincaltrials.gov lists several biologics and targeted synthetic DMARDs (tsDMARDS) that are currently in different stages of development in GCA (Table 3). Based on the pathogenesis of GCA potential, cytokine targets under study include: IL-1 (anakinra), IL-6 (sarilumab), IL-17 (secukinumab), IL-12/23 (ustekinumab), and GM-CSF (mavrililumab). In addition, RCTs on the tsDMARDS upadacitinib and baricitinib are ongoing. A number of trials are currently investigating TCZ combined with a short GC taper protocol (less than 26 weeks) or different GC taper regimens.
Other Medications
Antiplatelet and Anticoagulant Therapy
The risk for cardiovascular and cerebrovascular events is increased in GCA [55, 56]. In two retrospective studies, the use of low-dose aspirin before or at the time of diagnosis of GCA was associated with a reduced rate of vision loss or stroke, but the number of events was low [57, 58]. However, two more recent cohort studies and a meta-analysis did not confirm a protective effect of aspirin in GCA [59,60,61]. High-quality data from a RCT on this topic are lacking [62]. A potential but yet unproven protective effect of aspirin must be balanced against its potential harm such as bleeding [63]. In view of these considerations, EULAR and BSR do not recommend routine use of antiplatelet or anticoagulant therapy unless they are indicated for other reasons (e.g., coronary heart disease, cerebrovascular disease, etc.) [10, 11]. In contrast, the Swedish guidelines recommend to consider prescription of aspirin in newly diagnosed GCA unless there are contraindications [13].
Lipid-Lowering Agents
The routine use of cholesterol-lowering agents, such as statins for GCA, is not recommended in current guidelines. Our recent SLR [4] identified two population-based incident cases cohorts and two retrospective longitudinal cohorts that reported contradictory results. Therefore, a RCT on the potential role of statins for treatment of GCA would be needed.
Takayasu Arteritis
General Approach
The majority of principles for the general management of patients with GCA apply also for patients with TA. Unlike GCA, disease onset in patients with TA is rarely accompanied by acute ischemic complications. Therefore, a fast-track approach like in GCA has not been advocated. However, patients with TA should be managed by a specialist team with access to the multidisciplinary infrastructure and experience of a vasculitis center. Treatment recommendations for TAK are largely based on low-quality evidence retrieved from observational studies, whereas RCTs have only published on the use of TCZ and abatacept [3].
Glucocorticoids
Like in GCA, there are no RCTs in TA that have been specifically designed to evaluate dosing or tapering of GCs. The best available evidence is derived from two recently published RCTs on the use of ABA and TCZ, in which GC was tapered according to defined protocols [64, 65]. The TCZ study included only relapsing patients receiving different GC regimens at the time of inclusion into the study, but at least 0.2 mg/kg/day was administered to all patients [64]. The GC dose was then reduced by 10% per week from week 4 to a minimum of 0.1 mg/kg/day. This reduction regimen resulted in a high relapse rate of approximately 80% during weeks 8–16 in the GC monotherapy arm. The ABA study included newly diagnosed and relapsing patients receiving prednisone at doses of 40–60 mg/day [65]. After the dose was reduced to 20 mg/day by week 12 and then to 0 mg at week 28, 60% of patients relapsed within 1 year. Both studies did not include a second arm with a different GC taper protocol. Therefore, these studies do not allow final conclusions on the most appropriate GC starting dose and reduction protocol.
EULAR recommends starting GC therapy with a dose of 40 to 60 mg per day [10]. To date, there is no evidence that a higher starting dose improves outcome. Data from a recent cohort study suggest that in patients with limited vascular involvement who receive a conventional GC-sparing therapy, lower initial doses of 20–30 mg prednisolone equivalent per day may be sufficient [66]. Once symptoms related to the inflammatory activity appear to be controlled, a dose reduction to 15–20 mg per day after 2–3 months is recommended. Due to the high relapse rates following dose reductions below this threshold, the current EULAR recommendations advise a slower further GC dose reduction to 10 mg per day, or less, after 1 year compared to GCA [10].
Non-biologic Immunosuppressive Therapies
Around 60–80% of TA patients treated with GC monotherapy relapse [64, 65, 67]. In addition, many patients develop new vascular lesions and/or cannot reduce the GC dose to an acceptable level [3]. Therefore, EULAR experts recommend an early initiation of GC-sparing therapy. RCTs on the role of conventional, synthetic, disease-modifying anti-rheumatic drugs (csDMARDs) in TA have not been published to date. In a recently published retrospective study on 235 patients with early initiation of GC-sparing therapy (mainly with mycophenolate mofetil), only 7% of patients had at least one relapse after 1 year, 34% after 5 years, and 48% after 10 years [66]. New structural vascular lesions were observed in 20% of patients over the long term. In indirect comparison with other cohorts without early GC-sparing therapy, where relapses and/or new structural lesions occur in up to 80% of patients [3], this suggest a clinically relevant benefit of adding a csDMARD in newly diagnosed TAK patients, as pointed out by the EULAR recommendation of a first-line use of csDMARDs in TA [10].
Methotrexate
In an open prospective study involving patients with persistent or GC-refractory TA weekly, MTX (mean dose 17.1 mg) + GC resulted in remission in 13/16 (81%) of patients [68]. Relapses were common after GC taper, but 50% of patients remained in sustained remission for 18 months on average.
