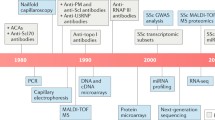
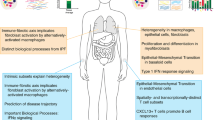
Abstract
Scleroderma is a heterogenous disease characterized by autoimmunity, a characteristic vasculopathy, and often widely varying extents of deep organ fibrosis. Recent advances in the understanding of scleroderma’s evolution have improved the ability to identify subgroups of patients with similar prognosis in order to improve risk stratification, enrich clinical trials for patients likely to benefit from specific therapies, and identify promising therapeutic targets for intervention. High-throughput technologies have recently identified fibrotic and inflammatory effectors in scleroderma that exhibit strong prognostic ability and may be tied to disease evolution. Increasingly, the use of collections of assayed circulating proteins and patterns of gene expression in tissue has replaced single-marker investigations in understanding the evolution of scleroderma and in objectively characterizing disease extent. Lastly, identification of shared patterns of disease evolution has allowed classification of patients into latent disease subtypes, which may allow rapid clinical prognostication and targeted management in both clinical and research settings. The concept of biomarkers in scleroderma is expanding to include nontraditional measures of aggregate protein signatures and disease evolution. This review examines the recent advances in biomarkers with a focus on those approaches poised to guide prospective management or themselves serve as quantitative surrogate disease outcomes.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001; 69(3):89–95. doi:10.1067/mcp.2001.113989.
Castelino FV, Varga J. Current status of systemic sclerosis biomarkers: applications for diagnosis, management and drug development. Expert Rev Clin Immunol. 2013;9(11):1077–90. doi:10.1586/1744666x.2013.848792.
Chora I, Guiducci S, Manetti M, Romano E, Mazzotta C, Bellando-Randone S, et al. Vascular biomarkers and correlation with peripheral vasculopathy in systemic sclerosis. Autoimmun Rev. 2015;14(4):314–22. doi:10.1016/j.autrev.2014.12.001.
Hummers LK. Biomarkers of vascular disease in scleroderma. Rheumatology (Oxford). 2008;47 Suppl 5:v21–2. doi:10.1093/rheumatology/ken281.
Hummers LK. The current state of biomarkers in systemic sclerosis. Curr Rheumatol Rep. 2010;12(1):34–9. doi:10.1007/s11926-009-0081-6.
Hummers LK, Hall A, Wigley FM, Simons M. Abnormalities in the regulators of angiogenesis in patients with scleroderma. J Rheumatol. 2009;36(3):576–82. doi:10.3899/jrheum.080516.
Lafyatis R. Application of biomarkers to clinical trials in systemic sclerosis. Curr Rheumatol Rep. 2012;14(1):47–55. doi:10.1007/s11926-011-0216-4.
O’Leary P, Waisman M. Acrosclerosis. Arch Intern Med. 1943; 47.
Rodnan GP, Benedek TG. An historical account of the study of progressive systemic sclerosis (diffuse scleroderma). Ann Intern Med. 1962;57(2):305–19.
Winterbauer RH. Multiple telangiectasia, Raynaud’s phenomenon, sclerodactyly, and subcutaneous calcinosis: a syndrome mimicking hereditary hemorrhagic telangiectasia. Bull Johns Hopkins Hosp. 1964;114:361–83.
LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger Jr TA, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15(2):202–5.
Cottrell TR, Wise RA, Wigley FM, Boin F. The degree of skin involvement identifies distinct lung disease outcomes and survival in systemic sclerosis. Ann Rheum Dis. 2014;73(6):1060–6. doi:10.1136/annrheumdis-2012-202849.
Nihtyanova SI, Schreiber BE, Ong VH, Rosenberg D, Moinzadeh P, Coghlan JG, et al. Prediction of pulmonary complications and long-term survival in systemic sclerosis. Arthritis Rheumatol. 2014;66(6):1625–35. doi:10.1002/art.38390.
Penn H, Howie AJ, Kingdon EJ, Bunn CC, Stratton RJ, Black CM, et al. Scleroderma renal crisis: patient characteristics and long-term outcomes. QJM. 2007;100(8):485–94. doi:10.1093/qjmed/hcm052.
Schieir O, Thombs BD, Hudson M, Boivin JF, Steele R, Bernatsky S, et al. Prevalence, severity, and clinical correlates of pain in patients with systemic sclerosis. Arthritis Care Res. 2010;62(3):409–17. doi:10.1002/acr.20108.
Jewett LR, Hudson M, Malcarne VL, Baron M, Thombs BD, Canadian Scleroderma Research G. Sociodemographic and disease correlates of body image distress among patients with systemic sclerosis. PLoS ONE. 2012;7(3):e33281. doi:10.1371/journal.pone.0033281.
Rodnan GP, Lipinski E, Luksick J. Skin thickness and collagen content in progressive systemic sclerosis and localized scleroderma. Arthritis Rheum. 1979;22(2):130–40.