Mycophenolate Mofetil
In a prospective open longitudinal study, MMF was initially combined with GC only and in case of an inadequate response to this treatment, MTX or azathioprine (AZA) was added. This step-wise approach improved the efficacy defined by a combined endpoint compared to MMF alone (80% vs. 40%) after a median follow-up of 17 months [69]. A meta-analysis of two observational studies showed that MMF significantly reduced acute phase parameters and the use of GC (mean difference in daily GC dose: − 17.96; 95% CI − 24.89; − 10.4 mg) [70].
Cyclophosphamide
The efficacy and safety of cyclophosphamide (CYC) was compared to MTX (+ GC) in a prospective cohort study in 58 TA patients without prior csDMARD therapy [71]. Induction treatment with CYC was followed by maintenance therapy with MTX or AZA. Remission (NIH criteria ≤ 1 and GC ≤ 15 mg/kg/day) was achieved with CYC (71.7%) and MTX (75%) at a similar rate. Although a significant decrease of humoral markers of inflammation and gadolinium enhancement in large vessel MRI was only observed in the CYC group, the study does not provide convincing evidence that induction treatment with CYC is superior to MTX, as the study was not randomized and baseline data differed significantly. Due to its gonadal toxicity and common long-term AEs, the use of CYC is limited in young women, who are predominantly affected by TA. Therefore, EULAR recommends avoiding the use of CYC except in patients where other treatments have failed or are not tolerated [10].
Other Drugs
In a prospective open-label study, a reduction of symptoms and humoral inflammation markers in patients treated with AZA (2 mg/kg/day) + GC (1 mg/kg/day) was reported [72]. No new structural vascular lesions were observed 1 year after the start of treatment. Long-term use of leflunomide was associated with persistent remission, in about half of the patients, in an open-label study [73]. However, after an average of 12 months, 7 of the 12 patients discontinued leflunomide therapy, mainly due to insufficient efficacy.
Biologic Therapies
In patients not responding adequately to therapy with csDMARDS + GC (refractory or relapse), EULAR recommends the use of TNF-α inhibitors (TNFi) or TCZ [10]. A descriptive prospective cohort study investigating the effects of escalating therapy with csDMARDs and then with bDMARDs (TNFi or TCZ) in TA patients that did not respond to GC showed that 64% of patients treated with bDMARDs achieved sustained remission [74].
Tocilizumab
The efficacy and safety of TCZ at a dose of 162 mg s.c. weekly was investigated in a double-blind, placebo-controlled RCT in 36 patients with relapsing TA [64]. Although this study failed to meet its primary endpoint in the intent-to-treat analysis (HR 0.41, 95% CI 0.15–1.10; p = 0.0596), it showed a significant difference in favor of TCZ versus placebo in the per-protocol analysis (HR 0.34, 95% CI 0.11–1.00; p = 0.0345). Since 44% of the patients receiving TCZ relapsed (vs. 61.1% on placebo), which resulted in an increase in the GC dose, no GC-sparing effect of TCZ could be shown during the blinded part of the study. However, during the long-term extension phase, in which 28 patients received open-label TCZ, the median GC dose was reduced from 0.223 mg/kg/day (study start) to 0.131 mg/kg/day (interquartile range 0.099, 0.207) after 48 weeks and 0.105 mg/kg/day (interquartile range 0.039, 0.153) after 96 weeks [75]. On imaging, 17.9% of patients showed improvement and 67.9% stable findings without progression at 96 weeks. An improvement in quality of life (SF-36) was also documented. The study showed no unexpected safety signals for TCZ.
The outcome in 144 patients with refractory TA treated with TCZ was reported from several cases series [76,77,78,79,80,81]. A decrease in clinical activity in response to TCZ was reported for the majority of patients. In the largest multicenter case series of 46 patients treated with TCZ, a reduction in median GC demand was reported from 15 (8–19) to 5 mg (4.5–9) daily after 6 months [78]. Event-free survival was significantly better on TCZ compared to DMARDs (p = 0.02). However, after TCZ was stopped, relapses occurred frequently, suggesting that the therapy reduces inflammatory activity but does not cure the disease. It is also unclear whether TCZ can improve structural vascular lesions such as stenoses or at least prevent further progression. In a case series, 4 out of 7 patients experienced a worsening of the vascular lesions observed by magnetic resonance angiography (MRA) and ultrasound [80]. An analysis of a case series of 5 patients plus a review 39 patients reported from the literature showed no effect of TCZ on radiological activity on imaging (defined as at least two of the following: (1) arterial wall thickening in angio-CT, (2) or arterial wall thickening with wall strengthening in MRA, (3) by PET-CT) after 6 months [76]. However, a significant decrease in arterial FDG uptake in PET-CT was demonstrated [76], which, in accordance with the long-term data of the prospective study [75], suggests that TCZ reduces inflammation in the vessel wall but does not necessarily lead to an improvement in structural vascular lesions.
TNF-α Inhibitors
In a prospective open-label study from the USA, ETA or IFX was given to patients with TA patients refractory to GC and csDMARDs [82]. The primary endpoint for efficacy was defined as the absence of clinical features of active disease or new lesions on sequential imaging with no GC therapy or GC dose reduced by ≥ 50%. Similar to TCZ studies, progression of imaging changes under TNFi was observed in 4 of 15 patients despite apparently complete clinical or partial remission. In the SLR for the EULAR recommendations, we analyzed 8 retrospective case series on the use of TNFi in patients with TA and several mixed cohorts (TA and GCA) [3]. In the predominantly refractory TA patients, a positive therapeutic effect or clinical symptoms was observed in most patients.
A retrospective, multicenter analysis of patients with TA (n = 49) treated with TNFi or TCZ showed no difference in safety and efficacy between the drugs [83], but the retrospective uncontrolled study design is unsuitable to rule out a possible difference between the two treatment arms.
RCTs on the use of TNFi in TA have not been published. Due to the retrospective design, the parallel use of other drugs, including continued GCs, the lack of control patients and the heterogeneity of the majority of studies, as well as the risk of a reporting bias (negative experiences are reported less frequently) and the different definitions of remission; thus, the formal level of evidence in supporting TNFi in TA is low. Several RCTs showed a lack of efficacy of TNFi in GCA, which makes a much better efficacy in a similar disease such as TA less likely. Therefore, a RCT on TNFi in TA is desirable.
Ustekinumab
A single nucleotide polymorphism of the IL-12B gene is associated with the clinical activity and complications of TA [84, 85]. In a small prospective observational study, an improvement in clinical symptoms and a decrease in inflammation markers was reported in 3 refractory TA patients who were already on treatment with conventional DMARD + GC and received the 12/23 antagonist ustekinumab on top [86]. All 3 patients did not show any change in intramural enhancement on MRA and no information on GC dose during therapy was provided. Therefore, further studies are needed to investigate a potential role of ustekinumab in TA.
Abatacept
In a double-blind RCT in newly diagnosed or relapsing TA, ABA did not reduce the relapse risk and had no GC-sparing effect [65].
Rituximab
In a retrospective case series, a progression of vascular lesions or persistent inflammatory activity was seen in 4 out of 7 patients following treatment with rituximab [87]. Individual case reports on the use of rituximab reporting favorable outcomes are limited by reporting bias [3, 88]. Very recently, autoantibodies against endothelial protein C receptor (EPCR) and scavenger receptor class B type 1 (SR-BI) were detected in 34.6% and 36.5% of patients with TA, respectively, with minimal overlap (3.8%) [89]. The antigens EPCR and SR-BI function as negative regulators of endothelial activation. EPCR has also an effect on human T cells and impair Th17 differentiation. The autoantibodies against EPCR and SR-BI were shown to block the functions of their targets, thereby promoting a pro-inflammatory phenotype. With the new discovery of pathogenetically relevant autoantibodies in a substantial proportion of TA patients, further studies on B cell targeted therapies such as rituximab are of interest.
JAK-Inhibition
Tofacitinib, a Janus kinase (JAK) inhibitor targeting JAK3 and JAK1, has been shown to suppress tissue-resident memory T cells and inhibit core effector pathways in an animal model in which human arteries were engrafted into immunodeficient mice that were reconstituted with T cells and monocytes from patients with GCA [90]. Recently, the successful treatment of 2 cases with TA by tofacitinib was reported [91, 92], while a lack of response was observed in two other cases [93].
Herbal Agents
Two RCTs from Asia reported a reduction of some proinflammatory cytokines after administration of plant TFNi (curcumin or resveratrol) in newly diagnosed TA [94, 95]. However, the primary endpoint of these studies (BVAS) is neither well suited nor fully validated for TA. In addition, the duration of treatment was very short (4 and 12 weeks, respectively) and there were no data reported on concomitant treatment of TA. Therefore, conclusions regarding a potential efficacy of curcumin or resveratrol cannot be drawn from these reports.
Future Perspectives of Immunomodulating Therapy in TA
In contrast to GCA, only a small number of trials in TA are listed on Clinicaltrials.gov lists (Table 4), some of them with unknown recruitment status. Two ongoing RCTs on JAK inhibition are of particular interest, given the potential role of the JAK/STAT pathway in LVV. In view of the low evidence available, well-designed RCTs in TA are desirable, particularly for IL-6- and TNFi, where signals of efficacy have been seen, but high-quality evidence showing a strong treatment effect for any of these drugs is still lacking. Furthermore, an RCT on the role of conventional immunosuppressants would be of importance.
Other Medications
Antiplatelet and Anticoagulant Therapy
Results of a retrospective study showed a reduction of acute ischemic events (HR 0.55; 95% CI 0.06–0.514) in TA patients taking antiplatelet therapy (62.5% of patients) [96]. Many patients in this cohort had cardiovascular risk factors such as hypertension (77.1%), hyperlipoproteinemia (45.8%), and obesity (16.7%), and in 44 of the 48 patients, cardiovascular disease was present in addition to TA. Anticoagulant therapy (12.5% of patients) was not associated with a reduction in the risk of ischemia. In view of the small sample size and high prevalence of cardiovascular comorbidities in this single study, antiplatelet therapy should be given in TA only if there is an indication for cardiovascular comorbidities, or if the vascular team identifies a potential benefit in an individual patient (e.g., critical vessel stenosis) [10].
Infection Prophylaxis
Like in GCA, data on the role of infection screening or prophylaxis for TA is lacking. A few cases of tuberculosis were reported in observational studies [3], so that screening and preventive measures should be considered before starting a biologic therapy.