Verrecchia F, Laboureau J, Verola O, Roos N, Porcher R, Bruneval P, et al. Skin involvement in scleroderma—where histological and clinical scores meet. Rheumatology (Oxford). 2007;46(5):833–41. doi:10.1093/rheumatology/kel451.
Furst DE, Clements PJ, Steen VD, Medsger Jr TA, Masi AT, D’Angelo WA, et al. The modified Rodnan skin score is an accurate reflection of skin biopsy thickness in systemic sclerosis. J Rheumatol. 1998;25(1):84–8.
Czirjak L, Nagy Z, Aringer M, Riemekasten G, Matucci-Cerinic M, Furst DE, et al. The EUSTAR model for teaching and implementing the modified Rodnan skin score in systemic sclerosis. Ann Rheum Dis. 2007;66(7):966–9. doi:10.1136/ard.2006.066530.
Kaloudi O, Bandinelli F, Filippucci E, Conforti ML, Miniati I, Guiducci S, et al. High frequency ultrasound measurement of digital dermal thickness in systemic sclerosis. Ann Rheum Dis. 2010;69(6):1140–3. doi:10.1136/ard.2009.114843.
Hesselstrand R, Kassner A, Heinegard D, Saxne T. COMP: a candidate molecule in the pathogenesis of systemic sclerosis with a potential as a disease marker. Ann Rheum Dis. 2008;67(9):1242–8. doi:10.1136/ard.2007.082099.
Hesselstrand R, Andreasson K, Wuttge DM, Bozovic G, Scheja A, Saxne T. Increased serum COMP predicts mortality in SSc: results from a longitudinal study of interstitial lung disease. Rheumatology (Oxford). 2012;51(5):915–20. doi:10.1093/rheumatology/ker442.
Fang F, Liu L, Yang Y, Tamaki Z, Wei J, Marangoni RG, et al. The adipokine adiponectin has potent anti-fibrotic effects mediated via adenosine monophosphate-activated protein kinase: novel target for fibrosis therapy. Arthritis Res Ther. 2012;14(5):R229. doi:10.1186/ar4070.
Tomcik M, Arima K, Hulejova H, Kuklova M, Filkova M, Braun M, et al. Adiponectin relation to skin changes and dyslipidemia in systemic sclerosis. Cytokine. 2012;58(2):165–8. doi:10.1016/j.cyto.2012.02.003.
Masui Y, Asano Y, Shibata S, Noda S, Aozasa N, Akamata K, et al. Serum adiponectin levels inversely correlate with the activity of progressive skin sclerosis in patients with diffuse cutaneous systemic sclerosis. J Eur Acad Dermatol Venereol. 2012;26(3):354–60. doi:10.1111/j.1468-3083.2011.04077.x.
Lakota K, Wei J, Carns M, Hinchcliff M, Lee J, Whitfield ML, et al. Levels of adiponectin, a marker for PPAR-gamma activity, correlate with skin fibrosis in systemic sclerosis: potential utility as biomarker? Arthritis Res Ther. 2012;14(3):R102. doi:10.1186/ar3827.
Arakawa H, Jinnin M, Muchemwa FC, Makino T, Kajihara I, Makino K, et al. Adiponectin expression is decreased in the involved skin and sera of diffuse cutaneous scleroderma patients. Exp Dermatol. 2011;20(9):764–6. doi:10.1111/j.1600-0625.2011.01310.x.
Marangoni RG, Korman BD, Wei J, Wood TA, Graham LV, Whitfield ML, et al. Myofibroblasts in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis Rheumatol. 2015;67(4):1062–73. doi:10.1002/art.38990.
Aidoudi S, Bikfalvi A. Interaction of PF4 (CXCL4) with the vasculature: a role in atherosclerosis and angiogenesis. Thromb Haemost. 2010;104(5):941–8. doi:10.1160/TH10-03-0193.
Zaldivar MM, Pauels K, von Hundelshausen P, Berres ML, Schmitz P, Bornemann J, et al. CXC chemokine ligand 4 (Cxcl4) is a platelet-derived mediator of experimental liver fibrosis. Hepatology. 2010;51(4):1345–53. doi:10.1002/hep.23435.
van Bon L, Affandi AJ, Broen J, Christmann RB, Marijnissen RJ, Stawski L, et al. Proteome-wide analysis and CXCL4 as a biomarker in systemic sclerosis. N Engl J Med. 2014;370(5):433–43. doi:10.1056/NEJMoa1114576. Dendritic cells isolated from persons with systemic sclerosis demonstrated a marked increase in CXCL4 expression, and the investigators go on to demonstrate differential hazards of clinical outcomes of interest which stratify by CXCL4 level in multiple external cohorts.
Farina G, Lafyatis D, Lemaire R, Lafyatis R. A four-gene biomarker predicts skin disease in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheum. 2010;62(2):580–8. doi:10.1002/art.27220.