Surgical/Interventional Treatment in GCA and TA
Surgical and interventional vascular therapies are not within the scope of this article. We therefore refer to the recently published results of two SLRs for the EULAR recommendations [3, 4]. In brief, such interventions need to be considered if a vascular lesion is symptomatic (e.g., claudication) or prognostically relevant (e.g., aortic aneurysm) and persists despite adequate medical therapy. All interventions should be performed during stable control of arterial inflammation [10].
Conclusion
We have reviewed the current evidence on the investigation and management of two forms of large vessel vasculitis. The evidence base is much larger for GCA than for TAK. There have been considerable advances in the use of imaging in the diagnostic process for both diseases. Pathogenetic mechanisms are beginning to form the basis for a more rational therapeutic approach to management with potentially less use of traditional therapy with glucocorticoids. While progress has been made, we still have no reliable measures to assess treatment response, predict disease severity or relapses.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Breuer GS, Poltorak V, Nesher G. Survival of patients with giant cell arteritis: a controversial issue. Clin Exp Rheumatol. 2020;38(Suppl 124):210–3.
Mirouse A, Biard L, Comarmond C, Lambert M, Mekinian A, Ferfar Y, et al. Overall survival and mortality risk factors in Takayasu’s arteritis: a multicenter study of 318 patients. J Autoimmun. 2019;96:35–9.
Agueda AF, Monti S, Luqmani RA, Buttgereit F, Cid M, Dasgupta B, et al. Management of Takayasu arteritis: a systematic literature review informing the 2018 update of the EULAR recommendation for the management of large vessel vasculitis. RMD Open. 2019;5:e001020.
Monti S, Agueda AF, Luqmani RA, Buttgereit F, Cid M, Dejaco C, et al. Systematic literature review informing the 2018 update of the EULAR recommendation for the management of large vessel vasculitis: focus on giant cell arteritis. RMD Open. 2019;5:e001003.
Restuccia G, Boiardi L, Cavazza A, Catanoso M, Macchioni P, Muratore F, et al. Flares in biopsy-proven giant cell arteritis in northern Italy: characteristics and predictors in a long-term follow-up study. Medicine (Baltimore). 2016;95:e3524.
Wilson JC, Sarsour K, Collinson N, Tuckwell K, Musselman D, Klearman M, et al. Incidence of outcomes potentially associated with corticosteroid therapy in patients with giant cell arteritis. Semin Arthritis Rheum. 2017;46:650–6.
Wilson JC, Sarsour K, Collinson N, Tuckwell K, Musselman D, Klearman M, et al. Serious adverse effects associated with glucocorticoid therapy in patients with giant cell arteritis (GCA): A nested case-control analysis. Semin Arthritis Rheum. 2017;46:819–27.
Stone JH, Tuckwell K, Dimonaco S, Klearman M, Aringer M, Blockmans D, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med. 2017;377:317–28. Large Phase-3 trial showing that tocilizumab plus a 26 week glococorticoid taper is superior in terms of disease control and glucocortiocid exposure compared to a 26 or 52 week glucocortioid taper.
Villiger PM, Adler S, Kuchen S, Wermelinger F, Dan D, Fiege V, et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2016;387:1921–7.
Hellmich B, Agueda A, Monti S, Buttgereit F, de Boysson H, Brouwer E, et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. 2020;79:19–30.
Mackie SL, Dejaco C, Appenzeller S, Camellino D, Duftner C, Gonzalez-Chiappe S, et al. British Society for Rheumatology guideline on diagnosis and treatment of giant cell arteritis. Rheumatology (Oxford). 2020;59:e1–e23.
Bienvenu B, Ly KH, Lambert M, Agard C, Andre M, Benhamou Y, et al. Management of giant cell arteritis: recommendations of the French Study Group for Large Vessel Vasculitis (GEFA). Rev Med Interne. 2016;37:154–65.
Turesson C, Borjesson O, Larsson K, Mohammad AJ, Knight A. Swedish Society of Rheumatology 2018 guidelines for investigation, treatment, and follow-up of giant cell arteritis. Scand J Rheumatol. 2019;48:259–65.
Patil P, Williams M, Maw WW, Achilleos K, Elsideeg S, Dejaco C, et al. Fast track pathway reduces sight loss in giant cell arteritis: results of a longitudinal observational cohort study. Clin Exp Rheumatol. 2015;33:S-103-106.
Diamantopoulos AP, Haugeberg G, Lindland A, Myklebust G. The fast-track ultrasound clinic for early diagnosis of giant cell arteritis significantly reduces permanent visual impairment: towards a more effective strategy to improve clinical outcome in giant cell arteritis? Rheumatology (Oxford). 2016;55:66–70.
Luqmani R, Lee E, Singh S, Gillett M, Schmidt WA, Bradburn M, et al. The role of ultrasound compared to biopsy of temporal arteries in the diagnosis and treatment of giant cell arteritis (TABUL): a diagnostic accuracy and cost-effectiveness study. Health Technol Assess. 2016;20:1–238.
Hauenstein C, Reinhard M, Geiger J, Markl M, Hetzel A, Treszl A, et al. Effects of early corticosteroid treatment on magnetic resonance imaging and ultrasonography findings in giant cell arteritis. Rheumatology (Oxford). 2012;51:1999–2003.