Farina G, Lemaire R, Pancari P, Bayle J, Widom RL, Lafyatis R. Cartilage oligomeric matrix protein expression in systemic sclerosis reveals heterogeneity of dermal fibroblast responses to transforming growth factor beta. Ann Rheum Dis. 2009;68(3):435–41. doi:10.1136/ard.2007.086850.
Kissin EY, Merkel PA, Lafyatis R. Myofibroblasts and hyalinized collagen as markers of skin disease in systemic sclerosis. Arthritis Rheum. 2006;54(11):3655–60. doi:10.1002/art.22186.
York MR, Nagai T, Mangini AJ, Lemaire R, van Seventer JM, Lafyatis R. A macrophage marker, Siglec-1, is increased on circulating monocytes in patients with systemic sclerosis and induced by type I interferons and toll-like receptor agonists. Arthritis Rheum. 2007;56(3):1010–20. doi:10.1002/art.22382.
Rice LM, Ziemek J, Stratton EA, McLaughlin SR, Padilla CM, Mathes AL, et al. A longitudinal biomarker for the extent of skin disease in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheumatol. 2015. doi:10.1002/art.39287. From among the subset of genes that correlate with the mRSS, the investigators develop and validate combination assays from skin biopsies which result in best model fit with mRSS.
Rice LM, Padilla CM, McLaughlin SR, Mathes A, Ziemek J, Goummih S, et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J Clin Invest. 2015;125(7):2795–807. doi:10.1172/JCI77958.
Milano A, Pendergrass SA, Sargent JL, George LK, McCalmont TH, Connolly MK, et al. Molecular subsets in the gene expression signatures of scleroderma skin. PLoS ONE. 2008;3(7):e2696. doi:10.1371/journal.pone.0002696.
Pendergrass SA, Lemaire R, Francis IP, Mahoney JM, Lafyatis R, Whitfield ML. Intrinsic gene expression subsets of diffuse cutaneous systemic sclerosis are stable in serial skin biopsies. J Invest Dermatol. 2012;132(5):1363–73. doi:10.1038/jid.2011.472.
Hinchcliff M, Huang CC, Wood TA, Matthew Mahoney J, Martyanov V, Bhattacharyya S, et al. Molecular signatures in skin associated with clinical improvement during mycophenolate treatment in systemic sclerosis. J Invest Dermatol. 2013;133(8):1979–89. doi:10.1038/jid.2013.130. Intrinsic subsets are applied to a cohort to demonstrate that mycophenolate-response is limited to patients in the inflammatory subset.
Parkes J, Guha IN, Roderick P, Harris S, Cross R, Manos MM, et al. Enhanced liver fibrosis (ELF) test accurately identifies liver fibrosis in patients with chronic hepatitis C. J Viral Hepat. 2011;18(1):23–31. doi:10.1111/j.1365-2893.2009.01263.x.
Abignano G, Cuomo G, Buch MH, Rosenberg WM, Valentini G, Emery P, et al. The enhanced liver fibrosis test: a clinical grade, validated serum test, biomarker of overall fibrosis in systemic sclerosis. Ann Rheum Dis. 2014;73(2):420–7. doi:10.1136/annrheumdis-2012-202843. A serologic assay consisting of three markers initially derived from patients with hepatic fibrosis demonstrates correlation with mRSS as well as physiologic and functional indices of activity.
Nagy Z, Czirjak L. Increased levels of amino terminal propeptide of type III procollagen are an unfavourable predictor of survival in systemic sclerosis. Clin Exp Rheumatol. 2005;23(2):165–72.
Kikuchi K, Kubo M, Sato S, Fujimoto M, Tamaki K. Serum tissue inhibitor of metalloproteinases in patients with systemic sclerosis. J Am Acad Dermatol. 1995;33(6):973–8.
Levesque H, Baudot N, Delpech B, Vayssairat M, Gancel A, Lauret P, et al. Clinical correlations and prognosis based on hyaluronic acid serum levels in patients with progressive systemic sclerosis. Br J Dermatol. 1991;124(5):423–8.
Simonneau G, Galie N, Jansa P, Meyer GM, Al-Hiti H, Kusic-Pajic A, et al. Long-term results from the EARLY study of bosentan in WHO functional class II pulmonary arterial hypertension patients. Int J Cardiol. 2014;172(2):332–9. doi:10.1016/j.ijcard.2013.12.179.
Humbert M, Yaici A, de Groote P, Montani D, Sitbon O, Launay D, et al. Screening for pulmonary arterial hypertension in patients with systemic sclerosis: clinical characteristics at diagnosis and long-term survival. Arthritis Rheum. 2011;63(11):3522–30. doi:10.1002/art.30541.
Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi:10.1038/nature10144.
Thakkar V, Stevens W, Prior D, Youssef P, Liew D, Gabbay E, et al. The inclusion of N-terminal pro-brain natriuretic peptide in a sensitive screening strategy for systemic sclerosis-related pulmonary arterial hypertension: a cohort study. Arthritis Res Ther. 2013;15(6):R193. doi:10.1186/ar4383.
Coghlan JG, Denton CP, Grunig E, Bonderman D, Distler O, Khanna D, et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis. 2014;73(7):1340–9. doi:10.1136/annrheumdis-2013-203301.
Allanore Y, Borderie D, Meune C, Cabanes L, Weber S, Ekindjian OG, et al. N-terminal pro-brain natriuretic peptide as a diagnostic marker of early pulmonary artery hypertension in patients with systemic sclerosis and effects of calcium-channel blockers. Arthritis Rheum. 2003;48(12):3503–8. doi:10.1002/art.11345.
Allanore Y, Borderie D, Avouac J, Zerkak D, Meune C, Hachulla E, et al. High N-terminal pro-brain natriuretic peptide levels and low diffusing capacity for carbon monoxide as independent predictors of the occurrence of precapillary pulmonary arterial hypertension in patients with systemic sclerosis. Arthritis Rheum. 2008;58(1):284–91. doi:10.1002/art.23187.
Williams MH, Handler CE, Akram R, Smith CJ, Das C, Smee J, et al. Role of N-terminal brain natriuretic peptide (N-TproBNP) in scleroderma-associated pulmonary arterial hypertension. Eur Heart J. 2006;27(12):1485–94. doi:10.1093/eurheartj/ehi891.
Allanore Y, Wahbi K, Borderie D, Weber S, Kahan A, Meune C. N-terminal pro-brain natriuretic peptide in systemic sclerosis: a new cornerstone of cardiovascular assessment? Ann Rheum Dis. 2009;68(12):1885–9. doi:10.1136/ard.2008.098087. Higher levels of the antiangiogenic splice variant VEGF-165b are cross-sectionally demonstrated among patients with scleroderma compared to controls, and those with a late nailfold capillary pattern compared to early pattern.
Avouac J, Meune C, Chenevier-Gobeaux C, Borderie D, Lefevre G, Kahan A, et al. Cardiac biomarkers in systemic sclerosis: contribution of high-sensitivity cardiac troponin in addition to N-terminal pro-brain natriuretic peptide. Arthritis Care Res. 2015;67(7):1022–30. doi:10.1002/acr.22547.
Farouk HM, Hamza SH, El Bakry SA, Youssef SS, Aly IM, Moustafa AA, et al. Dysregulation of angiogenic homeostasis in systemic sclerosis. Int J Rheum Dis. 2013;16(4):448–54. doi:10.1111/1756-185X.12130.
Riccieri V, Stefanantoni K, Vasile M, Macri V, Sciarra I, Iannace N, et al. Abnormal plasma levels of different angiogenic molecules are associated with different clinical manifestations in patients with systemic sclerosis. Clin Exp Rheumatol. 2011;29(2 Suppl 65):S46–52.
Glodkowska-Mrowka E, Gorska E, Ciurzynski M, Stelmaszczyk-Emmel A, Bienias P, Irzyk K, et al. Pro- and antiangiogenic markers in patients with pulmonary complications of systemic scleroderma. Respir Physiol Neurobiol. 2014. doi:10.1016/j.resp.2014.10.018.
Manetti M, Guiducci S, Romano E, Bellando-Randone S, Lepri G, Bruni C, et al. Increased plasma levels of the VEGF165b splice variant are associated with the severity of nailfold capillary loss in systemic sclerosis. Ann Rheum Dis. 2013;72(8):1425–7. doi:10.1136/annrheumdis-2012-203183. Cross-sectional and prospective analyses of the influence of endothelin receptor type A and Ang receptor type-1 stimulating antibodies on the prevalence and hazard of pulmonary arterial hypertension.
Distler O, Del Rosso A, Giacomelli R, Cipriani P, Conforti ML, Guiducci S, et al. Angiogenic and angiostatic factors in systemic sclerosis: increased levels of vascular endothelial growth factor are a feature of the earliest disease stages and are associated with the absence of fingertip ulcers. Arthritis Res. 2002;4(6):R11.
Dunne JV, Keen KJ, Van Eeden SF. Circulating angiopoietin and Tie-2 levels in systemic sclerosis. Rheumatol Int. 2013;33(2):475–84. doi:10.1007/s00296-012-2378-4.
Avouac J, Meune C, Ruiz B, Couraud PO, Uzan G, Boileau C, et al. Angiogenic biomarkers predict the occurrence of digital ulcers in systemic sclerosis. Ann Rheum Dis. 2012;71(3):394–9. doi:10.1136/annrheumdis-2011-200143. Cox regression is applied to a prospective cohort to determine risk factors for digital ulcers from a selection of circulating vascular markers and nailfold capillary pattern.