Nielsen BD, Gormsen LC, Hansen IT, Keller KK, Therkildsen P, Hauge EM. Three days of high-dose glucocorticoid treatment attenuates large-vessel 18F-FDG uptake in large-vessel giant cell arteritis but with a limited impact on diagnostic accuracy. Eur J Nucl Med Mol Imaging. 2018;45:1119–28.
Monti S, Delvino P, Bellis E, Milanesi A, Brandolino F, Montecucco C. Impact of delayed diagnoses at the time of COVID-19: increased rate of preventable bilateral blindness in giant cell arteritis. Ann Rheum Dis. 2020.
Mazlumzadeh M, Hunder GG, Easley KA, Calamia KT, Matteson EL, Griffing WL, et al. Treatment of giant cell arteritis using induction therapy with high-dose glucocorticoids: a double-blind, placebo-controlled, randomized prospective clinical trial. Arthritis Rheum. 2006;54:3310–8.
Chevalet P, Barrier JH, Pottier P, Magadur-Joly G, Pottier MA, Hamidou M, et al. A randomized, multicenter, controlled trial using intravenous pulses of methylprednisolone in the initial treatment of simple forms of giant cell arteritis: a one year followup study of 164 patients. J Rheumatol. 2000;27:1484–91.
Hayreh SS, Zimmerman B, Kardon RH. Visual improvement with corticosteroid therapy in giant cell arteritis. Report of a large study and review of literature. Acta Ophthalmol Scand. 2002;80:355–67.
Gonzalez-Gay MA, Blanco R, Rodriguez-Valverde V, Martinez-Taboada VM, Delgado-Rodriguez M, Figueroa M, et al. Permanent visual loss and cerebrovascular accidents in giant cell arteritis: predictors and response to treatment. Arthritis Rheum. 1998;41:1497–504.
Unizony SH, Dasgupta B, Fisheleva E, Rowell L, Schett G, Spiera R, et al. Design of the tocilizumab in giant cell arteritis trial. Int J Rheumatol. 2013;2013:912562.
Stone JH, Tuckwell K, Dimonaco S, Klearman M, Aringer M, Blockmans D, et al. Glucocorticoid dosages and acute-phase reactant levels at giant cell arteritis flare in a randomized trial of tocilizumab. Arthritis Rheum. 2019;71:1329–38. Analysis from the GIACTA trial showing that ESR and CRP are rarely elevated in relapsing GCA patients treated with tocilizumab.
Alba MA, Garcia-Martinez A, Prieto-Gonzalez S, Tavera-Bahillo I, Corbera-Bellalta M, Planas-Rigol E, et al. Relapses in patients with giant cell arteritis: prevalence, characteristics, and associated clinical findings in a longitudinally followed cohort of 106 patients. Medicine (Baltimore). 2014;93:194–201.
Labarca C, Koster MJ, Crowson CS, Makol A, Ytterberg SR, Matteson EL, et al. Predictors of relapse and treatment outcomes in biopsy-proven giant cell arteritis: a retrospective cohort study. Rheumatology (Oxford). 2016;55:347–56.
Martinez-Lado L, Calvino-Diaz C, Pineiro A, Dierssen T, Vazquez-Rodriguez TR, Miranda-Filloy JA, et al. Relapses and recurrences in giant cell arteritis: a population-based study of patients with biopsy-proven disease from northwestern Spain. Medicine (Baltimore). 2011;90:186–93.
Duru N, van der Goes MC, Jacobs JW, Andrews T, Boers M, Buttgereit F, et al. EULAR evidence-based and consensus-based recommendations on the management of medium to high-dose glucocorticoid therapy in rheumatic diseases. Ann Rheum Dis. 2013;72:1905–13.
Broder MS, Sarsour K, Chang E, Collinson N, Tuckwell K, Napalkov P, et al. Corticosteroid-related adverse events in patients with giant cell arteritis: a claims-based analysis. Semin Arthritis Rheum. 2016;46:246–52.
Serling-Boyd N, Stone JH. Recent advances in the diagnosis and management of giant cell arteritis. Curr Opin Rheumatol. 2020;32:201–7.
de Boysson H, Daumas A, Vautier M, Parienti JJ, Liozon E, Lambert M, et al. Large-vessel involvement and aortic dilation in giant-cell arteritis. A multicenter study of 549 patients. Autoimmun Rev. 2018;17:391–8.
Muratore F, Crescentini F, Spaggiari L, Pazzola G, Casali M, Boiardi L, et al. Aortic dilatation in patients with large vessel vasculitis: a longitudinal case control study using PET/CT. Semin Arthritis Rheum. 2019;48:1074–82.
Grayson PC, Alehashemi S, Bagheri AA, Civelek AC, Cupps TR, Kaplan MJ, et al. (18) F-Fluorodeoxyglucose-positron emission tomography as an imaging biomarker in a prospective, longitudinal cohort of patients with large vessel vasculitis. Arthritis Rheum. 2018;70:439–49.
Banerjee S, Quinn KA, Gribbons KB, Rosenblum JS, Civelek AC, Novakovich E, et al. Effect of treatment on imaging, clinical, and serologic assessments of disease activity in large-vessel vasculitis. J Rheumatol. 2020;47:99–107.
Dumont A, Parienti JJ, Delmas C, Boutemy J, Maigne G, Martin Silva N, et al. Factors associated with relapse and dependence on glucocorticoids in giant cell arteritis. J Rheumatol. 2020;47:108–16.