Hamaguchi Y, Hasegawa M, Tanaka C, Kumada S, Sato S, Takehara K, et al. Elevated serum placenta growth factor (PlGF) levels in patients with systemic sclerosis: a possible role in the development of skin but not lung fibrosis. J Dermatol Sci. 2010;58(3):229–31. doi:10.1016/j.jdermsci.2010.04.004.
Becker MO, Kill A, Kutsche M, Guenther J, Rose A, Tabeling C, et al. Vascular receptor autoantibodies in pulmonary arterial hypertension associated with systemic sclerosis. Am J Respir Crit Care Med. 2014;190(7):808–17. doi:10.1164/rccm.201403-0442OC.
Ciurzynski M, Bienias P, Irzyk K, Kostrubiec M, Bartoszewicz Z, Siwicka M, et al. Serum endothelin-1 and NT-proBNP, but not ADMA, endoglin and TIMP-1 levels, reflect impaired right ventricular function in patients with systemic sclerosis. Clin Rheumatol. 2014;33(1):83–9. doi:10.1007/s10067-013-2354-8.
Silva I, Almeida J, Vasconcelos C. A PRISMA-driven systematic review for predictive risk factors of digital ulcers in systemic sclerosis patients. Autoimmun Rev. 2015;14(2):140–52. doi:10.1016/j.autrev.2014.10.009.
Silva I, Teixeira A, Oliveira J, Almeida I, Almeida R, Aguas A, et al. Endothelial dysfunction and nailfold videocapillaroscopy pattern as predictors of digital ulcers in systemic sclerosis: a cohort study and review of the literature. Clin Rev Allergy Immunol. 2015. doi:10.1007/s12016-015-8500-0. Prospective analysis of several vascular biomarkers in scleroderma patients with PAH compared to those without demonstrates differential expression of soluble Flt-1 and PlGF.
Solanilla A, Villeneuve J, Auguste P, Hugues M, Alioum A, Lepreux S, et al. The transport of high amounts of vascular endothelial growth factor by blood platelets underlines their potential contribution in systemic sclerosis angiogenesis. Rheumatology (Oxford). 2009;48(9):1036–44. doi:10.1093/rheumatology/kep154.
Vempati P, Popel AS, Mac Gabhann F. Extracellular regulation of VEGF: isoforms, proteolysis, and vascular patterning. Cytokine Growth Factor Rev. 2014;25(1):1–19. doi:10.1016/j.cytogfr.2013.11.002.
De Vivo A, Baviera G, Giordano D, Todarello G, Corrado F, D’Anna R. Endoglin, PlGF and sFlt-1 as markers for predicting pre-eclampsia. Acta Obstet Gynecol Scand. 2008;87(8):837–42. doi:10.1080/00016340802253759.
Lucy C, Suzy D, Melanie G, Paul S, Christopher R, Andrew S. OP007. PLGF in combination with other commonly utilised tests and other biomarkers for predicting need for delivery for pre-eclampsia within 14 days in women presenting prior to 35 weeks’ gestation. Pregnancy Hypertens. 2013;3(2):64–5. doi:10.1016/j.preghy.2013.04.023.
McMahan Z, Schoenhoff F, Van Eyk JE, Wigley FM, Hummers LK. Biomarkers of pulmonary hypertension in patients with scleroderma: a case-control study. Arthritis Res Ther. 2015;17:201. doi:10.1186/s13075-015-0712-4.
Shi-wen X, Kennedy L, Renzoni EA, Bou-Gharios G, du Bois RM, Black CM, et al. Endothelin is a downstream mediator of profibrotic responses to transforming growth factor beta in human lung fibroblasts. Arthritis Rheum. 2007;56(12):4189–94. doi:10.1002/art.23134.
Jain R, Shaul PW, Borok Z, Willis BC. Endothelin-1 induces alveolar epithelial-mesenchymal transition through endothelin type A receptor-mediated production of TGF-beta1. Am J Respir Cell Mol Biol. 2007;37(1):38–47. doi:10.1165/rcmb.2006-0353OC.
Chung L, Ball K, Yaqub A, Lingala B, Fiorentino D. Effect of the endothelin type A-selective endothelin receptor antagonist ambrisentan on digital ulcers in patients with systemic sclerosis: results of a prospective pilot study. J Am Acad Dermatol. 2014;71(2):400–1. doi:10.1016/j.jaad.2014.04.028.
Matucci-Cerinic M, Denton CP, Furst DE, Mayes MD, Hsu VM, Carpentier P, et al. Bosentan treatment of digital ulcers related to systemic sclerosis: results from the RAPIDS-2 randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2011;70(1):32–8. doi:10.1136/ard.2010.130658.
Korn JH, Mayes M, Matucci Cerinic M, Rainisio M, Pope J, Hachulla E, et al. Digital ulcers in systemic sclerosis: prevention by treatment with bosentan, an oral endothelin receptor antagonist. Arthritis Rheum. 2004;50(12):3985–93. doi:10.1002/art.20676.
Humbert M, Barst RJ, Robbins IM, Channick RN, Galie N, Boonstra A, et al. Combination of bosentan with epoprostenol in pulmonary arterial hypertension: BREATHE-2. Eur Respir J. 2004;24(3):353–9. doi:10.1183/09031936.04.00028404. Prospective study examining associations among endostatin and deep organ manifestations in scleroderma and mixed connective tissue disease.
Oudiz RJ, Galie N, Olschewski H, Torres F, Frost A, Ghofrani HA, et al. Long-term ambrisentan therapy for the treatment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54(21):1971–81. doi:10.1016/j.jacc.2009.07.033. VEGF and circulating endothelial progenitor cells are examined cross-sectionally in consecutive scleroderma patients and compared across nailfold capillary patterns.
Galie N, Olschewski H, Oudiz RJ, Torres F, Frost A, Ghofrani HA, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117(23):3010–9. doi:10.1161/CIRCULATIONAHA.107.742510.
Wipff J, Avouac J, Borderie D, Zerkak D, Lemarechal H, Kahan A, et al. Disturbed angiogenesis in systemic sclerosis: high levels of soluble endoglin. Rheumatology (Oxford). 2008;47(7):972–5. doi:10.1093/rheumatology/ken100.
Hebbar M, Peyrat JP, Hornez L, Hatron PY, Hachulla E, Devulder B. Increased concentrations of the circulating angiogenesis inhibitor endostatin in patients with systemic sclerosis. Arthritis Rheum. 2000;43(4):889–93. doi:10.1002/1529-0131(200004)43:4<889::AID-ANR21>3.0.CO;2-5.
Reiseter S, Molberg O, Gunnarsson R, Lund MB, Aalokken TM, Aukrust P, et al. Associations between circulating endostatin levels and vascular organ damage in systemic sclerosis and mixed connective tissue disease: an observational study. Arthritis Res Ther. 2015;17(1):231. doi:10.1186/s13075-015-0756-5.
Avouac J, Vallucci M, Smith V, Senet P, Ruiz B, Sulli A, et al. Correlations between angiogenic factors and capillaroscopic patterns in systemic sclerosis. Arthritis Res Ther. 2013;15(2):R55. doi:10.1186/ar4217.
Distler JH, Akhmetshina A, Dees C, Jungel A, Sturzl M, Gay S, et al. Induction of apoptosis in circulating angiogenic cells by microparticles. Arthritis Rheum. 2011;63(7):2067–77. doi:10.1002/art.30361. Circulating levels of adhesion molecules are examined serially over 4 years and compared to indices of lung function, skin severity, quality of life, and rate of progression.
Trojanowska M. Cellular and molecular aspects of vascular dysfunction in systemic sclerosis. Nat Rev Rheumatol. 2010;6(8):453–60. doi:10.1038/nrrheum.2010.102.
Manetti M, Guiducci S, Romano E, Bellando-Randone S, Conforti ML, Ibba-Manneschi L, et al. Increased serum levels and tissue expression of matrix metalloproteinase-12 in patients with systemic sclerosis: correlation with severity of skin and pulmonary fibrosis and vascular damage. Ann Rheum Dis. 2012;71(6):1064–72. doi:10.1136/annrheumdis-2011-200837.
Serrati S, Cinelli M, Margheri F, Guiducci S, Del Rosso A, Pucci M, et al. Systemic sclerosis fibroblasts inhibit in vitro angiogenesis by MMP-12-dependent cleavage of the endothelial cell urokinase receptor. J Pathol. 2006;210(2):240–8. doi:10.1002/path.2048.
Kim WU, Min SY, Cho ML, Hong KH, Shin YJ, Park SH, et al. Elevated matrix metalloproteinase-9 in patients with systemic sclerosis. Arthritis Res Ther. 2005;7(1):R71–9. doi:10.1186/ar1454.
Hasegawa M, Asano Y, Endo H, Fujimoto M, Goto D, Ihn H, et al. Serum adhesion molecule levels as prognostic markers in patients with early systemic sclerosis: a multicentre, prospective, observational study. PLoS ONE. 2014;9(2):e88150. doi:10.1371/journal.pone.0088150.
Mihai C, Tervaert JW. Anti-endothelial cell antibodies in systemic sclerosis. Ann Rheum Dis. 2010;69(2):319–24. doi:10.1136/ard.2008.102400.
Villalta D, Morozzi G, Tampoia M, Alpini C, Brusca I, Salgarolo V, et al. Antibodies to fibrillarin, PM-Scl and RNA polymerase III detected by ELISA assays in patients with systemic sclerosis. Clin Chim Acta. 2010;411(9–10):710–3. doi:10.1016/j.cca.2010.01.037.
Aggarwal R, Lucas M, Fertig N, Oddis CV, Medsger Jr TA. Anti-U3 RNP autoantibodies in systemic sclerosis. Arthritis Rheum. 2009;60(4):1112–8. doi:10.1002/art.24409.