Mahr AD, Jover JA, Spiera RF, Hernandez-Garcia C, Fernandez-Gutierrez B, Lavalley MP, et al. Adjunctive methotrexate for treatment of giant cell arteritis: an individual patient data meta-analysis. Arthritis Rheum. 2007;56:2789–97.
Spiera RF, Mitnick HJ, Kupersmith M, Richmond M, Spiera H, Peterson MG, et al. A prospective, double-blind, randomized, placebo controlled trial of methotrexate in the treatment of giant cell arteritis (GCA). Clin Exp Rheumatol. 2001;19:495–501.
Hoffman GS, Cid MC, Hellmann DB, Guillevin L, Stone JH, Schousboe J, et al. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum. 2002;46:1309–18.
Jover JA, Hernandez-Garcia C, Morado IC, Vargas E, Banares A, Fernandez-Gutierrez B. Combined treatment of giant-cell arteritis with methotrexate and prednisone. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;134:106–14.
Koster MJ, Yeruva K, Crowson CS, Muratore F, Labarca C, Warrington KJ. Efficacy of methotrexate in real-world management of giant cell arteritis: a case-control study. J Rheumatol. 2019;46:501–8.
Stone J, Bao M, Han J, Aringer M, Blockmans D, Brouwer E, et al. Long-term outcome of tocilizumab for patients with giant cell arteritis: results from part 2 of a randomized controlled phase 3 trial [abstract]. Arthritis Rheum. 2019;71.
Adler S, Reichenbach S, Gloor A, Yerly D, Cullmann JL, Villiger PM. Risk of relapse after discontinuation of tocilizumab therapy in giant cell arteritis. Rheumatology (Oxford). 2019;58:1639–43.
Samson M, Bonnotte B. Analysis of IL-6 measurement in patients with GCA treated with tocilizumab should consider concomitant treatment with prednisone. Ann Rheum Dis 2019:annrheumdis-2019-215,697.
Berger CT, Daikeler T. Longitudinal versus cross-sectional IL-6 measurements in tocilizumab-treated GCA response to: ‘Analysis of IL-6 measurement in GCA patients treated with tocilizumab should consider concomitant treatment with prednisone’ by Samson et al. Ann Rheum Dis 2019:annrheumdis-2019-215,729.
Prieto-Gonzalez S, Terrades-Garcia N, Corbera-Bellalta M, Planas-Rigol E, Miyabe C, Alba MA, et al. Serum osteopontin: a biomarker of disease activity and predictor of relapsing course in patients with giant cell arteritis. Potential clinical usefulness in tocilizumab-treated patients. RMD Open. 2017;3:e000570.
Saito S, Okuyama A, Okada Y, Shibata A, Sakai R, Kurasawa T, Kondo T, et al. Tocilizumab monotherapy for large vessel vasculitis: results of 104-week treatment of a prospective, single-centre, open study. Rheumatology (Oxford) 2019; kez511.
Gloor AD, Yerly D, Adler S, Reichenbach S, Kuchen S, Seitz M, et al. Immuno-monitoring reveals an extended subclinical disease activity in tocilizumab-treated giant cell arteritis. Rheumatology (Oxford). 2018;57:1795–801.
Weyand CM, Goronzy JJ. Immune mechanisms in medium and large-vessel vasculitis. Nat Rev Rheumatol. 2013;9:731–40.
Conway R, O’Neill L, Gallagher P, McCarthy GM, Murphy CC, Veale DJ, et al. Ustekinumab for refractory giant cell arteritis: a prospective 52-week trial. Semin Arthritis Rheum. 2018;48:523–8.
Matza M, Stone J, Fernanadez A, Unizony S. Ustekinumab for the treatment of giant cell arteritis [abstract]. Arthritis Rheum. 2019;70.
Seror R, Baron G, Hachulla E, Debandt M, Larroche C, Puechal X, et al. Adalimumab for steroid sparing in patients with giant-cell arteritis: results of a multicentre randomised controlled trial. Ann Rheum Dis. 2014;73:2074–81.
Martinez-Taboada VM, Rodriguez-Valverde V, Carreno L, Lopez-Longo J, Figueroa M, Belzunegui J, et al. A double-blind placebo controlled trial of etanercept in patients with giant cell arteritis and corticosteroid side effects. Ann Rheum Dis. 2008;67:625–30.
Hoffman GS, Cid MC, Rendt-Zagar KE, Merkel PA, Weyand CM, Stone JH, et al. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med. 2007;146:621–30.
Gonzalez-Gay MA, Vazquez-Rodriguez TR, Gomez-Acebo I, Pego-Reigosa R, Lopez-Diaz MJ, Vazquez-Trinanes MC, et al. Strokes at time of disease diagnosis in a series of 287 patients with biopsy-proven giant cell arteritis. Medicine (Baltimore). 2009;88:227–35.
Uddhammar A, Eriksson AL, Nystrom L, Stenling R, Rantapaa-Dahlqvist S. Increased mortality due to cardiovascular disease in patients with giant cell arteritis in northern Sweden. J Rheumatol. 2002;29:737–42.
Nesher G, Berkun Y, Mates M, Baras M, Rubinow A, Sonnenblick M. Low-dose aspirin and prevention of cranial ischemic complications in giant cell arteritis. Arthritis Rheum. 2004;50:1332–7.
Lee MS, Smith SD, Galor A, Hoffman GS. Antiplatelet and anticoagulant therapy in patients with giant cell arteritis. Arthritis Rheum. 2006;54:3306–9.
Narvaez J, Bernad B, Gomez-Vaquero C, Garcia-Gomez C, Roig-Vilaseca D, Juanola X, et al. Impact of antiplatelet therapy in the development of severe ischemic complications and in the outcome of patients with giant cell arteritis. Clin Exp Rheumatol. 2008;26:S57–62.
Berger CT, Wolbers M, Meyer P, Daikeler T, Hess C. High incidence of severe ischaemic complications in patients with giant cell arteritis irrespective of platelet count and size, and platelet inhibition. Rheumatology (Oxford). 2009;48:258–61.
Martinez-Taboada VM, Lopez-Hoyos M, Narvaez J, Munoz-Cacho P. Effect of antiplatelet/anticoagulant therapy on severe ischemic complications in patients with giant cell arteritis: a cumulative meta-analysis. Autoimmun Rev. 2014;13:788–94.
Mollan SP, Sharrack N, Burdon MA, Denniston AK. Aspirin as adjunctive treatment for giant cell arteritis. Cochrane Database Syst Rev. 2014; CD010453.
McNeil JJ, Wolfe R, Woods RL, Tonkin AM, Donnan GA, Nelson MR, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509–18.
Nakaoka Y, Isobe M, Takei S, Tanaka Y, Ishii T, Yokota S, et al. Efficacy and safety of tocilizumab in patients with refractory Takayasu arteritis: results from a randomised, double-blind, placebo-controlled, phase 3 trial in Japan (the TAKT study). Ann Rheum Dis. 2018;77:348–54.
Langford CA, Cuthbertson D, Ytterberg SR, Khalidi N, Monach PA, Carette S, et al. A Randomized, double-blind trial of abatacept (CTLA-4Ig) for the treatment of takayasu arteritis. Arthritis Rheum. 2017;69:846–53.
Goel R, Danda D, Joseph G, Ravindran R, Kumar S, Jayaseelan V, et al. Long-term outcome of 251 patients with Takayasu arteritis on combination immunosuppressant therapy: single centre experience from a large tertiary care teaching hospital in Southern India. Semin Arthritis Rheum. 2018;47:718–26.
Comarmond C, Biard L, Lambert M, Mekinian A, Ferfar Y, Kahn JE, et al. Long-term outcomes and prognostic factors of complications in Takayasu arteritis: a multicenter study of 318 patients. Circulation. 2017;136:1114–22.
Hoffman GS, Leavitt RY, Kerr GS, Rottem M, Sneller MC, Fauci AS. Treatment of glucocorticoid-resistant or relapsing Takayasu arteritis with methotrexate. Arthritis Rheum. 1994;37:578–82.
Li J, Yang Y, Zhao J, Li M, Tian X, Zeng X. The efficacy of mycophenolate mofetil for the treatment of Chinese Takayasu’s arteritis. Sci Rep. 2016;6:38687.
Dai D, Wang Y, Jin H, Mao Y, Sun H. The efficacy of mycophenolate mofetil in treating Takayasu arteritis: a systematic review and meta-analysis. Rheumatol Int. 2017;37:1083–8.
Sun Y, Ma L, Ma L, Kong X, Chen H, Lv P, et al. Cyclophosphamide could be a better choice than methotrexate as induction treatment for patients with more severe Takayasu’s arteritis. Rheumatol Int. 2017;37:2019–26.
Valsakumar AK, Valappil UC, Jorapur V, Garg N, Nityanand S, Sinha N. Role of immunosuppressive therapy on clinical, immunological, and angiographic outcome in active Takayasu’s arteritis. J Rheumatol. 2003;30:1793–8.
de Souza AW, de Almeida Agustinelli R, de Cinque Almeida H, Oliveira PB, Pinheiro FA, Oliveira AC, et al. Leflunomide in Takayasu arteritis - a long term observational study. Rev Bras Reumatol Engl Ed. 2016;56:371–5.
Ohigashi H, Tamura N, Ebana Y, Harigai M, Maejima Y, Ashikaga T, et al. Effects of immunosuppressive and biological agents on refractory Takayasu arteritis patients unresponsive to glucocorticoid treatment. J Cardiol. 2017;69:774–8.
Nakaoka Y, Isobe M, Tanaka Y, Ishii T, Ooka S, Niiro H, Tamura N, et al. Long-term efficacy and safety of tocilizumab in refractory Takayasu arteritis: final results of the randomized controlled phase 3 TAKT study. Rheumatology (Oxford). 2020; kez630.
Abisror N, Mekinian A, Lavigne C, Vandenhende MA, Soussan M, Fain O, et al. Tocilizumab in refractory Takayasu arteritis: a case series and updated literature review. Autoimmun Rev. 2013;12:1143–9.
Kong X, Zhang X, Lv P, Cui X, Ma L, Chen H, et al. Treatment of Takayasu arteritis with the IL-6R antibody tocilizumab vs. cyclophosphamide. Int J Cardiol. 2018;266:222–8.