Cutolo M, Pizzorni C, Secchi ME, Sulli A. Capillaroscopy. Best Pract Res Clin Rheumatol. 2008;22(6):1093–108. doi:10.1016/j.berh.2008.09.001.
Bredemeier M, Xavier RM, Capobianco KG, Restelli VG, Rohde LE, Pinotti AF, et al. Nailfold capillary microscopy can suggest pulmonary disease activity in systemic sclerosis. J Rheumatol. 2004;31(2):286–94.
Hofstee HM, Vonk Noordegraaf A, Voskuyl AE, Dijkmans BA, Postmus PE, Smulders YM, et al. Nailfold capillary density is associated with the presence and severity of pulmonary arterial hypertension in systemic sclerosis. Ann Rheum Dis. 2009;68(2):191–5. doi:10.1136/ard.2007.087353.
Sato LT, Kayser C, Andrade LE. Nailfold capillaroscopy abnormalities correlate with cutaneous and visceral involvement in systemic sclerosis patients. Acta Reumatol Port. 2009;34(2A):219–27.
Riccieri V, Vasile M, Iannace N, Stefanantoni K, Sciarra I, Vizza CD, et al. Systemic sclerosis patients with and without pulmonary arterial hypertension: a nailfold capillaroscopy study. Rheumatology (Oxford). 2013;52(8):1525–8. doi:10.1093/rheumatology/ket168.
Castellvi I, Simeon-Aznar CP, Sarmiento M, Fortuna A, Mayos M, Geli C, et al. Association between nailfold capillaroscopy findings and pulmonary function tests in patients with systemic sclerosis. J Rheumatol. 2015;42(2):222–7. doi:10.3899/jrheum.140276.
Winstone TA, Assayag D, Wilcox PG, Dunne JV, Hague CJ, Leipsic J, et al. Predictors of mortality and progression in scleroderma-associated interstitial lung disease: a systematic review. Chest. 2014;146(2):422–36. doi:10.1378/chest.13-2626.
Tyndall AJ, Bannert B, Vonk M, Airo P, Cozzi F, Carreira PE, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69(10):1809–15. doi:10.1136/ard.2009.114264.
Walker UA, Tyndall A, Czirjak L, Denton C, Farge-Bancel D, Kowal-Bielecka O, et al. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials And Research group database. Ann Rheum Dis. 2007;66(6):754–63. doi:10.1136/ard.2006.062901.
Hamaguchi Y, Hasegawa M, Fujimoto M, Matsushita T, Komura K, Kaji K, et al. The clinical relevance of serum antinuclear antibodies in Japanese patients with systemic sclerosis. Br J Dermatol. 2008;158(3):487–95. doi:10.1111/j.1365-2133.2007.08392.x.
Assassi S, Sharif R, Lasky RE, McNearney TA, Estrada YMRM, Draeger H, et al. Predictors of interstitial lung disease in early systemic sclerosis: a prospective longitudinal study of the GENISOS cohort. Arthritis Res Ther. 2010;12(5):R166. doi:10.1186/ar3125.
Fertig N, Domsic RT, Rodriguez-Reyna T, Kuwana M, Lucas M, Medsger Jr TA, et al. Anti-U11/U12 RNP antibodies in systemic sclerosis: a new serologic marker associated with pulmonary fibrosis. Arthritis Rheum. 2009;61(7):958–65. doi:10.1002/art.24586.
Mitri GM, Lucas M, Fertig N, Steen VD, Medsger Jr TA. A comparison between anti-Th/To- and anticentromere antibody-positive systemic sclerosis patients with limited cutaneous involvement. Arthritis Rheum. 2003;48(1):203–9. doi:10.1002/art.10760. In a letter, the authors present Kaplan Meier curves for lung decline or mortality stratified by CCL18 levels.
Ceribelli A, Cavazzana I, Franceschini F, Airo P, Tincani A, Cattaneo R, et al. Anti-Th/To are common antinucleolar autoantibodies in Italian patients with scleroderma. J Rheumatol. 2010;37(10):2071–5. doi:10.3899/jrheum.100316. Using the GENISOS cohort, the ability of SP-D and CCL18 to predict short- and long-term lung restriction is examined.
Kodera M, Hasegawa M, Komura K, Yanaba K, Takehara K, Sato S. Serum pulmonary and activation-regulated chemokine/CCL18 levels in patients with systemic sclerosis: a sensitive indicator of active pulmonary fibrosis. Arthritis Rheum. 2005;52(9):2889–96. doi:10.1002/art.21257.
Prasse A, Pechkovsky DV, Toews GB, Schafer M, Eggeling S, Ludwig C, et al. CCL18 as an indicator of pulmonary fibrotic activity in idiopathic interstitial pneumonias and systemic sclerosis. Arthritis Rheum. 2007;56(5):1685–93. doi:10.1002/art.22559.