Mekinian A, Resche-Rigon M, Comarmond C, Soriano A, Constans J, Alric L, et al. Efficacy of tocilizumab in Takayasu arteritis: multicenter retrospective study of 46 patients. J Autoimmun. 2018;91:55–60.
Nakaoka Y, Higuchi K, Arita Y, Otsuki M, Yamamoto K, Hashimoto-Kataoka T, et al. Tocilizumab for the treatment of patients with refractory Takayasu arteritis. Int Heart J. 2013;54:405–11.
Tombetti E, Franchini S, Papa M, Sabbadini MG, Baldissera E. Treatment of refractory Takayasu arteritis with tocilizumab: 7 Italian patients from a single referral center. J Rheumatol. 2013;40:2047–51.
Xenitidis T, Horger M, Zeh G, Kanz L, Henes JC. Sustained inflammation of the aortic wall despite tocilizumab treatment in two cases of Takayasu arteritis. Rheumatology (Oxford). 2013;52:1729–31.
Hoffman GS, Merkel PA, Brasington RD, Lenschow DJ, Liang P. Anti-tumor necrosis factor therapy in patients with difficult to treat Takayasu arteritis. Arthritis Rheum. 2004;50:2296–304.
Mekinian A, Comarmond C, Resche-Rigon M, Mirault T, Kahn JE, Lambert M, et al. Efficacy of biological-targeted treatments in takayasu arteritis: multicenter, retrospective study of 49 patients. Circulation. 2015;132:1693–700.
Terao C, Yoshifuji H, Kimura A, Matsumura T, Ohmura K, Takahashi M, et al. Two susceptibility loci to Takayasu arteritis reveal a synergistic role of the IL12B and HLA-B regions in a Japanese population. Am J Hum Genet. 2013;93:289–97.
Terao C. Revisited HLA and non-HLA genetics of Takayasu arteritis--where are we? J Hum Genet. 2016;61:27–32.
Terao C, Yoshifuji H, Nakajima T, Yukawa N, Matsuda F, Mimori T. Ustekinumab as a therapeutic option for Takayasu arteritis: from genetic findings to clinical application. Scand J Rheumatol. 2016;45:80–2.
Pazzola G, Muratore F, Pipitone N, Crescentini F, Cacoub P, Boiardi L, et al. Rituximab therapy for Takayasu arteritis: a seven patients experience and a review of the literature. Rheumatology (Oxford). 2018;57:1151–5.
Mutoh T, Ishii T, Shirai T, Akita K, Kamogawa Y, Fujita Y, et al. Refractory Takayasu arteritis successfully treated with rituximab: case-based review. Rheumatol Int. 2019;39:1989–94.
Mutoh T, Shirai T, Ishii T, Shirota Y, Fujishima F, Takahashi F, et al. Identification of two major autoantigens negatively regulating endothelial activation in Takayasu arteritis. Nat Commun. 2020;11:1253.
Zhang H, Watanabe R, Berry GJ, Tian L, Goronzy JJ, Weyand CM. Inhibition of JAK-STAT signaling suppresses pathogenic immune responses in medium and large vessel vasculitis. Circulation. 2018;137:1934–48.
Yamamura Y, Matsumoto Y, Asano Y, Katayama Y, Hayashi K, Ohashi K, et al. Refractory Takayasu arteritis responding to the oral Janus kinase inhibitor, tofacitinib. Rheumatol Adv Pract. 2020;4:rkz050.
Sato S, Matsumoto H, Temmoku J, Fujita Y, Matsuoka N, Furuya M, et al. A case of Takayasu arteritis complicated by refractory ulcerative colitis successfully treated with tofacitinib. Rheumatology (Oxford). 2019.
Palermo A, Marvisi C, Casali M, Pipitone N, Muratore F, Salvarani C. Tofacitinib for the treatment of refractory Takayasu’s arteritis: description of 2 cases. Clin Exp Rheumatol. 2020;38(Suppl 124):234–5.
Shao N, Jia H, Li Y, Li J. Curcumin improves treatment outcome of Takayasu arteritis patients by reducing TNF-alpha: a randomized placebo-controlled double-blind clinical trial. Immunol Res. 2017;65:969–74.
Shi G, Hua M, Xu Q, Ren T. Resveratrol improves treatment outcome and laboratory parameters in patients with Takayasu arteritis: A randomized double-blind and placebo-controlled trial. Immunobiology. 2017;222:164–8.
de Souza AW, Machado NP, Pereira VM, Arraes AE, Reis Neto ET, Mariz HA, et al. Antiplatelet therapy for the prevention of arterial ischemic events in takayasu arteritis. Circ J. 2010;74:1236–41.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Bernhard Hellmich reports personal fees from Roche, personal fees from Chugai, personal fees from Abbvie, personal fees from Pfizer, personal fees from MSD, personal fees from Celgene, outside the submitted work. Raashid Luqmani reports grants from Vifor, grants from GSK, personal fees from Roche, grants from Roche, personal fees from AbbVie, grants from Celgene, outside the submitted work. Ana Agueda and Sara Monti declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Recent Advances in Large Vessel Vasculitis
Rights and permissions
About this article
Cite this article
Hellmich, B., Águeda, A.F., Monti, S. et al. Treatment of Giant Cell Arteritis and Takayasu Arteritis—Current and Future. Curr Rheumatol Rep 22, 84 (2020). https://doi.org/10.1007/s11926-020-00964-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s11926-020-00964-x