Tiev KP, Hua-Huy T, Kettaneh A, Gain M, Duong-Quy S, Toledano C, et al. Serum CC chemokine ligand-18 predicts lung disease worsening in systemic sclerosis. Eur Respir J. 2011;38(6):1355–60. doi:10.1183/09031936.00004711.
Schupp J, Becker M, Gunther J, Muller-Quernheim J, Riemekasten G, Prasse A. Serum CCL18 is predictive for lung disease progression and mortality in systemic sclerosis. Eur Respir J. 2014;43(5):1530–2. doi:10.1183/09031936.00131713. The authors develop and validate a mortality prediction tool using clinical data and the skin thickness progression rate (STPR).
Elhaj M, Charles J, Pedroza C, Liu X, Zhou X, Estrada YMRM, et al. Can serum surfactant protein D or CC-chemokine ligand 18 predict outcome of interstitial lung disease in patients with early systemic sclerosis? J Rheumatol. 2013;40(7):1114–20. doi:10.3899/jrheum.120997.
Lota HK, Renzoni EA. Circulating biomarkers of interstitial lung disease in systemic sclerosis. Int J Rheumatol. 2012;2012:121439. doi:10.1155/2012/121439.
Domsic RT, Rodriguez-Reyna T, Lucas M, Fertig N, Medsger Jr TA. Skin thickness progression rate: a predictor of mortality and early internal organ involvement in diffuse scleroderma. Ann Rheum Dis. 2011;70(1):104–9. doi:10.1136/ard.2009.127621.
Perera A, Fertig N, Lucas M, Rodriguez-Reyna TS, Hu P, Steen VD, et al. Clinical subsets, skin thickness progression rate, and serum antibody levels in systemic sclerosis patients with anti-topoisomerase I antibody. Arthritis Rheum. 2007;56(8):2740–6. doi:10.1002/art.22747. Serial adiponectin levels are examined in a cohort treated with cyclophosphamide and found to change in concert with changes in pFVC.
Domsic RT, Nihtyanova SI, Wisniewski SR, Fine MJ, Lucas M, Kwoh CK, et al. Derivation and validation of a prediction rule for two-year mortality in early diffuse cutaneous systemic sclerosis. Arthritis Rheumatol. 2014;66(6):1616–24. doi:10.1002/art.38381. A clinically-driven risk score was derived in a US cohort and validated in a UK cohort, with skin thickness progression rate, gastrointestinal severity, anemia, and age at first visit contributing to a two-year mortality risk score.
Man A, Davidyock T, Ferguson LT, Ieong M, Zhang Y, Simms RW. Changes in forced vital capacity over time in systemic sclerosis: application of group-based trajectory modelling. Rheumatology (Oxford). 2015. doi:10.1093/rheumatology/kev016.
Shand L, Lunt M, Nihtyanova S, Hoseini M, Silman A, Black CM, et al. Relationship between change in skin score and disease outcome in diffuse cutaneous systemic sclerosis: application of a latent linear trajectory model. Arthritis Rheum. 2007;56(7):2422–31. doi:10.1002/art.22721.
Schulam P, Wigley F, Saria S, editors. Clustering longitudinal clinical marker trajectories from electronic health data: applications to phenotyping and endotype discovery. The Twenty-Ninth AAAI Conference on Artificial Intelligence (AAAI-15). 2015.
Masui Y, Asano Y, Takahashi T, Shibata S, Akamata K, Aozasa N, et al. Clinical significance of monitoring serum adiponectin levels during intravenous pulse cyclophosphamide therapy in interstitial lung disease associated with systemic sclerosis. Mod Rheumatol. 2013;23(2):323–9. doi:10.1007/s10165-012-0660-7.
Hant FN, Ludwicka-Bradley A, Wang HJ, Li N, Elashoff R, Tashkin DP, et al. Surfactant protein D and KL-6 as serum biomarkers of interstitial lung disease in patients with scleroderma. J Rheumatol. 2009;36(4):773–80. doi:10.3899/jrheum.080633.
Hasegawa M, Fujimoto M, Hamaguchi Y, Matsushita T, Inoue K, Sato S, et al. Use of serum clara cell 16-kDa (CC16) levels as a potential indicator of active pulmonary fibrosis in systemic sclerosis. J Rheumatol. 2011;38(5):877–84. doi:10.3899/jrheum.100591.
Acknowledgments
We would like to acknowledge the Scleroderma Research Foundation for institutional support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial Support and Sponsorship
Colin Ligon was supported by the National Institute of Arthritis, Musculoskeletal, and Skin Diseases T32 Award AR048522.
Conflict of Interest
The authors declare that they have no competing interests.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
This article is part of the Topical Collection on Scleroderma
Rights and permissions
About this article
Cite this article
Ligon, C., Hummers, L.K. Biomarkers in Scleroderma: Progressing from Association to Clinical Utility. Curr Rheumatol Rep 18, 17 (2016). https://doi.org/10.1007/s11926-016-0565-0
Published:
DOI: https://doi.org/10.1007/s11926-016-0565-0